Abstract
Management of neonatal native coarctation is debated till now. Surgical therapy remains an option but may be unwarranted in critically sick infants with complex lesions. Balloon dilatation has been employed but with early re-stenosis. Stent angioplasty has also been used but as a bridge towards definitive surgical therapy. Four critically sick infants with complex coarctation and additional co-morbidity factors underwent primary stent therapy as surgical intervention was denied. One patient had died earlier due to reasons unrelated to the procedure. Three survivors underwent multiple dilatations of primary stents as indicated. One of the three survivors did not require any further dilatation after the age of 5 years and remained stable till the time of reporting. High-pressure Cheatham Platinum stents were implanted inside the primary stents in two infants, who developed re-stenosis due to somatic growth. These stents were further balloon dilated at high atmospheric pressure. Femoral arteries in both of them were blocked but were re-canalized after balloon dilatation in one and stent angioplasty in the other. After a follow-up of about 15 years, all of them have been doing fine with acceptable Doppler gradients. They were normotensive and on no cardiac medications. It can be concluded that, though surgical repair remains a standard of care, stent angioplasty in selected infants with complex lesions is feasible and effective. Multiple dilatations can be performed without added risk of stent migration. Bio-absorbable and growth stents hold a promise for future use in such situations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coarctation of the aorta is one of the four most common lesions requiring surgical or trans-catheter intervention early in life [1, 2]. About half of the children who presented earlier have a more complex lesion and demand immediate and aggressive intervention because of additional co-morbidity factors including renal failure and left ventricular dysfunction and/or failure. Management options for this group of patients are still debated. Surgical repair remains an option but is associated with a mortality rate ranging between 5 and 15% [3, 4]. Even an overall mortality of 25% has also been reported [5]. At the same time, the long-term outcome in the neonates and the risk factors for operative mortality remain controversial. Balloon angioplasty has also been effectively utilized as an alternate to standard surgical therapy, but its efficacy during infancy is still debated due to the risks of re-stenosis and aneurysm formation [6]. Palliative stent angioplasty for native aortic coarctation has occasionally been performed in critically ill, premature low-birth weight newborns, infants, and young children, but as a bridge towards corrective repair [7,8,9,10]. Occasionally, the complexity of the lesions and the presence of other co-morbidity factors further limit management options.
Palliative stent implantation was used in four young children with complex and/or high-risk aortic coarctation with additional cardiac and non-cardiac complicating factors and also due to failure of balloon angioplasty. Our initial experience (six procedures and seven stents) along with early results and short-term follow-up had already been published in 2007 [11]. We now present extended long-term follow-up of up to 15 years in the three survivors with reference to clinical course, further interventions, and the most recent clinical profile. A review of relevant literature is also provided.
Patients’ Data
Our initial publication [11] can be referred for detailed demographic data, earlier interventions (six procedures, seven stents), related technical information, immediate results, and short- to mid-term follow-up. Four patients had undergone the procedure. One of them (patient number three) died due to reasons unrelated to the procedure. Three of them had survived (patient number four in the past publication [11] will be referred to as patient number three in the current). Extended follow-up in terms of re-interventions and current clinical status is provided here.
Patient 1 (Tables 1, 2)
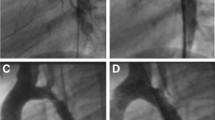
This 18-month-old boy had significant cavernous hemangioma of face, chest and mediastinum, mental retardation, severe developmental delay, and seizure disorder. He was confirmed to have tortuous aortic arch with significant stenosis (Fig. 1a). Surgical intervention was denied due to increased risk of bleeding due to hemangioma. After the initial stenting of the arch (using two stents Fig. 1b) at the age of 19 months (weight = 9 kg), balloon dilatation of the proximal stent was carried out at 28 months of age (weight = 10.6 kg). Second stent angioplasty (one stent) was carried out at 3.5 years of age (weight = 12 kg), and re-dilatation of the proximal stent was carried out at 5 years (weight = 15.6 kg) (Fig. 1c, d).
a–f. Aortic angiograms in multiple oblique angulations: before (a) and after (b) the deployment of proximal and middle stents. These show complex aortic arch lesions: distorted transverse arch, discrete critical coarctation along with irregular thoracic aorta. c Shows an aortograms after balloon dilatation of the proximal stent, while d after balloon dilatation of proximal, middle, and distal stents, depicting patent arch and no significant coarctation. e and f Show last aortic angiograms
Extended Follow-Up (Table 2)
An update of all of the trans-catheter interventions is provided in Table 2. Since the age of 5 years, he was regularly seen in outpatient clinic in a relatively stable condition without significant issues. Repeated 2D and Doppler interrogation at frequent intervals confirmed a peak systolic gradient of < 25 mmHg across the aortic arch without significant double envelope. Elective diagnostic catheterization for hemodynamic evaluation at 12 years of age (weight = 40 kg) was carried out, when no significant gradient was found on pull back from ascending to descending aorta (145/85–137/90 mmHg). So no intervention was offered (Fig. 1e, f).
