Abstract
In recent years, organophosphate esters (OPEs) have become one of the most common additives in various consumer products worldwide, therefore the exposure and impact of OPEs on human health are drawing a lot of attention. In this study, three metabolites of OPEs including bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), diphenyl phosphate (DPhP) and diethyl phosphate (DEP) were investigated in first-morning void urine samples taken from a population (age range: 3–76 years old) in Hanoi, Vietnam. The most dominant urinary OPE metabolite was DEP with the geometric mean of specific gravity adjust (SG-adjusted) concentration were 1960 ng mL−1 and detected frequency (DF) of 98%. Followed by DPhP (8.01 ng mL−1, DF: 100%) and BDCIPP (2.18 ng mL−1, DF: 51%). The results indicated that gender and age might have associations with the OPE metabolites variation in urine samples. The levels of OPE metabolites in urine samples from females were slightly higher than in males. An increase in age seems to have an association with a decrease in DPhP levels in urine. Exposure doses of parent OPEs were evaluated from the unadjusted urinary concentration of corresponding OPE metabolite. The estimated exposure doses of triethyl phosphate (TEP) (mean: 534,000 ng kg−1 d−1) were significantly higher than its corresponding reference dose, suggesting the high potential risk from the current exposure doses of TEP to human health. The results of this work provided the initial information on the occurrence of three OPE metabolites in urine from Hanoi, Vietnam and estimated exposure dose of corresponding parent OPEs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
From the mid-2000s, the consumption of organophosphate esters (OPEs) had been increased dramatically in many regions due to their application as the main replacement chemicals for polybrominated diphenyl ethers (PBDEs), which had been restricted in many countries (Butt et al. 2014) due to the toxic impacts on human health (Berghuis et al. 2015; Linares et al. 2015). In 2015, 680 thousand tons of OPEs were manufactured worldwide, and its estimated yearly increase was about 15% (Gustavsson et al. 2018). Worldwide, OPEs were applied as additives in various household and industrial items (plastic, textiles, mattresses, furniture, electronics, foam, resins, hydraulic fluids, lacquers and nail polish) (Mendelsohn et al. 2016; Salamova et al. 2014; Van der Veen and de Boer 2012). In addition, many OPEs are used as agricultural and household organophosphorus pesticides (Pundir et al. 2019), which are used widely to control insect crops and houses in many countries (Jayatilaka et al. 2019; Yang et al. 2019).
OPEs are additives without chemically bound to the main materials, therefore they can easily diffuse out of main products and go into surrounding environments during the life cycle of products (Wang et al. 2014). Combination with the common use of OPEs, these chemicals had been ubiquitously spread into living environments such as indoor air (Hoang et al. 2023), indoor and outdoor dust (Al-Omran et al. 2021; Hoang et al. 2023), atmospheres (Zhang et al. 2019), and natural environments (water, sediment, soil and biota) (Chen et al. 2022; Wang et al. 2019b; Ye et al. 2022; Zhang et al. 2022a). Furthermore, OPEs and some metabolites of OPE were also frequently presented in agro-foods (vegetables, fruit, meat, fish, egg, and milk) (He et al. 2018b; Yang et al. 2019; Zhang et al. 2022b) and drinking water (Choo and Oh 2020). There are concerns about OPEs exposure and the influence of these compounds on human health. OPEs exposure to humans mostly through ingestion of foods, drinking water and dust contaminated by OPEs, inhalation of OPEs in air and fine dust, and dermal contact with OPEs in goods and indoor environments (Cequier et al. 2015; He et al. 2018b; Lee et al. 2016). Previous reports have pointed out the negative influences on human health (including carcinogenic and neurotoxic impacts) of OPEs (Niu et al. 2019). Tri(1,3-dichloro-2-propyl) phosphate (TDCIPP) was recognized as a carcinogenic chemical with high biological and neurotoxicity (Lu et al. 2017; Niu et al. 2019). Triethyl phosphate (TEP) was recorded which may have some neurotoxic properties and/or potential mutagenic effects in humans at high exposure doses (Lai et al. 2022). Triphenyl phosphate (TPhP) has been pointed out to have associations with male reproductive system effects (Meeker and Stapleton 2010).
