Abstract
The objective of this manuscript was to explore if left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) could predict the efficacy of metoprolol therapy on vasovagal syncope (VVS) in children. Forty-nine children, including 30 with VVS and 19 gender- and age-matched healthy controls, were included in the study. Metoprolol was prescribed to the VVS subjects. The clinical data were obtained during follow-up at 2 and 6 months. The results showed that LVEF and LVFS of responders were significantly higher than those of non-responders both at the 2-month follow-up (LVEF: 72.5 ± 3.2% vs. 64.6 ± 3.4%; LVFS: 40.9 ± 2.3% vs. 34.9 ± 2.9%), and at the 6-month follow-up (LVEF: 72.8 ± 2.8% vs. 65.5 ± 4.6%; LVFS: 41.1 ± 1.9% vs. 35.8 ± 3.6%). The receiver operating characteristic curve (ROC) analysis demonstrated that 70.5% as a cutoff value of baseline LVEF yielded a sensitivity of 80% and a specificity of 100% in predicting the therapeutic effectiveness of metoprolol at 2 months. For baseline LVFS, 38.5% as a cutoff value yielded a sensitivity of 90% and a specificity of 90%. At the 6-month follow-up, the ROC analysis demonstrated that 70.5% as a cutoff value of baseline LVEF yielded a sensitivity of 81.3% and a specificity of 88.9% in the prediction of metoprolol efficacy. For baseline LVFS, 37.5% as a cutoff value yielded a sensitivity of 93.8% and a specificity of 66.7%. In conclusion, baseline LVEF and LVFS might be useful predictors of the efficacy of β-blocker therapy on VVS in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vasovagal syncope (VVS) is the most frequent type of neurally mediated syncope (NMS), and is also the most common type of childhood syncope [1, 2]. It is one of the forms of acute orthostatic intolerance (OI), with its frequent occurrence and remissions [3, 4]. Although most of the children with VVS have favorable prognosis, the recurrence rate of VVS is very high, with a 1-year recurrence rate of 25–35% [5]. In addition, studies have shown that children with VVS have a lower degree of psychological health compared with normal kids and have an increased risk of suffering from anxiety and depression [6,7,8]. The quality of learning and living may be seriously affected in these children. Therefore, the effective treatment should be given to children with VVS to reduce the rate of syncope recurrence. At present, the therapeutic options for VVS in children include conventional treatment (i.e., orthostatic training, inducement avoiding, and fluid and salt intake), peripheral alpha-agonists (i.e., midodrine hydrochloride), and beta-blockers (i.e., metoprolol) [9,10,11]. Our previous work showed that not all children could receive curative effect from beta-blockers. When followed up for 6 ~ 30 months, the syncope recurrence rate was only 30.7% [12]. The reason is likely attributed to the diverse and sophisticated pathogenesis of VVS, which has marked heterogeneity among children. Indeed, the status of high catecholamine is one of the proposed mechanisms for VVS [13]. Syncope is the result of an exaggerated neurocardiac reflex, which is also called the Bezold–Jarisch reflex [14]. Our hypothesis, therefore, is that adrenergic beta-antagonist therapy would likely be effective for VVS patients who have a predominantly high catecholamine status as the major mechanism for VVS. In line with the abovementioned hypothesis, our previous work showed that an over 30 bpm/min increase in heart rate (HR) from baseline to a positive response in the head-up tilt test (HUTT) might predict a favorable effect from adrenergic beta-antagonist therapy for VVS children, with a sensitivity of 81%, a specificity of 80%, and a diagnostic value of 81% [15, 16]. However, some children are nervous during the test, which may affect the fluctuation of HR caused by the tilting position and therefore obscure the estimation of the therapeutic effect of adrenergic beta-antagonists. As a result, looking for an effective, non-invasive, convenient and stable index for the prediction of the therapeutic effect of adrenergic beta-antagonists in pediatric patients with VVS is desired by pediatricians worldwide. Dobutamine is a type of catecholamine that can excite the cardiac β1-receptor, and a certain dose of injection can raise the numerical value of the left ventricular functional indices left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) [17, 18], suggesting that LVEF and LVFS measured by echocardiography may reflect the level of plasma catecholamine status in vivo. Therefore, we speculate that children with VVS with increased LVEF and LVFS might be predicted to have a favorable therapeutic effect from beta-blocker therapy. Therefore, the aim of our study was to explore the possible value of LVEF and LVFS in the prediction of the therapeutic response to beta-blocker in VVS in children.