Lately he was seen in outpatient department at 13 years of age (50 kg) when there were no complaints. He was not hypertensive and was not on any medications. There was a non-invasive blood pressure difference of 20 mmHg between upper and the lower limbs.
Doppler interrogation confirmed peak/mean gradients of 30/12 mmHg, respectively, across the arch with mild diastolic tailing of flow and good ventricular systolic function (FS = 38%).
Patient 2 (Tables 1, 3)
This boy presented at 1 month of age (3.1 kg) and was diagnosed to have coarctation of the aorta (Fig. 2a), large ventricular septal defect (VSD), small secundum atrial septal defect (ASD), pulmonary arterial hypertension (PAH), and sever biventricular systolic dysfunction (FS = 15%). Surgical intervention was denied because of left ventricular systolic dysfunction. First stent angioplasty for the distal narrowing was carried out at the age of 3 months (4.5 kg) after early failure of balloon dilatation (Fig. 2b). Still later, the stent was electively balloon dilated twice: at the age of 7 months (weight = 5.5 kg) and at 18 months (weight = 12.5 kg). A second stent was successfully deployed proximal to but overlapping the first one with acceptable results (Fig. 2c) [11].
a–i. Aortic angiograms in multiple oblique projections before the intervention a showing narrowing of the isthmus with a discrete distal coarctation. b An aortograms after the stent angioplasty of the distal discrete coarctation and c after the deployment of second stent just proximal to but overlapping the first one, while d after further balloon dilatation of stents, demonstrating the smooth walled arch without obstruction. e and f Aortograms after deployment of CP stents inside the primary stents and g shows the last aortograms after dilatation of CP stents with high-pressure balloon. Although the arch can be seen nicely patent, a waste can still be seen in the CP stent, while h and i are stills taken from the CMR
Extended Follow-Up (Table 3)
On subsequent follow-up in the outpatient clinic, he was confirmed to have developed re-stenosis. Doppler interrogation confirmed a peak systolic gradient of 55 mmHg, with a double envelope and diastolic tailing of abdominal aorta flow. Aortic angiography is shown (Fig. 2d). So a Cheatham Platinum (CP) stent (NuMed, Inc: Hopkinton, NY. USA) mounted on a 14 × 30 Cristal balloon (Cristal, Balt. Montmorency, France) was successfully deployed inside the initially deployed stents (inflated at 8 atm pressure), at the age of about 10 years (32 kg) (Fig. 2e, f). It was further balloon dilated using 12 × 20 Atlas balloon (Bard Peripheral Vascular, Inc, AZ. USA) and inflated to 20 atm pressure. The peak systolic gradient was reduced from 20 to 8 mmHg post procedure. Doppler interrogation confirmed peak/mean gradients of 30/20 mmHg across the arch. It also confirmed pulsatile abdominal aorta without diastolic tailing of flow.
Lately the CP stent was electively balloon dilated twice: initially at the age of 12 years and 3 months (weight = 41 kg), by using two balloons, a 12 × 40 mm Conquest balloon (Bard Peripheral Vascular, Inc. AZ. USA) and 14 × 40 Atlas Balloon (Bard Peripheral Vascular, Inc, AZ. USA), resulting in peak gradient reduction from 25 to 15 mmHg. It was re-dilated subsequently at the age of about 14 years (weight = 53 kg) by double balloon technique, initially using two Conquest balloons of 8 × 20 mm each and then two balloons of 12 × 20 mm each (Bard Peripheral Vascular, Inc. AZ. USA) and finally using a single Mullins-X balloon 18 × 30 mm (NuMed Canada Inc. ON, Canada) (Fig. 2g–i). The gradient dropped from pre-dilatation of 34 to 28 mmHg post dilatation. But a waste was still seen on arch angiography (Fig. 2h, i). The blocked Right femoral artery (RFA) was also balloon dilated at the same time using a TREK balloon of 2.5 × 20 mm size (Abbott Vascular, CA, USA). Post -dilatation angiography confirmed its recanalization. There were no procedure-related complications. Post-dilatation Doppler interrogation confirmed still a residual mean gradient of 20 mmHg across the stented arch segment. The abdominal aorta flow was a bit damped with mild tailing and there was mildly depressed LV systolic function (EF = 50% and FS = 24%). Right femoral artery pulse was feebly palpable. He was normotensive for his age and there was a non-invasive systolic blood pressure difference of 20 mmHg between the upper (129/70 mmHg) and the lower limbs (109/66 mmHg). He has been planned for another attempt at balloon dilatation using a Conquest balloon size 18 with a view to break the waste seen in the middle of the stent and a possible further reduction in the gradient to an acceptable level.
