Abstract
The clinical course of residual ventricular septal defects after congenital heart disease repair is not completely elucidated in the medical literature. This study assessed the incidence, size, and clinical course of residual defects.This single-center retrospective study included 132 patients who survived after ventricular septal defect patch closure (n = 107) and intracardiac repair of double-outlet right ventricle (n = 16) and tetralogy of Fallot (n = 9). Residual defect was evaluated on transthoracic echocardiogram upon hospital discharge and at outpatient clinic visits.The median age at surgery was 1.2 (0.3–13.9) years. In total, 45 (34.1%) patients presented with residual defects upon hospital discharge. The residual defects were within 2 mm (n = 27), 2–3 mm (n = 15), and > 3 mm (n = 3), and the median size was 1.5 (0.5–3.8) mm. There was no late mortality during a median follow-up of 5.4 years. Among 42 residual defects measuring < 3 mm upon hospital discharge, 37 (82.2%) spontaneously closed. Further, five defects decreased in size (1.8 ± 0.6 mm upon hospital discharge vs1.2 ± 0.8 mm at the latest visits, p = 0.15). However, the size of three residual defects measuring > 3 mm upon hospital discharge increased, and two patients required re-surgery for residual defect.Significant residual defect requiring reoperation was rare. In most cases, residual defects measuring < 3 mm upon hospital discharge spontaneously closed within 5 years, and the size of the other defects decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence rate of residual ventricular septal defect (VSD) after congenital heart surgery is 31%–57% [1,2,3]. Multiple theoretical complications, ranging from left ventricular volume overload in larger defects to aortic valve damage in smaller defects, can develop because of residual defects. Further, chronic residual intracardiac shunting can result in remodeling of the pulmonary vascular bed and a fixed increase in pulmonary vascular resistance. A residual defect measuring > 3 mm was considered the cutoff for hemodynamically significant residual shunt [4,5,6]. However, previous studies on the sizes and clinical course of residual defects after surgery are limited. Therefore, the current study aimed to assess the clinical course of residual defects, particularly with respect to defect sizes.
Material and Methods
Study Design
This single-center retrospective study included 132 patients who survived after repair of isolated VSD (n = 107), double-outlet right ventricle (DORV) (n = 16), and tetralogy of Fallot (TOF) (n = 9) from March 2012 to December 2022. Residual VSD was defined as the presence of postoperative interventricular shunt with measurable defect on transthoracic echocardiogram. Therefore, tiny shunts, with immeasurable size or flow velocity, were not considered as residual defects in this study. This study aimed to evaluate the clinical course of residual defects around the margin of the VSD patch. Therefore, patients with iatrogenic VSD caused by septal myectomy, shunt attributed to multiple muscular-type VSD, and left ventricle to right atrium shunt, those who underwent direct VSD closure, those with more complicated anatomies such as atrioventricular septal defect and transposition of the great arteries with VSD, and those with early mortality at the time of repair were excluded from the analysis. The size of the residual defect, with the maximal VSD diameter, which is a technical performance score after VSD closure, was measured [7]. The residual defect was obtained from the para-sternal long-axial view, short-axial view, and apical four-chamber view on transthoracic echocardiogram. Further, the residual defect indexed to the patient’s body surface area (BSA) was measured. Transthoracic echocardiogram was performed upon hospital discharge and at 3 months, 6 months, and every 1–3 years after the surgery. In patients with a larger residual VSD, the examination was performed at short intervals. Spontaneous closure was defined as residual defects with an invisible interventricular shunt or immeasurable defect size.
This study was approved and monitored by the research ethics committee of Matsudo City General Hospital (approval number and date: R4-23, 1/6/2023). The need for a patient consent was waived due to the retrospective nature of this study. The medical records of the patients were reviewed, and data such as demographic characteristics of the patients, anatomical information, surgical history, intraoperative details, and postoperative outcomes were retrieved and analyzed.
Statistical Analysis
Statistical analysis was performed using the SPSS software version 18.0 (SPSS, Inc., Chicago, IL). Continuous variables were expressed as mean and standard deviation or median and range. Comparative studies between two groups were performed using the student’s t-test or the generalized Wilcoxon signed-rank test. The Kaplan–Meier curve for the spontaneous closure of residual defects was created. The end point of residual defect was reoperation or spontaneous closure. All p-values were two-sided, and a p-value of < 0.05 was considered statistically significant. Risk analysis of residual VSD measuring > 2 mm upon hospital discharge was evaluated via a regression analysis. In the risk analysis, the right ventricular approach and other approaches for VSD closure were compared. Each variable was found to be significantly associated with based on the univariate analysis (p < 0.15) and was used in the multivariable analysis.