Methods
Thirty children (10 males and 20 females aged 6–15 years) diagnosed with VVS in the Department of Pediatrics at the Peking University First Hospital from February 2006 to October 2016 were included in this case–control study. The diagnostic criteria of VVS were as follows: (1) syncope or presyncope occurs with an upright posture or with exposure to emotional stress, pain, or medical settings; (2) cases have features dizziness, diaphoresis, nausea or pallor as accompanied symptoms; (3) cases have a positive HUTT response; and (4) exclusion of other diseases, such as organic cardiovascular diseases and cerebrovascular diseases [5, 19]. Nineteen healthy children (10 males and 9 females aged 6.5–15 years) with no history of dizziness and syncope; normal physical examination and electrocardiogram; and negative response in HUTT were recruited for the control group. Informed consent was obtained from the parents of the subjects, and the study has been approved by the Ethics Committee at Peking University First Hospital, China (Grant No. [2009]170).
HUTT was performed in children with VVS according to the previously described method [10,11,12, 15, 16].
Doppler color echocardiogram (SSD-5000SV, Aloka, Japan) with a linear 3–5 MHz transducer was used to measure the cardiac function parameters. All children were evaluated in the supine position. Left ventricular end-diastolic diameter (LVDD) and left ventricular end-systolic diameter (LVSD) were measured in the long-axis section of the parasternal ventricular sinister by M-mode echocardiography. LVEF and LVFS were computed accordingly with the following formulae:
All children with VVS took oral metoprolol as the treatment. Generally, the dose was 12.5 mg bid, but slight differences existed according to age and weight. The course of treatment was 2 (1, 2) months. The clinical data were obtained in an out-patient visit and over the telephone during follow-up at 2 and 6 months. All patients were followed up for 2 months (lost to follow up: 0), and 25 patients were followed up for 6 months (5 cases lost to follow up, miss rate of 16.7%). The syncope frequency and the adverse drug reactions were observed. The syncope frequency was graded as follows: 0, syncope did not occur; 1, syncope occurred 2–4 times per month; 3, syncope occurred 2–7 times per week; and 4, syncope occurred more than once per day [20, 21].
IBM-SPSS 22.0 software was used for statistical analysis. Measurement data are presented as the mean ± standard deviation, and the Shapiro–Wilk normality test was performed. Ranked data are presented as median (quartile). Two-sample t tests, non-parametric tests, and χ2 tests were employed to compare between two groups. Correlation analysis used the Spearman rank correlation analysis. Analysis of the predictors of treatment effect was done using the receiver operation characteristic (ROC) curve. A p < 0.05 was considered statistically significant.
Results
Gender composition, height, weight, body mass index (BMI), baseline HR, baseline SBP, baseline DBP, baseline LVEF, and baseline LVFS did not differ between the VVS group and the control group (Table 1).
Thirty cases were followed up, and the curative efficacy of adrenergic beta-antagonists was assessed 2 months after the start of metoprolol therapy. Within the 2-month period, children with a syncope frequency score decrease by ≥ 1 point compared with the baseline were classified as responders (20 cases, 66.7%), whereas children with a syncope frequency score decrease by < 1 point or with a syncope frequency score increase compared with the baseline were classified as non-responders (10 cases, 33.3%) [19]. The comparison between the two groups showed that the LVEF of responders was significantly higher than that of non-responders (72.5 ± 3.2% vs. 64.6 ± 3.4%, P < 0.001), and the LVFS of responders was significantly higher than that of non-responders (40.9 ± 2.3% vs. 34.9 ± 2.9%, P < 0.001, Table 2), whereas there was no difference in baseline parameters such as HR, SBP, DBP, and syncope frequency score between the two groups.
Twenty-five cases (five cases lost to follow up, miss rate of 16.7%) with VVS were followed up for 6 months, and the curative efficacy of adrenergic beta-antagonists was assessed 6 months after starting metoprolol therapy. Children with a syncope frequency score decrease by ≥ 1 point were classified as responders (16 cases, 64.0%), whereas children with a syncope frequency score decrease by < 1 point or with a syncope frequency score increase were classified as non-responders (9 cases, 36.0%). The results showed that the LVEF of responders was significantly higher than that of non-responders (72.8 ± 2.8% vs. 65.5 ± 4.6%, P = 0.001), and the LVFS of responders was significantly higher than that of non-responders (41.1 ± 1.9% vs. 35.8 ± 3.6%, P = 0.002, Table 2), whereas there was no difference in baseline parameters such as HR, SBP, DBP, and syncope frequency score between the two groups.
