Abstract
Background
The postnatal period in preterm infants involves multiple physiologic changes starting directly after birth and continuing for days or weeks. To recognize and treat compromise, it is important to measure cardiovascular function. We used a novel technique (speckle tracking echocardiography, STE) to measure cardiac function in this period.
Methods
We obtained cardiac ultrasound images at day 3, 7, 14, 21 and 28 in preterm infants <30-week gestation. Conventional measures included cardiac size, left ventricular stroke volume, atrial volume and the patent ductus arteriosus (PDA). Four chamber images were analyzed with STE, which provided parameters of left ventricular volume, longitudinal deformation and myocardial velocities.
Results
Images of 54 infants (gestational age 23–29 weeks) were analyzed. STE-derived stroke volume correlated well with conventional echocardiography-derived stroke volume, but agreement was suboptimal. Most STE parameters showed good reliability. All volume parameters and systolic and atrial velocities increased over time. Cardiac deformation and early diastolic velocity did not change. A PDA was associated with 33 % increased stroke volume at day 3 up to 98 % at day 28 with a spherically enlarged heart and increased filling pressure.
Conclusion
Speckle tracking echocardiography analysis is a feasible and reliable technique that can simultaneously obtain systolic and diastolic volumes, longitudinal deformation and myocardial velocities from one ultrasound window. Preterm hearts maintain cardiac function well during the first weeks of life, even with increased preload as a consequence of a PDA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The fetal circulation undergoes dramatic transition at birth. The circulation changes from a parallel system characterized by the presence of central shunts, right ventricular dominance and constricted pulmonary vasculature, to a circulation in series with cardiac output more evenly divided between the two ventricles and a greatly dilated pulmonary vascular bed. This transitional period can be challenging, especially for the very preterm infant. Significant changes in cardiac function can be observed in the first day of life, with further cardiovascular challenges to be expected from common complications after the immediate transitional phase such as a patent ductus arteriosus (PDA), infections and/or the development of chronic lung disease. An understanding of expected cardiovascular changes is important for recognition and management of circulatory disturbances in this population [10].
Blood pressure monitoring and conventional echocardiography are currently used to assess cardiovascular function in newborn infants, but these measures have limited diagnostic accuracy to detect subtle changes. There is a growing interest in novel echocardiography techniques in newborn infants to help monitor systolic and diastolic function during transition and in the period thereafter. Several investigators have used tissue Doppler imaging (TDI) to evaluate cardiac function [8, 19, 22, 24]. This technique uses Doppler principles to measure myocardial tissue velocity and can assess segmental wall shortening. TDI is relatively easy to use and has become well established in adult cardiology; however, it has its limitations [15]. The most important problem of TDI is its angle dependency.
Speckle tracking echocardiography (STE) is a novel technique that applies computer software analysis on images generated by conventional ultrasound techniques to track natural acoustic markers (speckles) from frame to frame, hence providing angle-independent segmental and global information on cardiac motion (velocity and displacement) and deformation [2, 12]. Deformation parameters are known as strain (the change of wall length during stress at end-systole compared to its original length in a relaxed state at end-diastole, expressed in %) and strain rate (the change of strain per unit time, expressed in s−1). STE software also provides automated volume measurements as additional parameters to describe cardiac function. The aim of this study is to explore longitudinal changes in left ventricular volume parameters, longitudinal deformation and myocardial velocities during the first few weeks after preterm birth using speckle tracking analysis.
Methods
Study Population
Preterm infants less than 30-week gestation >48 h of age in our neonatal intensive care were assessed for their eligibility for inclusion. Exclusion criteria were significant congenital abnormalities or congenital heart disease. After informed consent, the infants were measured at day 3, 7, 14, 21 and 28 after birth. Approval for this study was obtained from the Hunter New England Human Research Ethics Committee.
Echocardiographic Image Acquisition
A 12-MHz phased-array transducer was used with an iE33 echocardiographic scanner (Philips Medical Systems, the Netherlands). Images were acquired from two cardiac cycles triggered by the R wave and stored at acquired frame rate (typically 90–110 Hz). To minimize handling time in these small fragile infants, our scanning protocol was limited to long axis, four chamber and high parasternal view images.
