Abstract
The medical records of 2283 patients with ventricular septal defect (VSD) were reviewed to determine spontaneous closure, left ventricular-to-right atrial shunt, subaortic ridge, and aortic valve prolapse. One thousand eight hundred and twenty-three patients had been followed 1 month to 26 years (median 4 years) by echocardiography. Most of 460 patients could not be followed due to transportation of the institution. VSD was perimembranous in 68.8% (1255), trabecular muscular in 21.7% (395), muscular outlet in 6% (109), muscular inlet in 2.6% (48), and doubly committed subarterial in 0.9% (16). Defect size was classified in 66.8% (1218) as small, in 15.7% (286) as moderate, and in 17.5% (319) as large. VSD closed spontaneously in 18.8% (343 of 1823 patients) by ages 40 days to 24.9 years (median, 1.8 years). One hundred fifty-seven of 1255 perimembranous defects (12.5%) and 167 of 395 trabecular muscular defects (42%) closed spontaneously (p < 0.001). Defect size became small in 306 (16.8%) of patients with VSD at a median of 2.5 years. Aneurysmal transformation was detected in 32.9% (600), left ventricular-to-right atrial shunt in 9.7% (176), subaortic ridge in 2.6% (48) of 1823 patients who were followed. In 381 (20.9%) of the 1823 patients, the VSD had been closed by a surgical or transcatheter technique. Surgery is required in one-fifth of patients with subaortic ridge or aortic valve prolapse. In conclusion, isolated VSDs are usually benign abnormalities that tend to shrink and close spontaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventricular septal defect (VSD) is one of the most common congenital malformations of the heart, with an incidence of 1.75–4.48 per million live births [1]. During the last two decades, echocardiography has played a major role in diagnosing VSDs and their associated complications. The latter may include aneurysmal transformation, left ventricular-to-right atrial (LV-RA) shunt, a subaortic ridge, aortic valve prolapse, and aortic regurgitation, which may be detected and followed by echocardiography. Aneurysmal transformation is an important mechanism of closure and has a more favorable prognosis [2–4]. However, subaortic ridge or LV-RA shunt may develop from aneurysmal transformation [5]. Aneurysmal transformation generally occurs from tissue arising from the tricuspid valve. An LV-RA shunt may develop through a deformed tricuspid valve [5, 6]. Aortic valve prolapse may be seen in VSDs, reducing the size of the VSD [7]. Subaortic ridge and aortic valve prolapse may cause aortic regurgitation.
It is known that VSDs can close spontaneously [8–12]. The rates of spontaneous closure, aneurysmal transformation, appearance of a subaortic ridge, LV-RA shunt, aortic valve prolapse, and aortic regurgitation have been reported in various studies at different rates [9–12]. These variations may be due to the age of patients, the size and site of the defect, the population studied, methods employed, and length of the follow-up period. Our previous study focused on the evolution of VSDs with special reference to spontaneous closure rate, aneurysmal transformation, subaortic ridge, LV-RA shunt, aortic valve prolapse, and aortic regurgitation [13]. It described the echocardiographic follow-up of 685 patients with VSD for up to 10.9 years. In the current study, we reviewed the medical records of 1823 patients with isolated VSD who were diagnosed by echocardiography to assess the rates of spontaneous closure, aneurysmal transformation, subaortic ridge, LV-RA shunt, aortic valve prolapse, and aortic regurgitation.
Materials and Methods
The study population consisted of 2283 patients with an isolated VSD who had been studied at our institution between 1988 and 2014. The study was approved by Istanbul University Cerrahpasa Medical Faculty Ethics Committee (44538). One thousand eight hundred and twenty-three of 2283 patients were then followed by echocardiography. Most of 460 patients could not be followed due to transportation of the institution. Patients with other cardiac abnormalities were excluded. In 381 of 1823 patients, the VSD was closed by a surgical or transcatheter technique. In these patients, the evolution of the defect has been studied by echocardiography up to the point of surgical or transcatheter VSD closure.