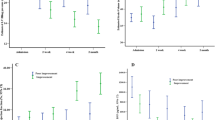
The Spearman’s correlation test indicated that when the subjects were followed up for 2 months, the syncope frequency score was negatively correlated with baseline LVEF (rs = − 0.712, P = 0.000, Fig. 1a) and baseline LVFS (rs = − 0.712, P = 0.000, Fig. 1b). When the subjects were followed up for 6 months, the syncope frequency score was negatively correlated with baseline LVEF (rs = − 0.660, P = 0.000, Fig. 1c) and baseline LVFS (rs = − 0.640, P = 0.001, Fig. 1d).
Correlation analysis of LVEF or LVFS and the syncope frequency score. a Scatter diagram of syncope frequency score and LVEF when children with VVS were followed up for 2 months. b Scatter diagram of syncope frequency score and LVFS when children with VVS were followed up for 2 months. c Scatter diagram of syncope frequency score and LVEF when children with VVS were followed up for 6 months. d Scatter diagram of syncope frequency score and LVFS when children with VVS were followed up for 6 months. The y-axis represents the syncope frequency score of VVS children with a follow-up period of 2 or 6 months; the x-axis represents the numerical value of LVEF (%) or LVFS (%). The oblique lines in each figure represent the correlation slope. “n” denotes the number of samples of each figure; “rs” denotes the correlation coefficient; and “P” denotes p value
The ROC analysis demonstrated an area under the curve (AUC) of 0.952 (95% CI 0.883–1.000, P = 0.000), and 70.5% as a cutoff value of baseline LVEF yielded a sensitivity of 80% and a specificity of 100% in the prediction of the therapeutic effect of metoprolol on VVS in children at the 2-month follow-up. For baseline LVFS, the AUC was 0.942 (95% CI 0.864–1.000, P = 0.000), and 38.5% as a cutoff value yielded a sensitivity of 90% and a specificity of 90% in the prediction of the therapeutic effect of metoprolol on VVS in children at the 2-month follow-up (Fig. 2a).
ROC curve of the predictive value of baseline LVEF and LVFS for predicting the efficacy of metoprolol on VVS in children. a The follow-up period of children with VVS was 2 months. b The follow-up period of children with VVS was 6 months. The y-axis represents the sensitivity to predict the ideal efficacy of metoprolol on VVS in children; the x-axis represents the false-positive rate (1-specificity). The reference line represents the sensitivity and the false-positive rate is equal, which means no predictive value completely. The curves of LVEF and LVFS are farther from the reference line and nearer to the upper-left corner of the diagram, indicating that the predictive value is higher
At the 6-month follow-up, the ROC analysis demonstrated an AUC of 0.906 (95% CI 0.783–1.000; P = 0.001), and 70.5% as a cutoff value of baseline LVEF yielded a sensitivity of 81.3% and a specificity of 88.9% in the prediction of the therapeutic effect of metoprolol on VVS in children. For baseline LVFS, the AUC was 0.903 (95% CI 0.781–1.000, P = 0.001), and 37.5% as a cutoff value yielded a sensitivity of 93.8% and a specificity of 66.7% in the prediction of the therapeutic effect of metoprolol on VVS in children (Fig. 2b).
Discussion
Our study showed that the baseline parameters of left ventricular systolic function LVEF and LVFS could predict the 2- and 6-month therapeutic effect of adrenergic beta-antagonists on VVS in children. For LVEF, when the children were followed up for 2 and 6 months, a cutoff value of 70.5% yielded a sensitivity of 80.0 and 81.3%, respectively, and a specificity of 100 and 88.9% respectively, for the prediction of therapeutic response of metoprolol in children with VVS. For LVFS, a cutoff value of 38.5 and 37.5% yielded a sensitivity of 90.0 and 93.8%, respectively, and a specificity of 90.0 and 66.7%, respectively, for the prediction of the therapeutic response of metoprolol in children with VVS.