Conventional Echocardiography Parameters
Left ventricular stroke volume (LV-SV) was calculated from the aortic valve area obtained from the parasternal long axis images and the average of 3–5 velocity time integrals of the left ventricular outflow Doppler pulse wave just distal to the aortic valve from the apical images.
The patent ductus arteriosus (PDA) was viewed from the high parasternal view. The minimum diameter of the color flow jet closest to the entry to the main pulmonary artery in end-systole was taken as PDA diameter [11].
The left atrium (LA) was imaged from the apical four chamber view. LA area was estimated by manually tracing the left atrium with exclusion of the appendage and pulmonary veins at end-systole. LA volume was then calculated using monoplane summation of disks method and indexed on body weight [17].
Left ventricular length was measured from four chamber images in end-diastole from the mitral annular hinge points to the apex, and left ventricular diameter as the endocardial distance just below the papillary muscles [20]. A sphericity index (SI) was calculated by dividing left ventricular diameter with length.
2D Speckle Tracking Analysis
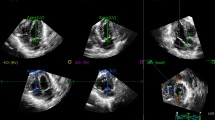
Speckle tracking analysis was performed using vendor-independent software (Cardiac Performance Analysis, version 1.1; TomTec Imaging Systems, Germany). A detailed description of our STE methodology in this population has been published elsewhere [6]. In short, after selecting an apical four chamber clip with optimal image quality, we traced the endocardial border as a sequence of points on a single frame where the trace was placed slightly within the endocardium border (Fig. 1, panel A). The software would then track the endocardial border from frame to frame throughout the cardiac cycle (supplemental video). The software automatically divided the cross-sectional image into six equidistant segments and rendered segmental and global curves for velocity, displacement, strain and strain rate (Fig. 1, panel B). The software would then report on longitudinal (base-to-apex wall shortening) and radial (inwards wall thickening) parameters. Previous research indicated that radial parameters were not reliable, so we report on longitudinal parameters only.
Screen capture of speckle tracking analysis of a four chamber clip with two cardiac beats. a The four chamber clip with added tracking line (green line) placed along the endocardial border. b Radial (top) and longitudinal (bottom) motion and deformation data for each segment and as global average. Strain data are shown in this example. c The data for volume (orange line) and rate of volume changes dVdt (blue line)
Myocardial velocities are reported by the software as average and for each segment. Basal septal and lateral velocities were averaged and presented as systolic (s’), early diastolic (e’) and atrial contraction (a’) peak velocities [30].
STE software provides an automated method of measuring left ventricular volume parameters. Using monoplane summation of disks method on the trace from the four chamber images, the software calculates volume for each frame (Fig. 1, panel C). The minimum volume (end-systolic volume, ESV) and maximum volume (end-diastolic volume, EDV) allow for the calculation of stroke volume (STE-SV) and ejection fraction. As volume is calculated for each frame, the software also reports on rate of volume changes (dVdt). dVdt of the left ventricle represents aortic outflow and mitral inflow patterns, with a systolic wave (S wave), early diastolic filling wave (E wave) and atrial contraction wave (A wave) [32]. We present peak rate of volume changes for each wave (dVdt S, dVdt E, dVdt A) and calculated an EA ratio (dVdt E/dVdt A). All volume parameters were indexed on body weight. An Ee’ ratio was calculated from dVdt E divided by early diastolic myocardial velocity (e’) as an estimation of left ventricular filling pressure [26, 30].
Statistical Analysis
All data were explored for normal distribution and reported accordingly. Repeated-measures ANOVA was conducted to examine differences between the measurement time points. Means were compared with independent sample t test for gestational age group (≤26 week vs >26 week), the use of mechanical ventilation at time of the scan and the presence of a PDA. There is a wide variety in the definition of a hemodynamically significant PDA, but for the purpose of this study, we used a PDA diameter > 1.5 mm as this is the most quoted cutoff in the neonatal literature [34]. Infants were grouped and analyzed at each measurement time point, i.e., with advancing age, the PDA > 1.5-mm group would only include infants in whom the PDA persisted.