Transthoracic echocardiography was performed with an appropriate transducer interfaced with an Acuson 128/XP 10 ultrasonography (US) system (Siemens Medical Solutions, Mountain View, CA), an Acuson CV70 US system (Siemens Medical Solutions, Mountain View, CA), and Philips IE 33 US system (Philips Healthcare, Andover, MA). Two-dimensional, M-mode, color-flow Doppler, pulsed Doppler, and continuous-wave Doppler echocardiography were used in all patients.
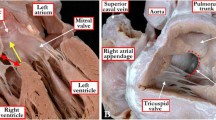
VSDs were classified according to their location and relation to the tricuspid annulus and semilunar valves (Figs. 1, 2) [14]. Defects located in the apical portion of the septum are especially likely to be multiple (so-called Swiss cheese defects) [15]. Defect size was expressed in terms of the size of the aortic root [16]. Defects approximating the size of the aorta were classified as large, defects one-third to two-thirds the diameter of the aorta were classified as moderate, and defects less than one-third the diameter of the aortic root were classified as small. VSDs can close spontaneously with aneurysmal transformation [8]. An LV-RA shunt was defined as the presence of a color Doppler turbulence from left ventricle to right atrium and a Doppler demonstration of high-velocity turbulence (with a pressure gradient of 50 mmHg) moving from the left ventricle, through the VSD, and directly into the right atrium. The pressure gradient of an LV-RA shunt was equivalent to the difference between the systolic left ventricle and right atrium pressure and could be readily differentiated from tricuspid regurgitation on the basis of the LV-RA shunt in the absence of any elevated right ventricular pressure [6]. Pathological pressure gradient for aortic stenosis was defined as more than 13 mmHg (4v2 = 4 × 1.82 = 13) [17].
An example of a perimembranous ventricular septal defect. a The five chamber view demonstrates the location of the defect. b Color flow imaging from the five chamber view. c Parasternal short axis view demonstrates the location of the defect. d Color flow imaging from the short axis view. Ao aorta, LV left ventricle, RA right atrium, RV right ventricle, VSD ventricular septal defect
Statistical analyses were performed using SPSS 15 (SPSS, Chicago, IL). Data were expressed as medians. The χ2 test was used to assess differences between groups for categoric variables. Spontaneous closure of perimembranous and muscular trabecular defects was analyzed by the Kaplan−Meier actuarial analysis. Statistical significance was inferred at p < 0.05.
Results
A total of 2283 patients with isolated VSD were diagnosed by echocardiography. Four hundred sixty patients could not be followed. Finally, 1823 (80%) of the 2283 patients (974 boys, 849 girls), aged 1 day–25.7 years (median 11 months), were followed 1 month to 26 years (median 4 years) by echocardiography. Altogether, 52.2% (n = 951) of the patients who were followed were <1 year old (Table 1). The median age at VSD diagnosis was 13 months (range 20 days–29 years). The types and sizes of the VSDs are shown in Figs. 3 and 4. There were two VSDs in 45 (2.5%) patients and Swiss cheese VSDs in 9 (0.5%) patients. Abnormalities associated with the 1823 VSDs are shown in Table 2.
Spontaneous VSD closure occurred in 18.8% (343/1823 patients) by age 40 days–24.9 years (median 1.8 years). Spontaneous closure occurred in 131 of 343 patients (38.1%) during the first year of life. The spontaneous closure rate was higher for muscular trabecular defects than for perimembranous defects (p < 0.001) (Table 3; Fig. 5). Defects had shrunk in 16.8% (306/1823) of the VSD patients at a median of 2.5 years (Table 3).
Aneurysmal transformation was detected in 600 of 1823 patients who were followed. Aneurysmal transformation was present in 348 patients at the initial echocardiographic examination and developed in 252 during the follow-up at ages 1–16 years (median 3 years). Aneurysmal transformation was detected in 550 of 1255 patients (43.8%) with a perimembranous defect, in 5 of 48 patients (10.4%) with a muscular inlet defect, in 23 of 395 patients (5.8%) with a muscular trabecular defect, and in 22 of 109 patients (20.2%) with muscular outlet VSD. A subaortic ridge was detected in 5.7% (n = 34) of patients who had aneurysmal transformation and in 1.1% (n = 14) of patients who did not (p < 0.001). LV-RA shunt was detected in 23.7% (n = 142) of patients who had aneurysmal transformation and in 2.8% (n = 34) of patients who did not (p < 0.001).