Previous studies have shown that catecholamine might play an active part in the pathogenesis of syncope. In the supine position, plasma adrenaline (AD) was slightly higher in patients with VVS compared with controls (P = 0.06) [22]. AD begins to increase before syncope, and the increase accelerates when syncope occurs. After syncope, the plasma concentration of AD is four times higher than that at baseline in the supine position [22,23,24]. In 2004, Zygmunt et al. found that the baseline parameters, such as the square root of the mean of the sum for the squares of differences between adjacent RR intervals, percentage of differences between adjacent RR intervals that are greater than 50 ms and the high-frequency index (HF) of children with VVS, were significantly lower than those of healthy children, whereas the low-frequency index (LF) was significantly higher than that of healthy children, which suggests that the baseline sympathetic impulse increases while the baseline vagal impulse decreases in children with VVS [25]. When stimuli, such as prolonged standing, emotional stress, and stuffiness, appear, and the pressor effect of the depressor reflex is triggered, the over-excitation of the adrenergic nervous system may occur in children with VVS, based on the high level of basal catecholamine. As a result, the ventricular myocardium contracts excessively, and the baroreceptor in the posterior inferior wall of the heart is stimulated. Nerve impulses are then generated and are transmitted to vasomotor centers through C-fibers, which triggers the Bezold–Jarisch reflex, leading to sympathetic inhibition and vagal excitation. As a result, blood pressure and HR decrease, resulting in cerebral ischemia and, thus, a syncopal attack [13, 14].
Therefore, for cases of VVS, metoprolol has been widely used [10,11,12, 26,27,28]. However, some children do not have a favorable therapeutic response to the drug [10,11,12, 26,27,28]. One of the possible reasons is the complexity and diversity of VVS pathogenesis. In fact, in addition to the high catecholamine status and sympathetic over-excitation, the pathogenesis of VVS in some of the children might involve hypovolemia or excessive vascular dilation [29, 30]. Therefore, the ability to predict children with high catecholamine levels and sympathetic over-excitation who might achieve an ideal therapeutic effect from adrenergic beta-antagonists is of great importance. Our previous research showed that the children with an HR increase > 30 bpm/min during a positive response in the HUTT might have a high catecholamine status and sympathetic over-excitation and, therefore, had an ideal therapeutic response to adrenergic beta-antagonist therapy [15]. However, due to the fact that children may have a strong sense of discomfort and become nervous during the HUTT, which may interfere with HR, HUTT may not be the ideal method to predict the therapeutic response to adrenergic beta-antagonist therapy in patients with VVS.
LVEF and LVFS, which are measured by echocardiography, can reflect the contractile function of the left ventricle and, sometimes, a high catecholamine status. LVEF and LVFS are easy to measure, relatively stable, reliable, non-invasive, and safe. The rationale of dobutamine stress echocardiography and isoproterenol stress echocardiography is that catecholamine such as dobutamine and isoproterenol can induce the heart to work at sufficient doses, and LVEF and LVFS increase accordingly in humans with normal cardiac function [17, 18, 31, 32]. Therefore, LVEF and LVFS may reflect the level of plasma catecholamine to an extent. In addition, studies have shown that from rest to 25% of the submaximal workload, a positive correlation exists between plasma catecholamine and LVEF changes or LV volumes [33,34,35,36,37]. Thus, we speculate that children with VVS who have relatively high levels of LVEF and LVFS might achieve ideal therapeutic efficacy with adrenergic beta-antagonist therapy. Our present study reveals that baseline LVEF and LVFS may help to predict the therapeutic response to metoprolol at 2 and 6 months after the initiation of metoprolol in children with VVS, which is meaningful and helpful for the individualized treatment of childhood VVS.
The study also has limitations. The sample size is relatively small, and the follow-up period is not long enough. Therefore, in the future studies, it is worthy to conduct multi-center studies with a large sample size and a long-term follow-up.