In human body, OPEs are hydrolyzed to their diester metabolites, and these OPE metabolites are excreted through urine (Liu et al. 2016). For instant, the main conversion products of TDCIPP, TPhP and TEP in human and/or animal bodies were BDCIPP, DPhP and DEP, respectively (Wang et al. 2016; Yao et al. 2021). Therefore, these OPE diester metabolites (BDCIPP, DPhP and DEP) in urine were commonly used as the biomarker of their parent chemicals (TDCIPP, TPhP and TEP, respectively) (Hoffman et al. 2014; Krystek et al. 2019). However, it is noticeable that DPhP and DEP are non-specific metabolites, DPhP is degraded from some other aryl-OPEs (Wang et al. 2019a; Zheng et al. 2021) and DEP is a metabolite of some organophosphorus pesticides (Yang et al. 2019). Some previous reports have shown the ubiquitous presence of OPE metabolites (such as BDCIPP, DPhP, DEP, bis(1-chloro-2-propyl) phosphat, bis-2(butoxyethyl) phosphate, bis(2-chloroethyl) hydrogen phosphate, di-cresyl phosphate, bis(butoxyethyl) phosphate, dipropyl phosphate, dibutyl phosphate) in human (children and/or adults) urine in many areas in the United States (U.S) (Dodson et al. 2014; Jayatilaka et al. 2019; Preston et al. 2017; Thomas et al. 2017; Wang et al. 2019a), Canada (Kosarac et al. 2016), Australia (Van den Eede et al. 2015), Norway (Cequier et al. 2015), and China (He et al. 2018b; Lu et al. 2017; Sun et al. 2018; Tao et al. 2018). Among those reports, the frequently detected metabolites of OPEs in urine were BDCIPP, DPhP and DEP (He et al. 2018a; Sun et al. 2018; Wang et al. 2019a).
In developing countries, such as Vietnam, the appearance of OPEs was reported in various environments (air, dust and water) (Hoang et al. 2023; Truong et al. 2023). Especially, in our recent studies in Hanoi city in Vietnam, the wide spread of OPEs were observed in indoor environment (air and dust) with high levels (median of total OPEs concentration were 101 and 7580 ng g−1, respectively) (Hoang et al. 2023), and in surface water in urban Hanoi with the total concentration was also at elevated levels (range: 46–3644 ng L−1, average: 1409 ng L−1) (Truong et al. 2023). However, the study on the organic pollutants in urine from Vietnam was limited. Previous studies had only focused on metals and some organic chemicals (polycyclic aromatic hydrocarbons, PBDEs, polychlorinated biphenyls, persistent pesticides and phthalate metabolites) in urine from a group of electronic waste recyclers or a small group of people in Vietnam (Schecter et al. 2018; Thai et al. 2015), which leads to the information shortage on the OPE metabolites levels in urine from Vietnam.
For a general view of the occurrence of urinary OPE metabolite levels and exposure to OPEs in the population of Hanoi citizens in Vietnam, the three most frequently detected urinary OPE metabolites (BDCIPP, DPhP, and DEP) were selected for analysis in urine samples taken randomly from Hanoi, Vietnam. This work aimed to (1) Determine the levels of urinary metabolites of OPE (BDCIPP, DPhP and DEP) from a population in Hanoi, Vietnam; (2) to determine the associations between gender/age and urinary OPE metabolites concentration of the study group; (3) estimate the exposure doses to parent OPEs (TDCIPP, TPhP and TEP) of citizen in Hanoi, Vietnam.
Materials and Methods
Chemicals and Materials
Three organophosphate metabolites standards BDCIPP, DPhP and DEP (Table S1) and internal standards BDCIPP-d10, DPhP-d10 and DEP-d10 were supplied by Toronto Research Chemicals Inc. (Canada). In this study, solvents (acetonitrile, methanol and acetone) were used at analytical grade and supplied by Merck Co. (Germany). The polymeric solid phase extraction Strata-X-AW (60 mg 3 mL−1) sorbents were purchased from Phenomenex Inc. (Italy), and the nylon filter (Millex® hydrophilic PTFE syringe filter, 13 mm, 0.2 µm) were provided by Merck Co. (Germany). All the glassware was washed and rinsed with solvents prior to usage. A mixed-stock solution containing all analytes was prepared in acetonitrile at a concentration of 10 mg L−1 and was kept at − 20 °C. Calibration solutions (0.02–100 μg L−1) used in routine applications were prepared in acetonitrile.
Sample Collection, Extraction and Analysis
Sixty-one urine samples were sampled in January 2023 from 61 volunteers, who have been living in Hanoi, Vietnam. The volunteers were chosen randomly, and the information about the participant’s gender (male, n = 26; female, n = 35) and ages (3–76 years old) was collected before sampling (Table S2). Female volunteers were not in their menstrual period during the sampling time. Each individual gave a single first-morning void urine sample into a 100 mL glass container, which was precleaned with heat at 450° C before use. The obtained samples were taken to the laboratory within 1 h and then preserved at − 20° C until analysis. The protocol of this study was reviewed and approved by the Hanoi University of Public Health Institutional Review Board, No: 337/2023/YTCC-HD3.
The method of extraction of OPEs metabolites in urine samples was carried out according to Sun's method (Sun et al. 2018) with small modifications. A 2 mL urine sample was spiked with an internal standards mixture (BDCIPP-d10, DPhP-d10 and DEP-d10: 20 μg mL−1), added 1 mL of ammonium acetate buffer (10 mM, pH = 5), and mixed with a vortex shaker in 1 min. Then the mixture was extracted by solid phase extraction with a cartridge Strata-X-AW (60 mg 3 mL−1) that had been conditioned with 2 mL of methanol, and 2 mL of ultrapure water, sequentially at a flow rate of 1 mL min−1, after that washed by 2 mL of ultrapure water, and vacuum-dried for 30 min before being eluted with 2 mL methanol containing 5% ammonium hydroxide. The eluent was then concentrated down to approximately dry under a stream of N2. Finally, re-dissolve the residue and diluted to a final volume of 500 µL with acetonitrile, and filtered through a nylon filter (13 mm, 0.2 µm) prior to LC–MS/MS analysis.