A subaortic ridge was detected in 48 of the 1823 patients who were followed. The VSD type was perimembranous in 45 and of the muscular outlet type in 3. In 14 patients, a subaortic ridge was detected during the initial echocardiographic examination. In 6 of these patients, the subaortic ridge gradient was <50 mmHg, and in 1 it was >50 mmHg. The pathological gradient (>13 mmHg) was absent in 7 patients. These patients were followed for 1−24 years (median 4.6 years). Surgery was performed in five because of subaortic stenosis. Among 34 patients in whom a subaortic ridge developed during follow-up (median 4 years, range 1−17 years), a subaortic ridge gradient was <50 mmHg in 9 patients and >50 mmHg in 1 patient. No pathological gradient (>13 mmHg) was seen in 24 patients. These patients were followed 1–17 years (median 10 years). Surgery was performed in 5 because of the subaortic stenosis. The VSD closed spontaneously in 2 (4.2%) of 48 patients with a subaortic ridge.
LV-RA shunt was detected in 176 of the 1823 patients who were followed. The VSD type was perimembranous in 158, muscular inlet in 5, muscular trabecular in 5, and muscular outlet in 8. Among 27 patients in whom an LV-RA shunt was detected at the initial echocardiographic examination, the shunt was mild in 21 and moderate in 6. These patients were followed 1 month–20 years (median 6 years). During that time, the degree of shunt progressed from mild to moderate in 3. Those with a moderate shunt showed no change. Among the 149 patients in whom an LV-RA shunt developed during follow-up (1 month–24 years, median 9.8 years), the shunt was mild in 132, moderate in 16, and severe in 1. These patients were followed 1.8–20 years (median 9 years), during which time the degree of the shunt progressed from mild to moderate in 19. Those with a moderate shunt showed no changes. Surgery was performed in 6 patients with the LV-RA shunt. The VSD closed spontaneously in 8 of 176 (4.5%) patients with an LV-RA shunt.
We detected aortic valve prolapse in 146 of 1255 perimembranous defects (11.6%) (in 33 at the initial echocardiographic examination and in 113 at follow-up) at ages 13 days–23 years (median 4.9 years). Aortic regurgitation due to aortic valve prolapse was detected in 95 of 1255 perimembranous defects (7.6%) (trivial in 40, mild in 47, moderate in 6, and severe in 2 at the last echocardiographic examination). Additionally, aortic valve prolapse was detected in 16 of 109 patients (14.7%) with muscular outlet defects. Among 10 patients with muscular outlet defects in whom aortic regurgitation due to aortic valve prolapse was diagnosed, it was trivial in 4, mild in 5, and moderate in 1. Aortic valve prolapse was detected in 4 (25%) of 16 patients with doubly committed subarterial defects and aortic regurgitation was developed in all of these patients. Aortic regurgitation was detected in 16 patients without aortic valve prolapse. Of these patients, 13 had perimembranous defect, 2 muscular outlet defect, and 1 muscular inlet defect. There was subaortic ridge in 2 of 13 patients with perimembranous defect and aortic regurgitation.
In 381 of the 1823 patients, the VSD had been closed by a surgical or transcatheter technique. In all, 20 of 176 patients with an LV-RA shunt underwent surgery. In 6 of these 20 patients, one of the operative indications was the LV-RA shunt. Surgery was indicated in the others for other reasons (pulmonary hypertension, heart failure). Of the 48 patients with a subaortic ridge, 17 underwent surgery. In 10 of these patients, the operative indication was the subaortic ridge. Altogether, 40 of 166 patients with aortic valve prolapse underwent surgery. The operative indication was aortic regurgitation in 33 patients. The other seven patients underwent surgery for other reasons (pulmonary hypertension, heart failure).