References
Yang JY, Wang C, Tian H, Wang HW, Liao Y, Chen L, Zhang FW, Wang YL, Liu P, Zhang QY, Chen YH, Zhang CY, Du JB, Jin HF (2014) The analysis of the underlying disease spectrum of transient loss of consciousness in children. Int J Pediatr 41:195–197,201. https://doi.org/10.3760/cma.j.issn.1673-4408.2014.02.024
McHarg ML, Shinnar S, Rascoff H, Walsh CA (1997) Syncope in childhood. Pediatr Cardiol 18:367–371. https://doi.org/10.1007/s002469900202
Stewart JM (2013) Common syndromes of orthostatic intolerance. Pediatrics 131:968–980. https://doi.org/10.1542/peds.2012-2610
Stewart JM (2009) Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr 154:481–485. https://doi.org/10.1016/j.jpeds.2009.01.004
Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K (2015) 2015 Heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12:e41-63
Ding YY, Wang C, Liu XY, Lin P, Wu LJ, Xue XH, Li MX, Kumar P, Hu CY (2009) The relationship between vasovagal syncope and anxiety in children. Chin J Pract Pediatr 24:536–538
Ding YY, Wang C, Wu LJ, Hu CY, Lin P (2010) Psychological factors of children with vasovagal syncope. J Appl Clin Pediatr 25:437–439
Ding YY, Wang C, Lin P, Li MX, Xue XH, Liu XY, Wu LJ, Hu CY, P Kumar (2009) Relationship between vasovagal syncope and depression in children. Chin J Crit Care Med 29:17–20. https://doi.org/10.3969/j.issn.1002-1949.2009.01.005
Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, Olshansky B, Raj SR, Sandhu RK, Sorajja D, Sun BC, Yancy CW (2017) 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Heart Rhythm 14:e218-254. https://doi.org/10.1016/j.hrthm.2017.03.005
Chen L, Du JB, Zhang QY, Wang C, Du ZD, Wang HW, Tian H, Chen JJ, Wang YL, Hu XF, Li WZ, Han L (2007) A multicenter study on treatment of autonomous nerve-mediated syncope in children with beta-receptor blocker. Chin J Pediatr 45:885–888. https://doi.org/10.3760/j.issn:0578-1310.2007.12.002
Zhang FW, Liao Y, Li XY, Chen L, Jin HF, Du JB (2011) Follow-up study of the efficacy of different therapies on vasovagal syncope in children with different hemodynamic patterns. Chin J Pract Pediatr 26:97–100
Zhang QY, Du JB, Li WZ, Wang YL (2006) The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. Chin J Pract Pediatr 21:826–828
Grubb BP (1999) Pathophysiology and differential diagnosis of neurocardiogenic syncope. Am J Cardiol 84:3Q–9Q
Kinsella SM, Tuckey JP (2001) Perioperative bradycardia and asystole: relationship to vasovagal syncope and the Bezold-Jarisch reflex. Br J Anaesth 86:859–868
Zhang QY, Du JB, Zhen JL, Li WZ, Wang YL (2007) Hemodynamic changes during head-up tilt test and predictive value thereof in predicting the efficacy of metoprolol therapy in children with vasovagal syncope. Natl Med J China 87:1260–1262. https://doi.org/10.3760/j.issn:0578-1310.2007.12.002
Lin J, Jin HF, Du JB (2014) Assessment of therapeutic biomarkers in the treatment of children with postural tachycardia syndrome and vasovagal syncope. Cardiol Young 24:792–796. https://doi.org/10.1017/S1047951114000316
Edner M, Brodin LK, Al-Khalili F, Svane B, Moor E, StÅhle A, Nordiander R (1998) Changes in systolic and diastolic function indexes throughout dobutamine stress echocardiography in healthy volunteers and patients with ischemic heart disease. Echocardiography 15:625–634
Zheng HP, Li WZ, Li Y, Ma YW, Chen YH, Du JB (1999) Evaluation of cardiac beta-adrenergic receptor responsiveness in children by dobutamine stress echocardiography. Chin Med J (Engl) 112:623–626. https://doi.org/10.3760/j.issn:0578-1310.1999.06.017
Chu WH, Wang C, Wu LJ, Lin P, Li F, Zou RM (2015) Oral rehydration salts: an effective choice for the treatment of children with vasovagal syncope. Pediatr Cardiol 36:867–872. https://doi.org/10.1007/s00246-015-1097-5
Winker R, Barth A, Bidmon D, Ponocny I, Weber M, Mayor O, Robertson D, Diedrich A, Maier R, Pilger A, Haber P, Rüdiger HW (2005) Endurance exercise training in orthostatic intolerance: a randomized, controlled trial. Hypertension 45:391–398. https://doi.org/10.1161/01.HYP.0000156540.25707.af
Li HX, Wang YL, Liu P, Chen YH, Feng XL, Tang CS, Du JB, Jin HF (2016) Body mass index (BMI) is associated with the therapeutic response to oral rehydration solution in children with postural tachycardia syndrome. Pediatr Cardiol 37:1313–1318. https://doi.org/10.1007/s00246-016-1436-1