The compounds BDCIPP, DPhP and DEP were analyzed with high-performance liquid chromatography (HPLC, Waters, model: Xevo TQ-XS) coupled with electrospray triple quadrupole mass spectrometer (Waters, USA), operated in the electrospray negative ionization (ESI-) for DPhP and DEP, and electrospray positive ionization (ESI +) for BDCIPP, with atmospheric-pressure chemical ionization (APCI) mode. Using an ACQUITY UPLC C18 column (leghth 150 mm, i.d. 2.1 mm and film thickness 1.7 μm, Phenomenex, Torrance, USA). 5 µL of extracted sample was injected into the chromatographic separation column at maintained temperature of 60 °C. The mobile phase consisted of 0.05% (v/v) trifluoroacetic acid in MilliQ water (A) and 0.01% (v/v) formic acid in acetonitrile (B), at a flow rate of 0.2 mL min−1. The gradient flow started at 80:20 A/B followed by an increase of phase B to 100% in 2 min, and held for 3 min. Then an increase of phase A to 80% and phase B decrease to 20% over 3 min and held for and equilibrated for 6 min. Details on the MS/MS parameters used are presented in Table S3.
The chemical parameters of urine samples such as pH, specific gravity (SG), total creatinine (Cr), and total protein were measured by using a digital ACON Mission U120 Smart urine refractometer (Acon Biotech, Hangzhou, Co., Ltd, China).
Quality Assurance and Quality Control
In each batch of samples, three procedural blanks, three spiked blanks (analytes added into solvents), and three spiked samples (analytes added into real urine samples) were extracted and analyzed according to the procedure of real urine samples. The blank samples were not found trace levels of BDCIPP, DPhP and DEP. The recoveries of BDCIPP, DPhP and DEP spiked in the urine matrix were 96.2, 85.1 and 102%, respectively. The limits of quantification (LOQs) and method detection limit (MDLs) of BDCIPP, DPhP and DEP from the spiked blanks and spiked matrix are shown in Table S4.
Data Analysis
Reported urinary BDCIPP, DPhP and DEP values were subtracted from the concentrations detected in blanks (average value). To justify for variances in dilution of urine caused by age, urine flow, disease, body mass index and sex (Braun et al. 2011), the raw (unadjusted) concentrations were converted to the specific gravity (SG)-adjusted concentrations using the following equation (Sun et al. 2018; Thomas et al. 2017):
where CSG is the SG-adjusted concentration of OPE metabolite (ng mL−1), C is the raw concentration of OPE metabolite (ng mL−1), and the specific gravity value is SG (Sun et al. 2018; Thomas et al. 2017). Descriptive statistics, statistical tests and discussion of the urinary OPE metabolites concentrations in this study used the SG-adjusted values. In addition, we also corrected the raw concentration for creatinine (Cr)-adjusted values (ng g−1 creatine) to serve as a reference for future studies.
A value of half of MDL was used for the concentration below method detection limit (MDL), and the non-detected (ND) was set as zero. Mann–Whitney U tests were performed to investigate the variances in urinary OPE metabolite concentrations of individuals with gender and age. Connections between each urinary OPE metabolites were examined by operating Spearman's correlation rank analysis. p < 0.05 was set as the statistical significance value. We used IBM SPSS Statistics software (Version 26.0, SPSS Inc., NY, U.S.) to conduct all the statistical analyses.
The unadjusted values of OPE metabolite in urine samples were used to estimate the exposure doses of corresponding OPEs, using Eq. (2) (Wang et al. 2019a) as follows:
where ED is the estimated exposure dose of parent OPE (ng kg−1 d−1), Cm is the unadjusted concentrations of OPE metabolite detected urine samples (ng mL−1). MWm is the OPE metabolite’s molecular weight and MWp is the molecular weight of corresponding OPEs. Vur is the daily urine excretion volume (22.2 and 20 mL kg−1 d−1 for children and adults, respectively) (Chen et al. 2018). Urine metabolite excreted molar fraction (MF) was 0.18 for DEP and 0.63 for DPhP and BDCIPP (Lynn et al. 1981; Suzuki et al. 1984).
Results and Discussion
Levels of OPE Metabolites in Urine
The chemical parameters of urine samples collected from 61 volunteers aged 3–76 years, including 33 females and 25 males were shown that pH values ranged from 5 to 8, with no glucose detected in urine samples, creatine content is between 0.1 and 1 g L−1, specific gravity between 1.005 and 1.030 and a few samples proteins are present (Table S5). The results showed that the volunteers were in normal health.