Discussion
Of all the defect types, the frequency of a perimembranous defect was highest in this study, similar to our previous study [13]. It was followed by trabecular muscular, muscular outlet, muscular inlet, and doubly committed subarterial defects. Likewise, in other large series [9–11, 18, 19], the frequency of perimembranous defect was higher than the other types of VSD. In some series in which the patients had been followed from birth, muscular septal defects were more common than perimembranous defects [20, 21]. Although the incidence of doubly committed subarterial defects in Japanese and Chinese populations was reported to be approximately 30% [22], our study showed a relatively smaller incidence (0.4%), similar to that in studies reported from the other regions of the world [9, 10].
The rate of spontaneous closure of VSD ranged from 11 to 84% in various studies [6, 8, 16, 19, 23–30]. Muscular defects are believed to be more likely to close spontaneously than perimembranous defects [31, 32]. In this study, the rate of spontaneous closure of all VSDs was 18.8%. Spontaneous closure was detected in 12.5% of perimembranous defects and 42.3% of the muscular trabecular defects. In our previous study, the rate of spontaneous closure of all VSDs was 27% [13]. The rate of spontaneous closure was lower in this study than in our 2003 study. Spontaneous closure was detected in 15% of perimembranous defects and 57% of trabecular muscular defects. Whereas the median age of patients at VSD diagnosis in our previous study was 6 months, the median age at VSD diagnosis in this study was 11 months. It is known that age has a significant influence on the incidence of spontaneous VSD closure. The reason for this difference between the rates of spontaneous closure was thought to be the older age at initial diagnosis in this study. Similar to the other reports, spontaneous closure occurred most commonly during the first year of life [31, 33].
It is known that aneurysmal transformation is an important mechanism responsible for spontaneous VSD closure [2–4], although aneurysmal transformation may be particularly likely to develop a subaortic ridge or LV-RA shunt [5, 6]. The rate of aneurysmal transformation has been reported at 48–81%, depending on the study population, in regard to perimembranous VSD [12, 34]. The incidence of aneurysmal transformation has not been reported for other VSD types. In this study, aneurysmal transformation was detected in 45.8% of patients with a perimembranous defect, 10.4% of those with a muscular inlet defect, 6% of those with a muscular trabecular defect, and 21.1% of those with a muscular outlet defect. The rates of subaortic ridge and LV-RA shunt were determined to be higher in patients with aneurysmal transformation than those without aneurysmal transformation.
The rate of subaortic ridge in patients with VSD has been reported to be between 0.7 and 6% in various studies [5, 10, 11]. Subaortic ridge may be asymptomatic or may cause subaortic stenosis and aortic regurgitation. In this study, subaortic ridge was detected in 2.6% of patients with VSD. Most of patients with subaortic ridge had a perimembranous defect and a few of them a muscular outlet defect.
In previous studies, LV-RA shunt was detected in 3–58% of patients with perimembranous defects [5, 13, 35, 36]. The rate and degrees of LV-RA shunt have not been reported for other VSD types. Additionally, the long-term prognosis of patients with an LV-RA shunt is unclear. The rate of LV-RA shunt with perimembranous defects was 15% in the study published by Ramaciotti et al. [4], whereas Weng et al. [36] reported the incidence of LV-RA shunt in perimembranous defects as 58%, most of which were trivial. In this study, LV-RA shunt was detected in 9.7% of patients who were followed with a VSD. Most of the patients with LV-RA shunt had a perimembranous defect, and the LV-RA shunt was generally mild. Nearly 15% of patients with mild LV-RA shunt progressed to moderate LV-RA, which then did not change.
Aortic valve prolapse may reduce the size of a VSD [22] but may cause aortic regurgitation. Aortic valve prolapse and aortic regurgitation are common in doubly committed subarterial defects [37]. Aortic valve prolapse is not as common in perimembranous and muscular outlet defects. In this study, aortic valve prolapse was detected in 9.1% of VSDs who were followed, 11.6% of perimembranous defects, 10% of muscular outlet defects, and 25% of patients with doubly committed subarterial defects. In VSD series, the frequency of aortic regurgitation has been reported to range from 5.5 to 25% [38, 39]. Aortic regurgitation was detected in 5.7% of all VSDs, 7.5% of perimembranous defects, 10% of muscular outlet defects, and 25% of doubly committed subarterial defects.