Bondanelli M, Alboni P, Margutti A, Franceschetti P, Dinelli M, Gruppillo P, Marchi P, Degli UE (2003) Plasma galanin response to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Metabolism 52:315–321. https://doi.org/10.1053/meta.2003.50051
Alboni P, Dinelli M, Gruppillo P, Bondanelli M, Bettiol K, Marchi P, Degli UE (2002) Haemodynamic changes early in prodromal symptoms of vasovagal syncope. Europace 4:333–338
Sra JS, Murthy V, Natale A, Jazayeri MR, Dhala A, Deshpande S, Sheth M, Akhtar M (1994) Circulatory and catecholamine changes during head-up tilt testing in neurocardiogenic (vasovagal) syncope. Am J Cardiol 73:33–37
Zygmunt A, Stanczyk J (2004) Heart rate variability in children with neurocardiogenic syncope. Clin Auton Res 14:99–106. https://doi.org/10.1007/s10286-004-0168-0
Jian PJ, Du JB, Zhang QY (2006) Effect of metoprolol treatment in vasovagal syncope in children. J Appl Clin Pediatr 21:305–306. https://doi.org/10.3969/j.issn.1003-515X.2006.05.022
Wang C, Xu Y, Liu XY, Hu CY, Praveen K, Wu LJ, Hu EL, Cui XL, Xie ZW (2009) Intervention study of midodrine hydrochloride and metoprolol in the treatment of children vasovagal syncope. Chin J Crit Care Med 29:196–199. https://doi.org/10.3969/j.issn.1002-1949.2009.03.002
Wang XR (2016) Effect of metoprolol on children with vasovagal syncope. Chin J Pract Med 43:10–11. https://doi.org/10.3760/cma.j.issn.1674-4756.2016.03.004
Zhang FW, Liao Y, Li XY, Chen L, Jin HF, Du JB (2012) The predictive value of flow-mediated vasodilation on therapeutic efficacy of midodrine hydrochloride for vasovagal syncope in children. Chin J Pract Pediatr 27:102–105
Younoszai AK, Franklin WH, Chan DP, Cassidy SC, Allen HD (1998) Oral fluid therapy. A promising treatment for vasodepressor syncope. Arch Pediatr Adolesc Med 152:165–168
Nishi I, Iida K, Kawano S, Masumi T, Yamaguchi I (2001) Using isoproterenol stress echocardiography to predict the response to carvedilol in patients with dilated cardiomyopathy. Jpn Circ J 65:514–518
Kawano S, Iida K, Fujieda K, Yukisada K, Magdi ES, Iwasaki Y, Tabei F, Yamaguchi I, Sugishita Y (1995) Response to isoproterenol as a prognostic indicator of evolution from hypertrophic cardiomyopathy to a phase resembling dilated cardiomyopathy. J Am Coll Cardiol 25:687–692. https://doi.org/10.1016/0735-1097(94)00432-P
Kelbaek H, Christensen NJ, Godtfredsen J (1988) Left ventricular volumes during graded upright exercise in healthy untrained subjects. Clin Physiol 8:51–56
Halter JB, Stratton JR, Pfeifer MA (1984) Plasma catecholamines and hemodynamic responses to stress states in man. Acta Physiol Scand Suppl 527:31–38
Stratton JR, Pfeifer MA, Ritchie JL, Halter JB (1985) Hemodynamic effects of epinephrine: concentration-effect study in humans. J Appl Physiol 58:1199–1206
Stratton JR, Halter JB, Hallstrom AP, Caldwell JH, Ritchie JL (1983) Comparative plasma catecholamine and hemodynamic responses to handgrip, cold pressor and supine bicycle exercise testing in normal subjects. J Am Coll Cardiol 2:93–104
Kanstrup IL, Marving J, Gadsbøll N, Lønborg-Jensen H, Høilund-Carlsen PF (1995) Left ventricle haemodynamics and vaso-active hormones during graded supine exercise in healthy male subjects. Eur J Appl Physiol Occup Physiol 72:86–94
Funding
This work was supported by Beijing Municipal Science and Technology Major Project (No. Z171100001017253, Beijing, China) and National Youth Top-notch Talent Support Program (China). There has been no other financial support for this work that could have influenced its outcome. The funding agencies had no other involvement, such as study design; the collection, analysis, and interpretation of data; writing the report; and in the decision to submit the article for publication in this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This manuscript is approved by all authors for publication.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Song, J., Li, H., Wang, Y. et al. Left Ventricular Ejection Fraction and Fractional Shortening are Useful for the Prediction of the Therapeutic Response to Metoprolol in Children with Vasovagal Syncope. Pediatr Cardiol 39, 1366–1372 (2018). https://doi.org/10.1007/s00246-018-1904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1904-x