Surgical Indication
VSD closure was indicated for a pulmonary-to-systemic blood flow ratio of > 1.5 or the presence of aortic valve prolapse caused by the venturi effect. Cardiac catheterization was aggressively performed on patients suspected with pulmonary arterial hypertension, defined as a mean pulmonary arterial pressure of > 20 mmHg, an end-expiratory pulmonary artery wedge pressure of < 15 mmHg, and pulmonary vascular resistance of > 3 wood units [8]. The indication for residual VSD re-closure was similar to that for the initial surgery.
Operative Surgeon and Technique
The surgeries were performed by four pediatric cardiac surgeons during the study period. Although the same surgical team performed the operation, the first operative surgeon was different on the era.
For VSD closure or intracardiac rerouting, a 0.4-mm-thick expanded polytetrafluoroethylene or a Dacron patch was used. The patch was applied with several pledgeted 5–0 or 6–0 polypropylene U-shaped interrupted sutures or with a 6–0 polypropylene continuous sutures at the ventricular septum and several 6–0 polypropylene U-shaped interrupted sutures reinforced with the pericardium strip at the valve annulus. The surgeon selected the approaches used for VSD closure.
Results
This study enrolled 132 patients. Table 1 shows the characteristics of the patients. The median age at surgery was 1.2 (0.3–13.9) years, and 46 (34.8%) patients were aged < 1-year-old. In total, 26 (19.7%) patients had preoperative pulmonary arterial hypertension, and 20 (15.2%) patients had Trisomy 21. Further, 45 (34.1%) patients presented with residual VSD upon hospital discharge (Table 2). The sizes of residual defects were < 2 mm (n = 27 [60%]), 2–3 mm (n = 15 [33.3%]), and > 3 mm (n = 3 [6.7%]), and the median size was 1.5 (0.5–3.8) mm. Among 25 patients with TOF or DORV, 8 (32%) presented with residual defect measuring > 2 mm upon hospital discharge. Moreover, among 107 patients with isolated VSD, only 10 (9.3%) had a residual defect measuring > 2 mm upon hospital discharge (p < 0.01, χ2 = 8.8). However, there was no significant difference in terms of residual defect among the four pediatric cardiac surgeons (p = 0.26, χ2 = 4.0).
Multivariable analysis showed that TOF or DORV was identified as a risk factor for residual VSD measuring > 2 mm upon hospital discharge (p = 0.04, hazard ratio = 5.1, 95% confidence interval = 1.7–15.2) (Table 3). Trisomy 21 was tended to be identified as a risk factor for residual VSD (p = 0.08, hazard ratio = 3.1, 95% confidence interval = 0.9–10.6). By contrast, the suture technique and the approach for VSD closure were not risk factors for residual VSD.
Univariate analysis showed that aged < 1 year at surgery was a risk factor for residual VSD sized measuring > 2 mm upon hospital discharge in 107 patients with isolated VSD alone (p = 0.04, hazard ratio = 4.3, 95% confidential interval = 1.1–17.8).
All patients with residual VSD upon hospital discharge completed the follow-up, and there were no cases of late mortality and infective endocarditis during a median follow-up of 5.4 (0.1–9.7) years (Table 4). In total, 37 (82.2%) patients presented with residual defect closure at a median duration of 0.5 (0.1–5.0) years from the surgery. The size of all defects that spontaneously closed were < 3 mm upon hospital discharge. Residual defects measuring < 2 mm spontaneously closed earlier than those measuring 2–3 mm. In particular, the incidence rates of spontaneous closure at 1 year after the surgery was 85.2% in patients with defects measuring < 2 mm and 34.0% in patients with defects measuring 2–3 mm (Fig. 1a). Among 42 residual defects measuring < 3 mm upon hospital discharge, five decreased in size at a median follow-up of 3.3 years (1.8 ± 0.6 mm upon hospital discharge vs.1.2 ± 0.8 mm at the latest visits, p = 0.15).
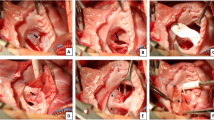
In contrast, three residual defects measuring > 3 mm upon hospital discharge remained patent. Then, their diameter increased to 5.7, 6.8, and 10 mm (Fig. 2). Postoperative cardiac catheterizations showed a pulmonary-to-systemic flow ratio of > 1.5 and a left ventricular volume of > 150%. Among them, two with Trisomy 21 and DORV or VSD required reoperation for residual defect at 0.7 and 2.1 years after the surgery. During reoperation, in both patients, the residual VSD was located beneath the anterior tricuspid valve, and was surrounded by the anterior limb of trabecula septomarginalis and the superior portion of the previous VSD patch.