Patient 3 (Tables 1, 4)
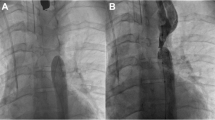
This patient was diagnosed at 1 month of age as complex congenital heart disease in the form of Taussig-Bing anomaly, multiple ventricular septal defects (VSDs), atrial septal defect (ASD) secundum, and a patent ductus arteriosus (PDA) and had undergone initially atrial septostomy and subsequently an arterial switch operation with coronary artery transfer, enlargement and closure of VSD, ligation of PDA, and primary closure of atrial septal defect (ASD) at an outside facility. She presented to us at the age of 4 months (4 kg) with severe aortic coarctation (Fig. 3a) with a peak systolic gradient of 70 mmHg, moderate aortic insufficiency (AI), moderate mitral regurgitation (MR), and severe left ventricular (LV) systolic dysfunction. Surgical intervention was denied due to LV systolic dysfunction and a major recent surgery in the recent past. She underwent an initial balloon angioplasty with immediate acceptable results and another elective re-dilatation at 10 months of age (6.9 kg) with good results in terms of gradient reduction. Still later at 15 months of age she underwent stent angioplasty (Fig. 3b) due to the development of re-stenosis because surgery was still denied due to persisting systolic dysfunction (FS = 25% in the presence of moderate MR) [11].
a–f. Aortograms showing significant juxta-ductal coarctation before any intervention, while b showing the aortic angiogram after the initial stent angioplasty. c Shows an aortic angiogram after the CP stent deployment and d after the final balloon dilatation of CP stent demonstrating nicely patent arch but with a mild waste in the middle. Final CMRI stills are shown in (e and f)
Extended Follow-Up (Table 4)
The primary stent was electively balloon dilated at the age of 7 years (weight = 24 kg) by using 12 × 20 mm Cristal balloon (Cristal, Balt. Montmorency, France). The pre-dilatation gradient of 25 mmHg was reduced to 5 mmHg. Upon subsequent follow-up at the age of 10.5 years (weight 43 kg), Doppler interrogation revealed peak gradient of 40 mmHg across the initially deployed stent. Post-stenotic dilatation of the arch was also noted. Cardiac catheterization was carried out, when a Cheatham Platinum (CP) stent (NuMed, Inc: NY. USA) mounted on a 14 × 30 Cristal balloon (Cristal, Balt. Montmorency, France) was successfully deployed inside the initially deployed stents (Fig. 3c). It was further balloon dilated using 12 × 20 Atlas balloon (Bard Peripheral Vascular, Inc, AZ. USA), inflated to 20 atm pressure. The gradient across the stent was reduced from pre-dilatation of 25 to 15 mmHg. Subsequently, the CP stent was balloon dilated at the age of 11 years and 9 months (weight = 50 kg): by using 18 × 30 mm Cristal balloon (Cristal, Balt. Montmorency, France) without significant reduction in gradient, and then by using two Conquest balloons of 12 × 20 mm each (Bard Peripheral Vascular, Inc. AZ. USA) through right femoral vein (RFV) due to weak arterial pulses. The pre-dilatation gradient of 38 mmHg was reduced to 20 mmHg. Still later, the stent was re-dilated at the age of 12.5 years (weight = 55 kg) by using 2 (12 × 30 mm) Cristal balloons (Cristal, Balt. Montmorency, France) through RFV. Lately an elective balloon dilatation of CP stent was carried out at the age of 14.5 years (weight = 64 kg) when she was found to have peak/mean gradients of 40/25 mmHg on Doppler interrogation. Two Conquest balloons (Bard Peripheral Vascular, Inc. AZ. USA) of 8 × 20 and 14 × 40 mm size were used in series through LFA (Fig. 3d–f). The pre-dilatation gradient of 22 mmHg dropped only to 19 mmHg. Stent angioplasty of blocked LFA was also carried out at the same time using long peripheral vascular stents.
Lately she was seen in the outpatient clinic, when she was doing fine, and was normotensive for her age, with non-invasive BP difference of 40 mmHg across right upper and right lower limbs. Echocardiography confirmed a residual mean gradient of 25 mmHg across the stents in the aortic arch. She also had moderate MR and good ventricular systolic function.