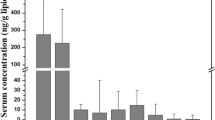
The occurrence of BDCIPP, DPhP and DEP detected in 61 urine samples from Hanoi, Vietnam ranged from not detected (ND) to 8080 ng mL−1 (uncorrected concentration) (Table S6). Due to extremely high of detected urinary OPE metabolite concentrations (especially DEP, uncorrected concentration of 503–8080 ng mL−1), a resurvey on the occupation of participants was undertaken to collect more information on OPEs exposure in working environments. Two urine samples with urinary DEP concentration at relatively high levels (sample: U55 and U58, uncorrected DEP concentration of 3700 and 8080 ng mL−1, respectively) were collected from two female workers (age 31–34 years old), who were frequently exposure to OPEs in their workplace (thermal cuts/reshape PU foam in a small workshop). Therefore, those two samples were not used for further discussion in this study. Table 1 shows the concentrations (unadjusted, SG-adjusted and Cr-adjusted) and the detected frequencies (DF) of three urinary OPE metabolites (BDCIPP, DPhP and DEP) in urine samples, with details are shown in Table S6. Comparison between the urinary OPE metabolites (unadjusted and SG-adjusted) values of this study with other studies is shown in Table 2. The detected urinary SG-adjusted concentration of BDCIPP, DPhP and DEP in Hanoi, Vietnam were from 0.02 to 120,000 ng mL−1. DEP was the most predominant urinary OPE metabolite observed in this study with the geometric mean (GM) SG-adjusted value of 1960 ng mL−1 (range: 402–120,000 ng mL−1, DF: 98%), which was remarkably greater than the levels of DPhP (SG-GM: 8.01 ng mL−1, range: 4.72–60.7 ng mL−1, DF: 100%) and BDCIPP (SG-GM: 2.18 ng mL−1, range: 0.02–238 ng mL−1, DF: 51%). The presence of all three targeted metabolites of OPE in urine samples, implies the widespread exposure of OPEs to the Hanoi citizen in Vietnam.
The high DF (98%) of DEP in urine samples in Vietnam were in agreement with some previous reports, such as in New York, U.S (DF: 100%) (Wang et al. 2019a), Atlanta, U.S (DF of DEP: 88–92%) (Jayatilaka et al. 2019) and Shanghai, China (91%) (Sun et al. 2018). However, the GM concentration of DEP in urine observed in Vietnam (SG-adjusted value: 1960 ng mL−1, unadjusted value: 1890 ng mL−1) was approximately 3–4 orders of magnitude higher than those appeared in the U.S (Jayatilaka et al. 2019; Wang et al. 2019a) and China (Sun et al. 2018). The extremely high levels and DF of DEP in urine samples indicated the frequency of exposure with high doses to its parent chemicals of citizens in Hanoi, Vietnam. DEP is the metabolite of TEP, which is a common flame retardant and plasticizer (Jayatilaka et al. 2019). TEP was found in indoor and outdoor dust samples in Hanoi, Vietnam (Hoang et al. 2023) and China (Wang et al. 2020). Furthermore, DEP can be metabolized from organophosphorus pesticides (Yang et al. 2019), such as diazinon and chlorpyrifos (Maravgakis et al. 2012). Diazinon and chlorpyrifos were commonly applied in agriculture in Vietnam (Phung et al. 2012; Van Cong et al. 2022). The residues of diazinon and/or chlorpyrifos were found on agricultural products such as vegetables, fruit, meat, beef, chicken and milk (Am 2019; Dallegrave et al. 2018; Gazzotti et al. 2009; Marete et al. 2020; Nematollahi et al. 2022; Słowik-Borowiec et al. 2012). In this research, urine samples were collected mostly from urban citizens, who have less contact with agricultural activities, therefore, the exposure sources of organophosphorus pesticides to the participants were probably from agro-foods and/or household insecticides (Jaga and Dharmani 2003). Therefore, the exposure sources of TEP and organophosphorus pesticides to citizens in Hanoi, Vietnam might come from industrial, domestic, and agricultural, and food origins.
The ubiquitous present of DPhP (DF: 100%) in urine samples same to this study was also observed in many areas of the U.S (DF: 92–100%) (Butt et al. 2014, 2016; Hoffman et al. 2014; Petropoulou et al. 2016; Preston et al. 2017; Thomas et al. 2017; Wang et al. 2019a), Canada (92%) (Kosarac et al. 2016), Australia (97–100%) (Van den Eede et al. 2015), Norway (97%) (Cequier et al. 2015) and China (99–100%) (Chen et al. 2018; Lu et al. 2017). In term of concentration, the urinary concentrations of DPhP in Hanoi, Vietnam (SG-adjusted value: 8.01 ng mL−1, unadjusted value: 7.74 ng mL−1) was approximately 1–2 order of magnitude greater than those observed in China (Chen et al. 2018; Lu et al. 2017; Sun et al. 2018; Tao et al. 2018) and it was at a higher level (3–18 times higher) than those recorded in many areas in the U.S (Butt et al. 2014, 2016; Cooper et al. 2011; Dodson et al. 2014; Hoffman et al. 2014; Jayatilaka et al. 2019; Petropoulou et al. 2016; Preston et al. 2017; Thomas et al. 2017; Wang et al. 2019a), Canada (Kosarac et al. 2016) and Norway (Cequier et al. 2015). However, when compared to the DPhP concentration in urine from Australia (24.4–63.4 ng mL−1 unadjusted concentration) (Van den Eede et al. 2015), the urinary DPhP values detected in this study were much lower (3–8 times lower). Since DPhP is a non-specific metabolite, its parent chemical is not only TPhP but several other aryl-OPEs (Funk et al. 2019; Wang et al. 2019a; Zheng et al. 2021). Therefore, the frequent appearances of DPhP in urine samples suggested the high frequent exposure to its parent chemicals of participants, and implied the common application of aryl-OPEs in daily items (such as plastic, polyurethane foams, hydraulic fluids, nail polish, varnishes and lacquers) in Hanoi, Vietnam.