Residual Defect Size Indexed the Patient’s BSA
The residual defect sizes indexed the patient’s BSA were < 5 mm/m2 (n = 28 [62.2%]), 5–10 mm/m2 (n = 14 [31.1%]), and > 10 mm/m2 (n = 3 [6.7%]). Two of three patients with residual defect indexed BSA measuring > 10 mm/m2 presented with a pulmonary-to-systemic flow ratio of > 1.5 after the surgery. The residual defects indexed BSA measuring < 5 mm/m2 was spontaneously closed earlier than that of defects measuring 5–10 mm/m2 (Incidence of spontaneous closure at 1 year after the surgery: 78.6% in residual defects indexed BSA of < 5 mm/m2 vs. 38.9% in residual defects indexed BSA of 5–10 mm/m2 (p = 0.03, χ2 = 5.1) (Fig. 1b).
Discussion
The incidence rate of residual defect for isolated VSD closure ranged from 31.2% to 57% [1,2,3], which is similar to the result of the current study (34.1%). Further, the remaining residual defect at 3–26 years after the surgery was 8%–10% [2, 9, 10]. The incidence of residual defect at a median follow up of 5.4 years was 4.5%. Previous studies have shown that a longer bypass time and a lower body weight at surgery were associated with residual defects [1, 11]. In our study, TOF or DORV had a significantly higher incidence of residual defects measuring > 2 mm upon hospital discharge. In the anatomy of TOF or DORV, the conal septum is anteriorly malaligned relative to the ventricular septum in general, and it may be challenging to suture. In previously reports about reoperations for TOF repair aged over 15 years, 9.4% of patients underwent reclosure for residual defect [12]. Cautious follow-up is required, especially for TOF or DORV with a larger residual VSD. In our study, trisomy 21 was considered a risk factor of residual defect, and two patients with trisomy 21 required re-surgery for residual defects because of congestive heart failure and pulmonary hypertension after the initial repair [13]. If the patient with trisomy 21 had persistent residual defects, early examination is required to evaluate the residual shunt flow before the exacerbation of pulmonary hypertension. For suture techniques such as continuous or interrupted sutures, previous reports have revealed the different incidence of residual defect based on suture techniques [14, 15]. However, in our study, there was no significant difference in the incidence of residual defects based on suture techniques or approaches. A size of > 3 mm was used as a cutoff for residual defect sizes in hemodynamically significant residual shunt [4,5,6], and the defect did not spontaneously close 3 years after the surgery [2]. The incidence rate of residual defects measuring > 3 mm was 4.7% [4]. In our study, the incidence rate was 2.2%. The frequent portion of residual defect was reported to be the transition from aortic valve annulus to the tricuspid valve annulus [2], and the superior portion of the VSD patch [16]. In our three patients with residual defects measuring > 3 mm, the residual defect was also located at the superior portion of the previously placed patch. Therefore, the area, particularly at the superior portion of the VSD, should be sutured to prevent persistent residual defect.
The incidence rate of re-surgery for residual defects after VSD or TOF repair was 0.5%–3.2% [3, 10, 12, 17], and the incidence rate of reoperation was 1.5%. If the residual defects measuring > 2 mm are detected during the surgery, return to cardiopulmonary bypass is recommended [2]. In our study, the residual defects measuring 2–3 mm upon hospital discharge were spontaneously closed or became smaller. However, the timing of spontaneous closure was later than that in defects measuring < 2 mm. We believe that the cutoff size for re-closure of residual defects is 3 mm, because the residual defects measuring > 3 mm are likely to increase in size. Further, the residual defect size indexed BSA might be useful in identifying the clinical course of residual defects. In residual defect size indexed BSA of > 10 mm/m2, it is important to monitor the development or exacerbation of congestive heart failure and pulmonary hypertension.
The current study had several limitations. It was retrospective in nature, and the number of surgeries performed by four pediatric cardiac surgeons during the study period was low.
Conclusion
Significant residual defect requiring re-surgery was rare. In most cases, residual defects measuring < 3 mm upon hospital discharge spontaneously closed within 5 years, and the others decreased in size. Further, residual defects measuring > 3 mm were unlikely to close.