Discussion
Endovascular stent therapy has now been well established as a primary mode of intervention in older children and onwards for all stenotic vascular lesions. The use of stent implantation in small children remains controversial due to the challenges in accommodating for the somatic growth and the requirement for relatively larger introducing sheath size. The ideal stent in such circumstances would be the one requiring not only a smaller sheath size but also to retain the ability to be dilated to the adult vessel size. According to the American Heart Association (AHA) guidelines [12], Class IIa indications for use of stent angioplasty for native coarctation include failure of response to balloon angioplasty and the use of stents that can be dilated to adult size diameters. The recommended age and/or weight for such intervention have lowered during the past 20 years. It is attributed not only to improvements in delivery techniques, availability of smaller delivery systems, the refinements of existing devices, and evolution of modern stent designs, but also to the technical improvement [13]. The published literature and current standard of care continues to advocate surgical repair in newborns and infants with native aortic coarctation. So implementing stent therapy in such situations still remains controversial. Demand of the situation due to the critical clinical condition, compounded by the refusal or non-availability of surgical therapy, may mandate the use of stents implantation, who otherwise would be at high risk of dying without treatment. We have shown earlier [11] that the procedures had been successful when the gradient across the coarcted segment in the three survivors was reduced to acceptable level immediately and also on short- to early mid-term follow-up. The lumen size of the coarcted segment and the dysfunctional left ventricle also had improved.
All three children remained normotensive and were on no cardiac medications. Two of them needed multiple interventions. None of them had undergone surgical therapy. This fact though remains satisfying on one hand but raises some legitimate questions about the risks, possible complications, and benefits/drawbacks of multiple trans-catheter interventions required in such situations. These aspects and the related issues will be further discussed with a review of current and most recent literature.
Stenting of Aortic Coarctation and the Age/Weight of Patients
Stent angioplasty for coarctation of aorta had been used in children since early 1990s [7, 14,15,16]. Earlier in 1995, Suarez de Lezo et al. [17] reported use of stent angioplasty successfully in ten patients including eight children and one infant with optimal immediate relief. Over time and since our initial such publication in 2007 [11], there have been many such reports of endovascular stent implantation in younger children and infants.
In 2008, Holzer et al. [18] reported stenting of the complex and long segment aortic arch lesions. They reported safety and efficacy of the procedure with excellent, immediate, and mid-term results. But they also reported increased risk of peripheral vascular complications in patients weighing 10 kg or less. Rao in 2009 [19] reviewed the subject and clearly recommended trans-catheter intervention in newborns with severe coarctation in specialized circumstances like severe myocardial dysfunction, severe cardio-respiratory decompensation, and prior cerebral hemorrhage. Some more conditions could safely be added in this list: severe sepsis, continuous dependency on artificial ventilation, and refusal to surgical therapy, etc. [20]. In another report published in the same year by Francis et al. [21], they reported endovascular stent implantation for severe aortic coarctation with left ventricular dysfunction in five infants. The conclusion was that balloon dilatation with or without stent angioplasty was effective but temporary palliation for sick infants with severe aortic coarctation and a re-dilatation was inevitable. Another report published by Dimas et al. in [22] described use of 4 × 8 mm pre-mounted coronary stent for severe aortic coarctation in a premature infant of 25 weeks of gestation (weight = 875 g), as a palliation to bridge to final surgical repair at 6 months of age (weight 5.4 kg). Similarly, Bentham et al. in [23] had described endovascular stent implantation in 11 infants with aortic arch obstruction. The median age and weight of patients were 46 days (range 3–399 days) and 4 kg, respectively (range 0.4–5.5 kg). Ten of their patients had undergone surgical intervention before. They concluded that stent placement in infants was technically feasible with good results even in small babies and recommended it as a therapeutic option in complex cases when surgical alternatives were less favorable. Sreeram et al. [10] in 2012 have also reported early palliative stent implantation for coarctation in five newborns and infants with age range of 6–68 days and weight range of 1650–4000 g at the time of intervention. All but one of their patients were unstable hemodynamically and had other co-morbidity factors. Lately in 2014, Greig et al. [24] described use of stent angioplasty as a palliative/bridging procedure, when surgical intervention could be delayed by a median of 13.5 months. Nine out of 19 of their cases had severe aortic obstruction in whom the surgical correction was contraindicated due to various unfavorable factors. Similarly some comparative studies have also been reported: Mohan et al. [25] in 2009 and Bondanza et al. [26] in 2016 in their reports concluded that stent therapy for of aortic coarctation in younger children was safe and effective and results were similar to that seen in older patients but younger patients would require re-dilatations and were more prone to femoral vessel-related immediate injury or thrombosis.
It can be seen from the above citations that the relative age of children requiring stent implantation for coarctation has lowered over time. If surgical intervention is not available or if is contraindicated then stent implantation is safe and effective without much added risks except injury to the peripheral accessing artery. Our patients also fell into the same group, when they were critically sick, had other co-morbidity factors—including severe left ventricular dysfunction and surgical intervention, and were either denied or were not considered due to their critical condition. That justified us for using early stent deployment.