The DF (51%) of BDCIPP in urine in Hanoi, Vietnam was higher than those observed in Canada (DF: 29%) (Kosarac et al. 2016), Shanghai (21%) (Sun et al. 2018) and South China (29%), China (Chen et al. 2018), but similar to urine collected in Norway (52–61%) in 2012 (Cequier et al. 2015). However, many studies in the U.S (DF > 90%) (Butt et al. 2014, 2016; Cooper et al. 2011; Hoffman et al. 2014; Petropoulou et al. 2016; Preston et al. 2017; Thomas et al. 2017; Wang et al. 2019a) and Australia (> 92%) (Van den Eede et al. 2015) showed much higher DF of BDCIPP in urine than those reported in this study. The GM value of BDCIPP (SG-adjusted value: 2.18 ng mL−1, unadjusted value: 2.20 ng mL−1) in this study was higher (about 1–3 orders of magnitudes) than those reported in some cities in the U.S (Cooper et al. 2011; Dodson et al. 2014; Hoffman et al. 2014; Jayatilaka et al. 2019; Wang et al. 2019a), Canada (Kosarac et al. 2016), Australia (Van den Eede et al. 2015), Norway (Cequier et al. 2015) and China (Chen et al. 2018; Lu et al. 2017; Sun et al. 2018; Tao et al. 2018), comparable to those recorded for Atlanta firefighter (unadjusted value: 3.3 ng mL−1) (Jayatilaka et al. 2019), mothers (SG-adjusted value: 3.3 ng mL−1) (Butt et al. 2016) and adults (unadjusted value: 2.5 ng mL−1) from California, U.S (Petropoulou et al. 2016), and mothers from New Jersey (SG-adjusted value: 2.4 ng mL−1) (Butt et al. 2014). In comparison with the urinary BDCIPP levels from children in the U.S (SG-adjusted GM value: 5.6–10.9 ng mL−1) (Butt et al. 2014, 2016; Thomas et al. 2017) the values detected in Hanoi, Vietnam were 2–5 times lower. This difference in the DF and concentration of BDCIPP in urine between countries is probably due to the variation of OPE usage habits of between these regions. While in the U.S, alternative flame retardants (mostly OPEs) had been replaced PBDEs in consumer products since the 2000s (Butt et al. 2016; Cristale et al. 2013; Gustavsson et al. 2018; Thomas et al. 2017; Xu et al. 2021), it seems that PBDEs were still the additives to in many items (Hoang et al. 2021), and OPEs may have been not yet completely substituted PBDEs in Vietnam. In general, the urinary BDCIPP, DPhP and DEP values detected in Hanoi, Vietnam were at greater levels than most of those reported in some other regions, these results might reflect the high exposure to TDCIPP, TPhP and TEP in Hanoi, Vietnam.
Relationship of Gender and Age with Urinary OPE Metabolite Levels
In order to determine the variation in urinary values of BDCIPP, DPhP and DEP by sex and age, GM of urinary BDCIPP, DPhP and DEP SG-adjusted values of each sex/age groups were compared (Table 3). Results of nonparametric statistical tests indicated no significant differences in the urinary BDCIPP, DPhP and DEP concentrations and DFs between gender (p = 0.299–0.757) (Table S7) or age (p = 0.079–0.943) (Table S8). However, when comparing the GM of BDCIPP, DPhP and DEP levels between gender and age groups, there were some slight differences. The GM urinary levels of BDCIPP, DPhP and DEP from females (4.64; 8.56 and 2050 ng mL−1, respectively) were slightly higher than males (0.92; 7.37 and 1860 ng mL−1, respectively). The higher urinary levels of BDCIPP and DPhP in female than males were also observed from a popular in New York, U.S. (Wang et al. 2019a). These results demonstrate the difference in exposure to OPEs between genders, which may be affected by their daily habits and behavior. For instance, females generally consume more cosmetic products than males, and some OPEs are applied in cosmetics (Mendelsohn et al. 2016; Ye et al. 2022) or a part of cosmetic products (such as plastic parking items) (Marklund et al. 2003; Shi et al. 2015), consequently, leading to the higher exposure to OPEs of female.