Conventional derived stroke volume was compared to STE-derived stroke volume with regression analysis and using a Bland–Altman approach. A 10 % portion of the total amount of scans was selected blindly. All parameters were blindly re-measured by two investigators (KW and PT) for interrater reliability analysis and coefficient of variation (the standard deviation of the difference of paired samples divided by the average of the paired samples). One investigator (KW) repeated the measurements in the same set of scans 1 week later for intrarater reliability. p values < 0.05 were considered to indicate significance. Statistical analyses were performed using SPSS for Windows version 16.0 (SPSS, Inc., Chicago, IL).
Results
Fifty-four preterm infants (78 % of eligible) were included over a 10-month period. The mean gestational age was 27 weeks (range 23–29 weeks) with a birth weight of 965 grams (range 550–1530 g). Males constituted 53 and 7 % were small for gestational age (<third percentile of weight for age). Three babies died, two of necrotizing enterocolitis (day 13 and 19) and one of a viral pneumonia (day 10). Further clinical characteristics are presented in Table 1. Twenty-one scans could not be obtained at day 21 and/or day 28 as infants were transferred back to their referral hospital. Twelve scans (5 %) could not be analyzed with STE due to poor image quality.
Cardiac function and size parameters are presented in Table 2. Left atrial volume, left ventricular volume and size, rate of volume changes and systolic and atrial myocardial velocities increased significantly over time. EDV and rate of volume changes were significantly increased from day 7, and most other parameters from day 14 when compared to the day 3 values. Deformation parameters and early diastolic velocity did not change between the measurement time points.
Infants of younger gestational age (≤26 weeks) had similar myocardial velocities compared to older infants, but higher stroke volume (1.75 vs 1.40 ml/kg, p < 0.001) and longitudinal strain (−23.4 vs −22.5 %, p = 0.013).
Infants on mechanical ventilation at time of the scan were younger (gestational age 25 vs 27 weeks, p < 0.001) and had a larger PDA (1.7 vs 1.1 mm, p < 0.01), but neither mechanical ventilation nor mean airway pressure influenced deformation or myocardial velocities.
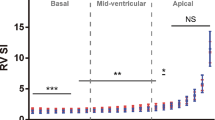
The presence of a PDA > 1.5 mm at time of the scan was found in 78 scans, with a median PDA diameter of 2.1 mm (range 1.6–3.8 mm). A PDA > 1.5 mm was associated with significantly higher atrial and end-diastolic volumes (p < 0.01, Fig. 2), stroke volume (p < 0.01), ejection fraction (p < 0.01, Fig. 3), systolic and diastolic dVdt (p < 0.01), sphericity (p < 0.01, Fig. 4) and STE-derived Ee’ ratio (p < 0.01, Fig. 5) at all measurement time points. Longitudinal deformation parameters were consistently higher in the infants with a PDA > 1.5 mm, but this only reached significance at day 3 (strain −23.6 vs −21.3 %, strain rate −2.68 vs −2.41 s−1, p < 0.01). Myocardial velocities were not significantly different between the groups.
LV-SV showed good correlation with STE-SV (r 2 = 0.639, p < 0.001). However, LV-SV was higher than STE-SV, with a bias of 0.77 ml/kg and limits of agreement between −0.36 and 1.90 ml/kg. Inter- and intrarater variability of conventional and STE parameters is presented in Table 3.
Discussion
This study presents serial data on left ventricular volume, longitudinal cardiac deformation and myocardial velocities obtained with speckle tracking analysis in very preterm infants after the immediate transitional period.
Speckle tracking as a technique has been well validated in vitro, in animals and in adults. The possibility of segmental analysis with increased sensitivity in detecting abnormal myocardium helped establish STE as diagnostic modality in detecting and quantifying myocardial ischemia and reperfusion viability [16]. Other clinical applications in adults include early detection of global ventricular dysfunction in valvular disease, cardiomyopathy and volume overload [21]. In the pediatric population, STE is used for functional assessment of congenital heart disease [12]. Recent reports in preterm infants show its additional value in the assessment of PDA treatments [7, 29].