Stent Angioplasty as a Palliative Versus Semi-Definitive to Definitive Therapy
Although there have been many studies similar to ours, reporting the use of stent therapy in young and sick children as an alternate to standard surgical therapy, this has been used only as a palliation [10, 21,22,23,24, 27, 28]. In all of these reports, the patients had undergone surgical intervention at a later age, when stents were removed and coarctation was repaired. In contrast, none of our three patients underwent surgical therapy to the time of filing this report. The main reason had been the refusal on the part of the parents or patients to undergo surgery and also the fact that the results continued to be satisfactory, were relatively asymptomatic, and were normotensive for their age. Patient 1 actually did not require any further intervention after the initial reporting [11]. Patient 2 and 3 underwent dilatation of the primary stents to a maximum diameter of 10–12 mm and subsequently underwent deployment of CP stents inside the primary stents to break these, so as to increase the vessel diameter to that of adult caliber aorta. These CP stents were re-dilated using two balloon technique. The final angiographic picture shows much better vessel diameter compared to what was before, but still with a waste in the middle of CP stent. A further dilatation of the CP stents with a high-pressure balloon would be planned to break the waist, hoping that could finally save the patients from surgical therapy.
Stent Dilatation
Early stent implantation in most of the circumstances requires re-dilatation, before final surgical therapy. This has been the case in our patients also. Ewert et al. [29] have in fact suggested in their review about cases of severe native or post-operative coarctation that early dilatation less than optimum actually may help. They have suggested avoiding full dilatation of the stent at the initial procedure: to minimize vascular damage and to avoid uncontrolled intimal tear and aneurysm formation. They were also of the opinion that a residual waste would better secure the stent in its final position, which would also reduce the risk of stent migration. Subsequently, the stent could be dilated to desired full diameter after complete endothelialization of the stent in about 6 months’ time. In our patients, we had deployed pre-mounted stents of 6 × 13 or 8 × 13 mm size (Palmaz Corinthian, Cordis). These were balloon dilated initially only up to the size mentioned on the pamphlets and subsequently further to a diameter of 10 and 12 mm; by then the endothelial healing had been completed. Re-dilatation of intravascular stents has also been reported by others. Earlier, Morrow et al. [30] in their report on animal studies have concluded that re-expansion of intravascular stents was feasible after growth in juvenile swine without significant injury to intima, media, or adventitia. Later, Peters et al. [13] in their state-of-the-art paper had very nicely and comprehensively discussed the issue. They have mentioned that recurrence of stenosis after stent angioplasty has been reported in about 11% of cases. Two conditions were attributed to the re-stenosis and late failure after primary successful stent implantation. One was “full growth” stenosis which depended upon the initial age and size of patients, which had been the cause of re-dilatation in our cases. The other was hyperplasia of endothelial intima of the vessel which occurs in less than 25% of the cases [15, 30]. Risk factors promoting neointimal proliferation included younger age, lower weight, recurrent coarctation, and stent over dilation [9, 31]. Zanjani et al. [32] in a large reported series (comprising 28 patients) of stent re-dilatation claimed a success rate of 93% in achieving satisfactory results. They have identified four main factors leading to failure of coarctation re-dilatation: severe neointimal hyperplasia, prior surgical repair, association of Williams’s syndrome, and rarely the aortic dissection. Peters et al. [13] had also concluded in his report that re-dilatation remained unproblematic, safe, and effective but limited to the maximal stent diameter.
We have gone a step ahead in terms of maximal diameter for re-dilatation, when we dilated stents of 6 and 8 mm to maximum of 10 and 12 mm without untoward results of stent breakage. When the breakage of the stent was felt inevitable due to the somatic outgrowth of vessel above and below the coarcted segment, we deployed a strong and high-pressure bearing CP stent inside the initially deployed smaller stents in two patients, knowing the fact that CP stents could be finally dilated to a maximum of 25 mm diameter. So far we had maximally balloon dilated these CP tents using one balloon of 18 × 30 mm and subsequently by using two simultaneous balloons of 12 × 30 mm, with acceptable results in terms of drop of pressure gradient but sub-optimal angiographic results.
Draw Backs and Possible Complications of Early Stent Implantation and Multiple Re-Dilatations
Femoral Artery Injury or Blockage
The biggest drawback of using stent implantation at a young age is the need of multiple re-dilatations to match the lumen size of the stent to the somatic growth of the children. It can be seen that our first patient underwent five interventions all in all, while the second and the third patients required eight interventions each within the full follow-up period. This means that the price for having an early stent implantation necessitates an almost yearly re-intervention. This puts a burden on patients and family. This can be inferred that in the younger age group, repeat intervention is much more common than if the stents were implanted at an older age, which not only could easily compete with surgical results but also would require much less re-interventions.