To examine the variation of BDCIPP, DPhP and DEP levels in urine with age, participants were divided into two major age groups: Children and teenagers (under 18 years old), and adults (from above 18 years old). Between the two age groups, children and teenagers had a higher urinary DPhP level (GM: 9.74 ng mL−1) than did the adults (GM: 7.41 ng mL−1), which suggested the higher exposure to TPhP and some other aryl-OPEs in younger ages. In addition, the results of Spearman's correlation rank analysis suggested a decrease in urinary DPhP levels associated with an increase in age (r = − 0.255, p = 0.051) (Table S9). This seems to be an international trend since many previous reports showed the same result (Butt et al. 2014; Butt et al. 2016; Sun et al. 2018; Van den Eede et al. 2015). Elevated exposure to aryl-OPEs in younger ages may associate with the hand-to-mouth behaviors, metabolism and demographic characteristic difference between children and adults (Butt et al. 2014; Butt et al. 2016; Sun et al. 2018; Van den Eede et al. 2015). Exposure to TPhP may impact the reproductive system (Fang et al. 2003; Meeker and Stapleton 2010), and it could be more susceptible when the exposure dose is elevated during childhood, because there may be potential adverse consequences appear later in life (WHO 2006). However, the knowledge on toxicities of TPhP and other aryl-OPEs is still limited, more information on the long-time influence of OPEs on human health need to be filled in future.
In terms of BDCIPP and DEP, the urinary GM concentrations of these chemicals appeared to be slightly higher in the elder age group (working ages) than those in younger age group. These age groups comprised university students and workers, who probably have longer contact time with many electronic items daily (Sun et al. 2018). Furthermore, exposure to OPEs in working environments could probably be one of the important sources attributed to the higher urinary BDCIPP and DEP levels among these working age groups.
Daily Exposure Assessment
In this work, uncorrected urinary values of BDCIPP, DPhP and DEP were used to estimate the exposure doses to their parent compounds (TDCIPP, TPhP and TEP, respectively), and each OPE metabolite was assumed to be degraded from single parent chemical (parent/metabolite chemical: TDCIPP/BDCIPP, TPhP/DPhP and TEP/DEP) (Table 4, detailed in Table S10). The mean and 95th percentile of exposure doses of TEP (534,000 and 897,000 ng kg−1 d−1, respectively) were the highest, followed by TPhP (342 and 508 ng kg−1 d−1, respectively) and TDCIPP (236 and 1740 ng kg−1 d−1, respectively). Higher exposure doses to OPEs were observed for females (mean: 303–555000 ng kg−1 d−1) than males (mean: 151–508,000 ng kg−1 d−1).
The 95th percentile of calculated exposure doses of TEP, TPhP and TDCIPP in this study were at greater levels than those calculated in the U.S (79.8; 118 and 61.9 ng kg−1 d−1, respectively) (Wang et al. 2019a). The mean calculated doses of TPhP and TDCIPP were also noticeably higher than those observed for children from South China (65 and 15 ng kg−1d−1, respectively) (Chen et al. 2018). However, the exposure doses of TDCIPP in Hanoi, Vietnam were lesser than those calculated for infants in the U.S. (330 ng kg−1d−1) (Hoffman et al. 2017).
According to previous studies, the reference dose (RfD) for TEP were 125,000 ng kg−1 d−1 (Ding et al. 2015), TPhP was 7000 ng kg−1 d−1 (Van den Eede et al. 2011), and TDCIPP was 20,000 ng kg−1 d−1 (Gbadamosi et al. 2022). In the present study, the calculated exposure doses of TPhP and TDCIPP were 1–2 orders of magnitude below their corresponding RfDs, indicating the insignificant risks to citizen health from the current exposure doses of these OPEs. However, the estimated exposure doses of TEP in Hanoi, Vietnam exceeded its RfD, demonstrated the high exposure level to TEP of Hanoi citizens. Although the information on the toxicity of TEP is limited, high exposure dose to TEP may cause certain neurotoxic properties and/or potential mutagenic effects in humans (Lai et al. 2022). Therefore, more attention should be put on the sources, fate and exposure of DEP parent compounds to Hanoi residents in Vietnam.
The information and inferences presented in this study pertain to BDCIPP, DPhP and DEP in human urine and estimated OPEs exposure in a population and at a particular time. Though this study yielded interesting findings, it has some limitations. Small sample size was one of the main limitations of this study, urine sampling was conducted on only a few individuals from a popular in Hanoi, Vietnam. In addition, there was only one single first-morning void urine sample taken from each participant. Thus, the results could just reflect relatively recent exposure (within 24 h) (Hyland et al. 2021; Van den Eede et al. 2015) and the information on the variation of urine OPE metabolite intra-day was lacking. Furthermore, the lack of some individual information (such as health conditions, living environments, and eating habits) was also a limitation. Therefore, interpreting results from this study needs to be cautious. More comprehensive research with bigger sample size in larger areas and more target chemicals are needed to produce comprehensive information on the occurrence, behavior and effects of OPEs in the human body in Vietnam.