Deformation parameters measure relative contractility (not absolute) and therefore reflect global cardiac function [2, 12]. Myocardial velocities are related to acceleration, which is a measure of force and thus also a measure of cardiac function [15, 30]. Changes in deformation or velocity reflect changes in contractility and load of the right and left ventricle depending on where it is measured. Longitudinal deformation did not change and systolic myocardial velocities gradually increased over time, suggesting that systolic cardiac function and contractility were maintained throughout the study period. These findings compare well with other investigators. Helfer et al. [13] measured TDI-derived strain and strain rate in a similar postnatal period and found no changes in wall shortening of the lateral wall of the left ventricle. The same research group also reported on deformation parameters obtained with STE and showed no change in global longitudinal strain and a small increase in strain rate [5]. Saleemi et al. [28] used TDI to measure myocardial velocities and found comparable changes in systolic velocities, except in the youngest gestational age group (24–27 weeks). Deformation parameters are fractional changes in length of a cardiac segment relative to its original length, and thus, values are normalized for size [2, 12]. Myocardial velocities are not normalized for size, possibly explaining the differences in postnatal course found. Some investigators suggest that normalization of myocardial velocities by heart size may be of value when assessing myocardial function in newborn infants [9]. Velocities in this study did not change over time when they were normalized for left ventricular length (data not shown). Overall, it seems that very preterm infants are capable of maintaining relative constant systolic myocardial function and contractility after the immediate transitional period.
Diastolic function during the study period resembled those of the fetal heart, with higher dependency on atrial contraction and impaired early filling [25]. Early diastolic function did not improve during early preterm life. This finding is consistent with a previous report that showed delayed early diastolic maturation in preterm infants up till term corrected age [14].
The most prominent effect on cardiac function in the measured time period was due to the presence of a PDA. A PDA > 1.5 mm was associated with increased volumes. Stroke volume was 33 % higher already at day 3 and almost doubled if the PDA persisted until day 28. As a consequence, the heart became more spherical in shape. In the early phase of increased volume load, longitudinal wall shortening was increased to help maintain stroke volume. Strain and strain rate are influenced by preload (positively) and afterload (negatively) [3, 6]. The presence of a reasonably sized PDA and falling pulmonary pressures presents as physiologic situation with increased preload and decreased afterload and could explain the higher wall shortening found. The increase in sphericity helped maintain high stroke volumes at later time points, as a larger diameter of the ventricle would require less longitudinal shortening to maintain stroke volume.
The increased amount of volume flowing through left ventricle in infants with a PDA did not lead to obvious cardiac dysfunction compared to infants who did not have a PDA. Longitudinal deformation and systolic velocities were maintained throughout the study period in all infants. The EA ratio as indicator of diastolic function remained unchanged, and early diastolic and atrial velocities in the infants with a PDA showed postnatal changes comparable to the infants without a PDA. Rate of volume changes was higher in the infants with a PDA; hence, our STE-derived Ee’ ratio was increased as indication of increased left ventricular filling pressure due to the increased volume throughput. We expected more cardiac dysfunction with a persistent PDA, especially after prolonged exposure. It is possible that the PDA cutoff used in this study did not adequately differentiated infants with a large shunt size. We also cannot comment if other (unmeasured) components of cardiac function such as circumferential deformation, torsion or radial mechanics were affected by a PDA.
The absolute values of STE-derived parameters were not directly comparable to values obtained with conventional or tissue Doppler measurements. Our STE-derived myocardial velocities were lower compared to TDI velocities of other investigators [8, 22, 28], and STE-SV was consistently lower compared to LV-SV. Nagasawa et al. [23] compared four methods to determine LV stroke volume in 70 healthy term neonates with 3D echocardiography as gold standard and showed that monoplane summation of disks method underestimated 3D-SV by 15 % and that the Vti method overestimated 3D-SV by 75 %. The authors concluded that 3D and summation of disks methods were appropriate for measuring SV in neonates during the early neonatal period, but it is unclear whether 3D echocardiography can be regarded as gold standard in very preterm infants due to the technical limitations to accommodate measurements at high frame rates [1].