Although repeated interventions are effective in terms of results, they come at the cost of injury to vessel wall. So loss of femoral pulse is more likely to occur in younger patients especially under 6 years of age [26, 33, 34]. Baykan A et al [35] also have reported femoral artery injury in 3 of his 35 patients after CP stent implantation. We encountered blocking of femoral artery in both of the patients in whom CP stents were deployed. One of them was re-canalized by balloon angioplasty, while in the other two peripheral vascular stents were needed to be deployed to re-canalize the femoral and common iliac arteries. This will remain a concern in future also till further miniaturization of delivery system size is achieved.
Stent Migration
The stent migration has been reported as the most encountered technical problem occurring in up to 5% of cases [34]. Bayken et al. [35] has also reported CP stent migration in four children. We did not encounter such issue because these stents were deployed inside the already deployed primary smaller stents, which helped to fix and maintain the CP stent position even after re-dilatations.
Radiation Issues
Since our patients needed multiple interventions, therefore they were exposed to radiation repeatedly. We have provided the radiation exposure time of all the procedures in the relevant tables. Although during the follow-up period none of our patients showed any signs of hemic malignancies and we did not encounter any other issue clinically implicating to radiation, the long-term effects of radiation should not be overlooked and cannot be ruled out based on our follow-up time period. The long-term implications of radiation need to be further studied by subject specialists.
Stents: Present and Future
Cheatham Platinum Stents
Cheatham Platinum stent has a unique design with platinum-iridium wires welded together rather than slotted stainless steel tubes. The rounded edges of these wires are less traumatic to the edges of the stented vessel. Since these stents can be dilated to 25 mm, these were originally designed to be used in large vessels such as aorta and to allow for dilatation to larger dimensions with limited shortening [36]. Baykan et al. [35] have recently reported their single-center experience of using CP stents in 35 children (38 stents) and have shown satisfactory results in reducing coarctation gradients, efficient enlargement of coarcted area diameter, and resolution of hypertension even in those weighing less than 20 kg (five patients) without additional risks of complications.
Biodegradable and Cobalt Chromium Stents
Over the years, new stents have been developed in terms of design and the metallic composition. Metal stents remain in the vessel wall, and get incorporated into it and do not have the potential to grow. We faced the same difficulty in balloon dilating our small primary stents to larger sizes. Biodegradable stents may address these issues. Such materials (polymers) have now been incorporated in stent construction, which have been used in the treatment of coronary and peripheral artery disease. An ideal biodegradable material would provide sufficient radial strength to prevent recoil, and be reabsorbed within weeks to months without toxic byproducts. Use of such stents in the treatment of aortic coarctation might help to keep the coarcted aortic segment open for a period of 3–6 months. Schranz et al. [37] in 2006 reported the use of bio-absorbable magnesium stent (AMS) in a newborn of 3 weeks of age who had severe long segment complex re-coarctation after surgical repair. But again, this remains a palliative and a bridging procedure towards a later surgical or possibly another TC intervention.
The more recent innovative Andrastent-first Cobalt Chromium stent technology allows very low-profile designs and the hybrid close/open cell design may be very helpful when placing the stent in a curved part of the aorta.
Growth Stents
Modification of the stent design by creating an “open-ring” has made over dilatation of the stents a possibility and has been tested in animal models. Ewert et al. [38] have planted 20 such stents in different vessel position in piglets and upon mean follow-up of 18 weeks have shown no significant stenosis or pressure gradient, as documented by angiography and catheter pullback. The growth stent has the potential to be non-restrictive during vessel growth, and thus is a promising new device for the permanent treatment of stenotic vessels in infancy and childhood.
Conclusions
The standard of care so far continues to advocate one-time surgical therapy for neonatal native aortic coarctation with mortality in most instances between 3 and 5%. Stenting in such cases can occasionally be required in critically sick children with complex coarctation and especially those with other co-morbidity factors. On short term, it is successful in improving the pressure gradient across the narrowing and to improve the vessel diameter. Multiple dilations are indicated to cope with the somatic growth. Subsequent surgical repair is an established option with acceptable results and should be performed, but occasionally this option remains void due to patients’ or parents’ choice. In such a situation when surgical option cannot be implemented, then as an alternate a bigger high-pressure stent can be deployed inside the primary one to break it. Injury to the accessing vessel wall with or without blockage is a potential risk of multiple interventions especially in younger children. Biodegradable or growth stents hold the key to future of early stent implantation and may modify the indications in future. No recommendations can be made based on our limited experience but it has opened a door for further experimentations. More comprehensive data are needed to further augment our approach.