Conclusion
Data on the occurrence of three OPE metabolites (BDCIPP, DPhP and DEP) in urine samples collected in Hanoi, Vietnam were presented in this study. In general, the urinary BDCIPP, DPhP and DEP concentrations measured in Hanoi, Vietnam were at a relatively high level when compared to those reported in developed countries. Among the urine samples, high detected frequencies were observed for DPhP and DEP (100 and 98%, respectively). DEP was the dominant urinary OPE metabolite with an extremely high concentration in urine samples. Gender and sex are possible factors that may influence OPEs exposure and/or the variation of urinary OPE metabolites in humans. The calculated exposure doses of TEP were higher than its corresponding RfD, indicated that the current exposure doses of TEP may pose significant impacts to human health. Although the study has limitations, these are initial preliminary results on OPE metabolites in the urine of people in Hanoi (Vietnam's major metropolis) and indicate the extent of exposure of people to OPEs present in the environment. Thereby, there is a need for further research on the behavior and exposure pathways of OPEs in humans to evaluate their effects on Vietnamese people's health. More attention must be paid to the origin and dispersion of OPEs in the environment and the use of OPEs in goods and foods to ensure people's health.
References
Al-Omran LS, Gbadamosi MR, Stubbings WA, Drage DS, Abdallah MAE, Harrad S (2021) Organophosphate esters in indoor and outdoor dust from Iraq: implications for human exposure. J Emerg Contam 7:204–212. https://doi.org/10.1016/j.emcon.2021.10.003
Am S (2019) Monitoring of some organophosphorus and organochlorine pesticides residue in beef meat from Khartoum State Slaughterhouses. Am J Biomed Sci Res 6:405–409
Berghuis SA, Bos AF, Sauer PJ, Roze E (2015) Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 89:687–709
Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP (2011) Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 119(1):131–137. https://doi.org/10.1289/ehp.1002366
Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM (2014) Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol 48:10432–10438. https://doi.org/10.1021/es5025299
Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM (2016) Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int 94:627–634. https://doi.org/10.1016/j.envint.2016.06.029
Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C (2015) Human exposure pathways to organophosphate triesters-A biomonitoring study of mother-child pairs. Environ Int 75:159–165. https://doi.org/10.1016/j.envint.2014.11.009
Chen Y, Fang J, Ren L et al (2018) Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ Pollut 235:358–364. https://doi.org/10.1016/j.envpol.2017.12.092
Chen Z, An C, Elektorowicz M, Tian X (2022) Sources, behaviors, transformations, and environmental risks of organophosphate esters in the coastal environment: a review. Mar Pollut Bull 180:113779. https://doi.org/10.1016/j.marpolbul.2022.113779
Choo G, Oh JE (2020) Seasonal occurrence and removal of organophosphate esters in conventional and advanced drinking water treatment plants. Water Res 186:116359. https://doi.org/10.1016/j.watres.2020.116359
Cooper E, Covaci A, Van Nuijs A, Webster T, Stapleton HM (2011) Analysis of the flame retardant metabolites bis (1, 3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 401:2123–2132
Cristale J, García Vázquez A, Barata C, Lacorte S (2013) Priority and emerging flame retardants in rivers: Occurrence in water and sediment, Daphnia magna toxicity and risk assessment. Environ Int 59:232–243. https://doi.org/10.1016/j.envint.2013.06.011
Dallegrave A, Pizzolato TM, Barreto F et al (2018) Residue of insecticides in foodstuff and dietary exposure assessment of Brazilian citizens. Food Chem Toxicol 115:329–335. https://doi.org/10.1016/j.fct.2018.03.028
Ding J, Shen X, Liu W, Covaci A, Yang F (2015) Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci Total Environ 538:959–965. https://doi.org/10.1016/j.scitotenv.2015.08.101
Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA (2014) Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol 48:13625–13633. https://doi.org/10.1021/es503445c
Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM (2003) Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol 16:1338–1358. https://doi.org/10.1021/tx030011g
Funk SP, Duffin L, He Y, McMullen C, Sun C, Utting N, Martin JW, Goss GG, Alessi DS (2019) Assessment of impacts of diphenyl phosphate on groundwater and near-surface environments: Sorption and toxicity. J Contam Hydrol 221:50–57. https://doi.org/10.1016/j.jconhyd.2019.01.002
Gazzotti T, Sticca P, Zironi E, Lugoboni B, Serraino A, Pagliuca G (2009) Determination of 15 organophosphorus pesticides in Italian raw milk. Bull Environ Contam Toxicol 82:251–254
Gbadamosi MR, Abdallah MAE, Harrad S (2022) Organophosphate esters in UK diet; exposure and risk assessment. Sci Total Environ 849:158368. https://doi.org/10.1016/j.scitotenv.2022.158368