Limitations of the Study
This study did have some limitations. It was a retrospective review of medical records. The images of echocardiographic examinations were not reviewed. Our patients were not followed from birth, although 52.2% were younger than 1 year old and the median age at initial echocardiographic examination was 13 months.
Conclusion
Isolated VSDs are usually benign abnormalities that tend to shrink and close spontaneously.
The rate of spontaneous closure was higher for muscular trabecular defects than for perimembranous defects. The rates of associated abnormalities—LV-RA shunt, subaortic ridge, aortic valve prolapse, aortic regurgitation—are low. LV-RA shunt is a more benign condition than subaortic ridge or aortic valve prolapse. Surgery is required in one-fifth of patients with subaortic ridge or aortic valve prolapse.
References
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39:1890–1900
Freedom RM, White RD, Pieroni DR et al (1974) The natural history of the so-called aneurysm of the membranous septum in childhood. Circulation 49:375–384
Nugent EW, Freedom RM, Rowe RD, Wagner HR, Rees JK (1977) Aneurysm of the membranous septum in ventricular septal defect. Circulation 56(1):I82–I84
Ramaciotti C, Keren A, Silverman NH (1986) Importance of (perimembranous) ventricular septal aneurysm in the natural history of isolated perimembranous ventricular septal defect. Am J Cardiol 57:268–272
Wu MH, Wu JM, Chang C et al (1993) Implication of aneurysmal transformation in isolated perimembranous ventricular septal defect. Am J Cardiol 72:596–601
Wu MH, Wang JK, Lin MT et al (2006) Ventricular septal defect with secondary left ventricular-to-right atrial shunt is associated with a higher risk for infective endocarditis and a lower late chance of closure. Pediatrics 117:e262–e267
Anderson RH, Lenox CC, Zuberbuhler JR (1983) Mechanisms of closure of perimembranous ventricular septal defect. Am J Cardiol 52:341–345
Zhang J, Ko JM, Guileyardo JM, Roberts WC (2015) A review of spontaneous closure of ventricular septal defect. Proceedings 28:516–520
Kazmi U, Sadiq M, Hyder SN (2009) Pattern of ventricular septal defects and associated complications. J Coll Physicians Surg Pak 19:342–345
Chaudhry TA, Younas M, Baig A (2011) Ventricular septal defect and associated complications. J Pak Med Assoc 61:1001–1004
Erdem S, Ozbarlas N, Küçükosmanoğlu O, Poyrazoğlu H, Salih OK (2012) Long term follow-up of 799 children with isolated ventricular septal defects. Turk Kardiyol Dern Ars 40:22–25
Sun J, Sun K, Chen S, Yao L, Zhang Y (2014) A new scoring system for spontaneous closure prediction of perimembranous ventricular septal defects in children. PLoS ONE 9:e113822
Eroğlu AG, Oztunç F, Saltik L, Bakari S, Dedeoğlu S, Ahunbay G (2003) Evolution of ventricular septal defect with special reference to spontaneous closure rate, subaortic ridge and aortic valve prolapse. Pediatr Cardiol 24:31–35
Moe DG, Guntheroth WG (1987) Spontaneous closure of uncomplicated ventricular septal defect. Am J Cardiol 60:674–678
Feigenbaum H (1994) Echocardiography, 5th edn. Lea&Febiger, Philadelphia, p 384–392
Graham TP, Gutgesell HP (1995) Ventricular septal defects. In: Emmanouilides GC, Riemenschneider TA, Allien HD, Gutgesell HP (eds) Heart disease in infants, children and adolescents: including the fetus and young adult. Williams & Wilkins, Baltimore, p 724–746
Feigenbaum H (1994) Echocardiography, 5th edn. Lea&Febiger, Philadelphia, p 675
Kidd L, Driscoll DJ, Gersony WM, et al (1993) Second natural history of congenital heart defects. Results of treatment of patients with ventricular septal defects. Circulation 87:I38–I51
Sutherland GR, Godman MJ, Smallhorn JF et al (1982) Ventricular septal defects: two-dimensional echocardiographic and morphological correlations. Br Heart J 47:316–328