References
Norman S, Talner (1998) Report of the New England Regional Infant Cardiac Program, by Donald C. Fyler. Pediatrics 102:258–259
Martin ML, Adams MM, Mortensen ML (1990) Descriptive epidemiology of selected malformations of the aorta, Atlanta, 1970–1983. Teratology 42:273–283
Uguz E, Ozkan S, Akay HT, Gultekin B, Aslamaci S (2010) Surgical repair of coarctation of aorta in neonates and infants: a 10 year experience. Turk J Thorac Cardiovasc Surg 18(2):94–99
Walhout RJ, Lekkerkerker JC, Oron GH, Hitchcock FJ, Meijboom EJ, Bennink GB (2003) Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg 126(2):521–528
Pandey R, Jackson M, Ajab S, Gladman G, Pozzi M (2006) Subclavian flap repair: review of 399 patients at median follow up of fourteen years. Ann Thorac Surg 81:1420–1428
Galal MO, Schmaltz AA, Joufan M, Benson L, Samatou L, Halees Z (2003) Balloon dilatation of native aortic coarctation in infancy. Z Kardiol 92:735–741
Radtke WA, Waller BR, Hebra A, Bradley SM (2002) Palliative stent implantation for aortic coarctation in premature infants weighing < 1500 gm. Am J Cardiol 90:1409–1412
Suárez de Lezo J, Pan M, Romero M, Medina A, Segura J, Lafuente M, Pavlovic D, Hernández E, Melián F, Espada J (1999) Immediate and follow up findings after stent treatment for severe coarctation of aorta. Am J Cardiol 83:400–406
Suárez de Lezo J, Pan M. Romero M, Segura J, Pavlovic J, Ojeda D, AlgarJ S, Ribes R, Lafuente M, Lopez-Pujol J (2005) Percutaneous intervention on severe coarctation of the aorta: a 21 year experience. Pediatr Cardiol 26:176–189
Sreeram I, Sreeram M, Bennink G (2012) Palliative stent implantation for coarctation in neonates and young infants. Ann Pediatr Cardiol 5:145–150
Al-Ata J, Arfi AM, Hussain A, Kouatly A, Galal MO (2007) Stent angioplasty: an effective alternative in selected infants with critical native coarctation. Pediatr Cardiol 28:183–192
Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, Mullins CE, Taubert KA, Zahn EM (2011) Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from American Heart association. Circulation 123:2607–26520020
Peters B, Ewert P, Berger F (2009) The role of stents in the treatment of congenital heart disease. Current status and future perspectives. Ann Pediatr Cardiol 2:3–23
Ebeid MR, Prieto LR, Latson LA (1997) Use of balloon-expandable stents for coarctation of aorta: initial results and intermediate-term follow up. JACC 30:1847–1852
Thanopoulos B, Hadjinikolaou L, Konstadopoulou G, Tsaousis G, Triposkiadis F, Spirou P (2000) Stent treatment for coarctation of aorta: Intermediate term follow up and technical considerations. Heart 84:65–67
Shah L, Hijazi J, Sandhu S, Joseph A, Cao QL (2005) Use of endovascular stents for the treatment of coarctation of the aorta in children and adults: immediate and mid-term results. J Invasive Cardiol 17:614–618
Suarez de Lezo J, Pan M, Romero M, Medina A, Segura J, Pavlovic D, Martinez C, Tejero I, Juan Navero P, Torres F, Lafuente M, Hernandez E, Melian F, Concha M (1995) Balloon expandable stent repair of severe coarctation of aorta. Am Heart J 129:1002–1008
Holzer RJ, Chisolm JL, Hill SL, Cheatham JP (2008) Stenting complex aortic arch obstructions. Catheter Cardiovasc Interv 71:375–382
Rao PS (2009) Transcatheter intervention in critically ill neonates and infants with aortic coarctation. Ann Pediatr Cardiol 2:116–119
Jadoon S, El-Segaier M, Galal MO (2016) Percutaneous balloon angioplasty for aortic coarctation in newborns and infants: is it still an option? J Struct Heart Dis 2(4):91–97
Francis E, Gayarthi S, Vaidynathan B, Kannan BR, Kumar RK (2009) Emergency balloon dilatation or stenting of critical coarctation of aorta in newborns and infants: an effective interim palliation. Ann Pediatr Cardiol 2:111–115
Dimas VV, Leonard SR, Guleserian KJ, Forbess JM, Zellers TM (2010) Stent implantation for coarctation of the aorta in a premature infant through carotid cutdown as a bridge to surgical correction. J Thorac Cardiovasc Surg 139:1070–1071
Bentham J, Shettihalli N, Orchard E, Westaby S, Wilson N (2010) Endovascular stent placement is an acceptable alternative to reoperation in selected infants with residual or recurrent aortic arch obstruction. Catheter Cardiovasc Interv 76:852–859