The longitudinal deformation parameters in our study were higher compared to values obtained in the same population but with different equipment [5, 14], and higher compared to 3D deformation in healthy term neonates measured with the same equipment [33]. Deformation values can be influenced by inherent population dissimilarities such as differences in heart rate, blood pressure and chamber geometry, but also by technical issues such as ultrasound equipment, acquired frame rate and software package used [4, 6, 18, 27]. The industry is aware of the need to standardize deformation imaging and has taken steps to reduce intervendor variability [31]. For now, each technique and software package have its own advantages and drawbacks and this study cannot comment on which is most accurate.
The limitations of our study include a relative small sample size and the lack of data on right ventricular function. We used monoplane volume and deformation measurements. Accuracy may be improved with biplane measurements but should be balanced against the burden of increased handling time of these small infants. Our study was not designed to investigate the acute cardiac effects of common pathology and management in the postnatal period. Further prospective studies are needed to evaluate the full clinical potential of STE measurements to help establish when pathology occurs and the subsequent effect of various management choices.
References
Balluz R, Liu L, Zhou X, Ge S (2013) Real time three-dimensional echocardiography for quantification of ventricular volumes, mass, and function in children with congenital and acquired heart diseases. Echocardiography 30(4):472–482
Blessberger H, Binder T (2010) Non-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart 96(9):716–722
Burns AT, La Gerche A, D’hooge J, MacIsaac AI, Prior DL (2010) Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr 11(3):283–289
Cantinotti M, Kutty S, Giordano R, Assanta N, Murzi B, Crocetti M, Marotta M, Iervasi G (2015) Review and status report of pediatric left ventricular systolic strain and strain rate nomograms. Heart Fail Rev 20(5):601–612
Czernik C, Rhode S, Helfer S, Schmalisch G, Bührer C, Schmitz L (2004) Development of left ventricular longitudinal speckle tracking echocardiography in very low birth weight infants with and without bronchopulmonary dysplasia during the neonatal period. PLoS ONE 9(9):e106504
de Waal K, Lakkundi A, Othman F (2014) Speckle tracking echocardiography in very preterm infants: feasibility and reference values. Early Hum Dev 90(6):275–279
El-Khuffash AF, Jain A, Dragulescu A, McNamara PJ, Mertens L (2012) Acute changes in myocardial systolic function in preterm infants undergoing patent ductus arteriosus ligation: a tissue Doppler and myocardial deformation study. J Am Soc Echocardiogr 25(10):1058–1067
Eriksen BH, Nestaas E, Hole T, Liestøl K, Støylen A, Fugelseth D (2013) Myocardial function in premature infants: a longitudinal observational study. BMJ Open 3(3) pii: e002441
Eriksen BH, Nestaas E, Hole T, Liestøl K, Støylen A, Fugelseth D (2014) Myocardial function in term and preterm infants. Influence of heart size, gestational age and postnatal maturation. Early Hum Dev 90(7):359–364
Evans N (2006) Assessment and support of the preterm circulation. Early Hum Dev 82(12):803–810
Evans N, Iyer P (1995) Longitudinal changes in the diameter of the ductus arteriosus in ventilated preterm infants: correlation with respiratory outcomes. Arch Dis Child Fetal Neonatal Ed 72(3):F156–F161
Forsey J, Friedberg MK, Mertens L (2013) Speckle tracking echocardiography in pediatric and congenital heart disease. Echocardiography 30(4):447–459
Helfer S, Schmitz L, Buhrer C, Czernik C (2014) Tissue Doppler-derived strain and strain rate during the first 28 days of life in very low birth weight infants. Echocardiography 31(6):765–772
Hirose A, Khoo NS, Aziz K, Al-Rajaa N, van den Boom J, Savard W, Brooks P, Hornberger LK (2015) Evolution of left ventricular function in the preterm infant. J Am Soc Echocardiogr 28(3):302–308