Abbreviations
- VSD:
-
Ventricular septal defect
- DORV:
-
Double outlet right ventricle
- TOF:
-
Tetralogy of Fallot
- BSA:
-
Body surface area
References
Deng X, Huang P, Luo J, Chen R, Yang G, Chen W et al (2020) Residual shunts following isolated surgical ventricular septal defect closure: risk factors and spontaneous closure. Pediatr Cardiol 41:38–45
Bibevski S, Ruzmetov M, Mendoza L, Decker J, Vandale B, Jayakumar KA et al (2020) The destiny of postoperative residual ventricular septal defects after surgical repair in infants and children. World J Pediatr Congenit Heart Surg 11:438–443
Schipper M, Slieker MG, Schoof PH, Breur JM (2017) Surgical repair of ventricular septal defect; contemporary results and risk factors for a complicated course. Pediatr Cardiol 38:264–270
Abdelrehim AR, Al-Muhaya M, Alkodami AA, Baangood LS, Al-Mutairi M, Quadeer A, Alabsi FA et al (2022) Predictors of major adverse events and complications after ventricular septal defects surgical closure in children less than 10 kg. J Cardiothorac Surg 17(1):232
Dodge-Khatami A, Knirsch W, Tomaske M, Prêtre R, Bettex D, Rousson V et al (2007) Spontaneous closure of small residual ventricular septal defects after surgical repair. Ann Thorac Surg 83(3):902–905
Yang SG, Novello R, Nicolson S, Steven J, Gaynor JW, Spray TL, Rychik J (2000) Evaluation of ventricular septal defect repair using intraoperative transesophageal echocardiography: frequency and significance of residual defects in infants and children. Echocardiography 17:681–684
Larrazabal LA, del Nido PJ, Jenkins KJ, Gauvreau K, Lacro R, Colan SD, Pigula F, Benavidez OJ, Fynn-Thompson F, Mayer JE Jr, Bacha EA (2007) Measurement of technical performance in congenital heart surgery: a pilot study. Ann Thorac Surg 83:179–184
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R (2019) Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53:1801913
Bol Raap G, Meijboom FJ, Kappetein AP, Galema TW, Yap SC, Bogers AJ (2007) Long-term follow-up and quality of life after closure of ventricular septal defect in adults. Eur J Cardiothorac Surg 32:215–219
Roos-Hesselink JW, Meijboom FJ, Spitaels SEC, Van Domburg R, Van Rijen EHM, Utens EMWJ et al (2004) Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22–34 years. Eur Heart J 25:1057–1062
Bol-Raap G, Weerheim J, Kappetein AP, Witsenburg M, Bogers AJ (2003) Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg 24:511–515
Mizuno A, Niwa K, Matsuo K, Kawada M, Miyazaki A, Mori Y et al (2013) Survey of reoperation indications in tetralogy of fallot in Japan. Circ J 77:2942–2947
Nakayama Y, Horimoto Y, Shinkawa T (2023) Atrioventricular conduction recovery immediately after the re-operation in a repaired CHD patient. Cardiol Young 33:2438–2439
Tanveer R, Khan AU, Siddiqi TA, Siddique S, Nasreen A, Salman-ur-Rehman, et al (2010) Continuous versus interrupted technique of ventricular septal defect (VSD) closure in total correction for tetrology of Fallot pertaining to residal VSD. J Pak Med Assoc 60:253–256
Sen O, Kadirogullari E, Aydin U, Guler S, Haydin S (2018) Comparison of continuous and interrupted suturing techniques in ventricular septal defect closure. Heart Surg Forum 21:E418–E422
Zhou W, Li F, Fu L, Gao W, Guo Y, Liu T, Huang M, Zhang Y (2016) Clinical experience of transcatheter closure for residual ventricular septal defect in pediatric patients. Congenit Heart Dis 11:323–331
Ergün S, Genç SB, Yildiz O, Öztürk E, Kafalı HC, Ayyıldız P et al (2019) Risk factors for major adverse events after surgical closure of ventricular septal defect in patients less than 1 year of age: a single-center retrospective. Braz J Cardiovasc Surg 34:335–343
Acknowledgements
We thank you for Ms. Motoko Kiuchi, Megumi Kobayashi, Tomomi Wakao and Teruko Takahashi as contributing to examine the transthoracic echocardiogram.
Author information
Authors and Affiliations
Contributions
Dr. Yuki Nakayama designed the study, and wrote the main manuscript text and figures. Dr. Takeshi Shinkawa supervised and edited the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral presentation at the 49th annual meeting of Western Thoracic Surgical Association, June 21-24, 2023, IDAHO, USA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakayama, Y., Horimoto, Y., Suzuki, K. et al. Clinical Course of Residual Ventricular Septal Defects After Congenital Heart Disease Repair. Pediatr Cardiol (2024). https://doi.org/10.1007/s00246-024-03542-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00246-024-03542-5