Greig C, Buys DG, Brown SC, Smit FE (2014) The use of small stents to delay surgical intervention in very young children with critical congenital heart disease. SA Heart 11:128–134
Mohan UR, Danon S, Levi D, Connolly D, Moore JW (2009) Stent implantation for coarctation of the aorta in children < 30 kg. JACC Cardiovasc Interv 2:877–883
Bondanza S, Calevo MG, Marasini M (2016) Early and long term results of stent implantation for aortic coarctation in pediatric patients compared to adolescents: a single center experience. Cardiol Res Pract. https://doi.org/10.1155/2016/4818307
Gorenflo M, Boshoff DE, Heying R, Eyskens B, Rega F, Meyns B, Gewillig M (2010) Bailout stenting for critical coarctation in premature/critical/complex/early re-coarcted neonates. Catheter Cardiovasc Interv 75:553–561
Haponiuk I, Chojnicki M, Steffens M, Jaworski R, Szofer-Sendrowska A, Juscinski J, Kwasniak E, Paczkowski K, Zielinski J, Gierat-Haponiuk K (2013) Miniinvasive interventional bridge to major surgical repair of critical aortic coarctation in a newborn with severe multiorgan failure. Wideochir Inne Tech Maloinwazyjne 8:244–248
Ewert P, Abdul-Khaliq H, Peters B, Nagdyman N, Schubert S, Lange PE (2004) Transcatheter therapy of long extreme subatretic aortic coarctations with covered stents. Catheter Cardiovasc Interv 63:236–239
Morrow WR1, Palmaz JC, Tio FO, Ehler WJ, VanDellen AF, Mullins CE (1993) Re-expansion of balloon-expandable stents after growth. JACC 22:2007–2013
Forbes TJ1, Moore P, Pedra CA, Zahn EM, Nykanen D, Amin Z, Garekar S, Teitel D, Qureshi SA, Cheatham JP, Ebeid MR, Hijazi ZM, Sandhu S, Hagler DJ, Sievert H, Fagan TE, Ringwald J, Du W, Tang L, Wax DF, Rhodes J, Johnston TA, Jones TK, Turner DR, Pass R, Torres A, Hellenbrand WE (2007) Intermediate follow up following intravascular stenting for treatment of coarctation of aorta. Catheter Cardiovasc Interv 70:569–577
Zanjani KS, Sabi T, Moysich A, Ovroutski S, Peters B, Miera O, Kühne T, Nagdyman N, Berger F, Ewert P (2008) Feasibility and efficacy of stent re-dilatation in aortic coarctation. Catheter Cardiovasc Interv 72:552–556
Golden AB1, Hellenbrand WE (2007) Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc Interv 69:289–299
Forbes TJ, Garekar S, Amin Z, Zahn EM, Nykanen D, Moore P, Qureshi SA, Cheatham JP, Ebeid MR, Hijazi ZM, Sandhu S, Hagler DJ, Sievert H, Fagan TE, Ringewald J, Du W, Tang L, Wax DF, Rhodes J, Johnston TA, Jones TK, Turner DR, Pedra CA, Hellenbrand WE, Congenital Cardiovascular Interventional Study Consortium (CCISC). (2007) Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv 70:276–285
Baykan A, Narin N, Ozyurt A, Argun M, Pamukcu O, Mavili E, Sezer S, Onan SH, Uzum K (2014) Cheatham platinum stent implantation in children with coarctation of the aorta: single-centre short-term, intermediate-term, and long-term results from Turkey. Cardiol Young 24:675–684
Cheatham JP (2001) Stenting of coarctation of aorta. Catheter Cardiovasc Interv 54:112–125
Schranz D, Zartner P, Michel-Behnke I, Akintürk H (2006) Bioabsorbable metal stents for percutaneous treatment of critical re-coarctation of the aorta in a newborn. Catheter Cardiovasc Interv 67:671–673
Ewert P, Riesenkampff E, Neuss M, Kretschmar O, Nagdyman N, Lange PE (2004) Novel growth stent for the permanent treatment of vessel stenosis in growing children: an experimental study. Catheter Cardiovasc Interv 62:506–510
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical Approval
This article does not contain any studies involving human and animal participants performed by any of the authors.
Informed Consent
Informed consent, either from patients or parents, was legibly obtained according to the institution policy in whom interventional procedures were performed and who were included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arfi, A.M., Galal, M.O., Kouatli, A. et al. Stent Angioplasty for Critical Native Aortic Coarctation in Three Infants: Up to 15-Year Follow-Up Without Surgical Intervention and Review of the Literature. Pediatr Cardiol 39, 1501–1513 (2018). https://doi.org/10.1007/s00246-018-1922-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1922-8