Trowitzsch E, Braun W, Stute M, Pielemeier W (1990) Diagnosis, therapy and outcome of ventricular septal defects in the 1st year of life: a two-dimensional color-Doppler echocardiography study. Eur J Pediatr 149:758–761
Mehta AV, Chidambaram B (1992) Ventricular septal defect in the first year of life. Am J Cardiol 70:364–366
Hisatomi K, Kosuga K, Isomura T et al (1987) Ventricular septal defect associated with aortic regurgitation. Ann Thorac Surg 43:363–367
Miyake T, Shinohara T, Inoue T, Marutani S, Takemura T (2011) Spontaneous closure of muscular trabecular ventricular septal defect: comparison of defect positions. Acta Paediatr 100:e158–e162
Ahunbay G, Onat T, C¸ elebi A, Batmaz G (1999) Regression of right ventricular pressure in ventricular septal defect in infancy: a longitudinal color-flow Doppler echocardiographic study. Pediatr Cardiol 20:336–342
Alpert BS, Cook DH, Varghese PJ, Rowe RD (1979) Spontaneous closure of small ventricular septal defects: ten-year follow-up. Pediatrics 65:204–206
Alpert BS, Mellits ED, Rowe RD (1973) Spontaneous closure of small ventricular septal defects. Probability rates in the first five years of life. Am J Dis Child 125:194–196
Collins G, Calder L, Rose V, Kidd L, Keith J (1972) Ventricular septal defect: clinical and hemodynamic changes in the first five years of life. Am Heart J 84:695–705
Corone P, Doyon F, Gaudeau S et al (1977) Natural history of ventricular septal defect. A study involving 790 cases. Circulation 55:908–915
Onat T, Ahunbay G, Batmaz G, Celebi A (1998) The natural course of isolated ventricular septal defect during adolescence. Pediatr Cardiol 79:230–234
Yokoyama M, Takao A, Sakakibara S (1970) Natural history and surgical indications of ventricular septal defect. Am Heart J 80:597–605
Meberg A, Otterstad JE, Frøland G, Lindberg H, Sørland SJ (2000) Outcome of congenital heart defects—a population-based study. Acta Paediatr 89:1344–1351
Turner SW, Hunter S, Wyllie JP (1999) The natural history of ventricular septal defects. Arch Dis Child 81:413–416
Erol O, Sevket O, Keskin S, Yazıcıoğlu HF, Gül A (2014) Natural history of prenatal isolated muscular ventricular septal defects. J Turk Ger Gynecol Assoc 15:96–99
Miyake T, Shinohara T, Fukuda T, Ikeoka M, Takemura T (2008) Spontaneous closure of perimembranous ventricular septal defect after school age. Pediatr Int 50:632–635
Wu MH, Lue HC, Wang JK, Hung CR (1989) Color flow mapping in perimembranous ventricular septal defect with left ventricular-to-right atrial shunts. Taiwan Yi Xue Hui Za Zhi 88:38–42
Weng KP, Huang SH, Lin CC, Huang SM, Chien KJ, Ger LP, Hsieh KS (2008) Reappraisal of left ventricular to right atrial (LV-RA) shunt in pediatric patients with isolated perimembranous ventricular septal defect. Circ J 72:1487–1491
Tohyama K, Satomi G, Momma K (1997) Aortic valve prolapse and aortic regurgitation associated with subpulmonic ventricular septal defect. Am J Cardiol 79:1285–1289
Ishikawa S, Morishita Y, Sato Y et al (1994) Frequency and operative correction of aortic insufficiency associated with ventricular septal defect. Ann Thorac Surg 57:996–998
Karpawich PP, Duff DF, Mullins CE, Cooley DA, McNamara DG (1981) Ventricular septal defect with associated aortic valve insufficiency. J Thorac Cardiovasc Surg 82:182–189
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Eroglu, A.G., Atik, S.U., Sengenc, E. et al. Evaluation of Ventricular Septal Defect with Special Reference to the Spontaneous Closure Rate, Subaortic Ridge, and Aortic Valve Prolapse II. Pediatr Cardiol 38, 915–921 (2017). https://doi.org/10.1007/s00246-017-1597-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1597-6