Ho CY, Solomon SD (2006) A clinician’s guide to tissue Doppler imaging. Circulation 113(10):e396–e398
Hoit BD (2011) Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 4(2):179–190
Jantzen DW, Aldoss O, Sanford B, Fletcher SE, Danford DA, Kutty S (2010) Is combined atrial volumetrics by two-dimensional echocardiography a suitable measure for quantitative assessment of the hemodynamic significance of patent ductus arteriosus in neonates and infants? Echocardiography 27(6):696–701
Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, Friedberg MK (2011) Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr 24(1):37–44
Lee A, Nestaas E, Liestøl K, Brunvand L, Lindemann R, Fugelseth D (2014) Tissue Doppler imaging in very preterm infants during the first 24 h of life: an observational study. Arch Dis Child Fetal Neonatal Ed 99(1):F64–F69
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23(5):465–495
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12(3):167–205
Murase M, Morisawa T, Ishida A (2013) Serial assessment of left-ventricular function using tissue Doppler imaging in premature infants within 7 days of life. Pediatr Cardiol 34(6):1491–1498
Nagasawa H, Kohno Y, Yamamoto Y, Kondo M, Sugawara M, Koyama T, Terazawa D, Miura R (2014) Methodologic comparison of left ventricular stroke volumes in the early neonatal period by echocardiography. Pediatr Cardiol 35(8):1415–1420
Negrine RJ, Chikermane A, Wright JG, Ewer AK (2012) Assessment of myocardial function in neonates using tissue Doppler imaging. Arch Dis Child Fetal Neonatal Ed 97(4):F304–F306
Nii M, Roman KS, Kingdom J, Redington AN, Jaeggi ET (2006) Assessment of the evolution of normal fetal diastolic function during mid and late gestation by spectral Doppler tissue echocardiography. J Am Soc Echocardiogr 19(12):1431–1437
Park JH, Marwick TH (2011) Use and limitations of E/e’ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound 19(4):169–173
Rösner A, Bijnens B, Hansen M, How OJ, Aarsaether E, Müller S, Sutherland GR, Myrmel T (2009) Left ventricular size determines tissue Doppler-derived longitudinal strain and strain rate. Eur J Echocardiogr. 10(2):271–277
Saleemi MS, El-Khuffash A, Franklin O, Corcoran JD (2014) Serial changes in myocardial function in preterm infants over a four week period: the effect of gestational age at birth. Early Hum Dev 90(7):349–352
Sehgal A, Doctor T, Menahem S (2014) Cyclooxygenase inhibitors in preterm infants with patent ductus arteriosus: effects on cardiac and vascular indices. Pediatr Cardiol 35(8):1429–1436
van Dalen BM, Bosch JG, Kauer F, Soliman OI, Vletter WB, ten Cate FJ, Geleijnse ML (2009) Assessment of mitral annular velocities by speckle tracking echocardiography versus tissue Doppler imaging: validation, feasibility, and reproducibility. J Am Soc Echocardiogr 22(11):1302–1308
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, Badano LP (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 28(2):183–193
Zeidan Z, Erbel R, Barkhausen J, Hunold P, Bartel T, Buck T (2003) Analysis of global systolic and diastolic left ventricular performance using volume-time curves by real-time three-dimensional echocardiography. J Am Soc Echocardiogr 16(1):29–37
Zhang L, Gao J, Xie M, Yin P, Liu W, Li Y, Klas B, Sun J, Balluz R, Ge S (2013) Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: feasibility, reproducibility, maturational changes, and normal ranges. J Am Soc Echocardiogr 26(8):853–859
Zonnenberg I, de Waal K (2012) The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr 101(3):247–251
Acknowledgments
Financial support was obtained through a grant of the John Hunter Hospital Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Waal, K., Phad, N., Lakkundi, A. et al. Cardiac Function After the Immediate Transitional Period in Very Preterm Infants Using Speckle Tracking Analysis. Pediatr Cardiol 37, 295–303 (2016). https://doi.org/10.1007/s00246-015-1277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1277-3