Gustavsson J, Wiberg K, Ribeli E, Nguyen MA, Josefsson S, Ahrens L (2018) Screening of organic flame retardants in Swedish river water. Sci Total Environ 625:1046–1055. https://doi.org/10.1016/j.scitotenv.2017.12.281
He C, English K, Baduel C, Thai P, Jagals P, Ware RS, Li Y, Wang X, Sly PD, Mueller JF (2018a) Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ Res 164:262–270. https://doi.org/10.1016/j.envres.2018.02.040
He C, Wang X, Tang S, Thai P, Li Z, Baduel C, Mueller JF (2018b) Concentrations of organophosphate esters and their specific metabolites in food in Southeast Queensland, Australia: is dietary exposure an important pathway of organophosphate esters and their metabolites? Environ Sci Technol 52:12765–12773. https://doi.org/10.1021/acs.est.8b03043
Hoang MTT, Anh HQ, Kadokami K et al (2021) Contamination status, emission sources, and human health risk of brominated flame retardants in urban indoor dust from Hanoi, Vietnam: the replacement of legacy polybrominated diphenyl ether mixtures by alternative formulations. Environ Sci Pollut Res 28:43885–43896. https://doi.org/10.1007/s11356-021-13822-9
Hoang MTT, Le GT, Kiwao K et al (2023) Occurrence and risk of human exposure to organophosphate flame retardants in indoor air and dust in Hanoi, Vietnam. Chemosphere 328:138597. https://doi.org/10.1016/j.chemosphere.2023.138597
Hoffman K, Daniels JL, Stapleton HM (2014) Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int 63:169–172. https://doi.org/10.1016/j.envint.2013.11.013
Hoffman K, Gearhart-Serna L, Lorber M, Webster TF, Stapleton HM (2017) Estimated tris (1, 3-dichloro-2-propyl) phosphate exposure levels for US infants suggest potential health risks. Environ Sci Technol Lett 4:334–338. https://doi.org/10.1021/acs.estlett.7b00196
Hyland C, Kogut K, Gunier RB, Castorina R, Curl C, Eskenazi B, Bradman A (2021) Organophosphate pesticide dose estimation from spot and 24-hr urine samples collected from children in an agricultural community. Environ Int 146:106226. https://doi.org/10.1016/j.envint.2020.106226
Jaga K, Dharmani C (2003) Sources of exposure to and public health implications of organophosphate pesticides. Rev Panam Salud Publica 14:171–185
Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, Ospina M (2019) Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere 235:481–491. https://doi.org/10.1016/j.chemosphere.2019.06.181
Kosarac I, Kubwabo C, Foster WG (2016) Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J Chromatogr B 1014:24–30. https://doi.org/10.1016/j.jchromb.2016.01.035
Krystek P, Beeltje H, Noteboom M, van den Hoeven EM, Houtzager MM (2019) Analytical human biomonitoring method for the identification and quantification of the metabolite BDCPP originated from the organophosphate flame retardant TDCPP in urine. J Pharm Biomed Anal 170:169–175. https://doi.org/10.1016/j.jpba.2019.03.036
Lai YJ, Wang XW, Liu JF (2022) Occurrence of trimethyl phosphate and triethyl phosphate in a municipal wastewater treatment plant and human urine. Env Pollut Bioavail 34:146–153. https://doi.org/10.1080/26395940.2022.2064338
Lee S, Jeong W, Kannan K, Moon HB (2016) Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res 103:182–188. https://doi.org/10.1016/j.watres.2016.07.034
Linares V, Bellés M, Domingo JL (2015) Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 89:335–356. https://doi.org/10.1007/s00204-015-1457-1
Liu LY, He K, Hites RA, Salamova A (2016) Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ Sci Technol 50:3065–3073. https://doi.org/10.1021/acs.est.5b05073
Lu SY, Li YX, Zhang T et al (2017) Effect of e-waste recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress. Environ Sci Technol 51:2427–2437. https://doi.org/10.1021/acs.est.6b05462
Lynn R, Wong K, Garvie-Gould C, Kennish J (1981) Disposition of the flame retardant, tris (1, 3-dichloro-2-propyl) phosphate, in the rat. Drug Metab Dispos 9:434–441
Maravgakis G, Tzatzarakis MN, Alegakis AK, Stivaktakis PD, Tsatsakis AM (2012) Diethyl phosphates accumulation in rabbits’ hair as an indicator of long term exposure to diazinon and chlorpyrifos. Forensic Sci Int 218:106–110. https://doi.org/10.1016/j.forsciint.2011.10.017
Marete GM, Shikuku VO, Lalah JO, Mputhia J, Wekesa VW (2020) Occurrence of pesticides residues in French beans, tomatoes, and kale in Kenya, and their human health risk indicators. Environ Monit Assess 192:1–13. https://doi.org/10.1007/s10661-020-08662-y
Marklund A, Andersson B, Haglund P (2003) Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53:1137–1146. https://doi.org/10.1016/S0045-6535(03)00666-0
Meeker JD, Stapleton HM (2010) House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect 118:318–323. https://doi.org/10.1289/ehp.0901332
Mendelsohn E, Hagopian A, Hoffman K et al (2016) Nail polish as a source of exposure to triphenyl phosphate. Environ Int 86:45–51. https://doi.org/10.1016/j.envint.2015.10.005
Nematollahi A, Rezaei F, Afsharian Z, Mollakhalili-Meybodi N (2022) Diazinon reduction in food products: a comprehensive review of conventional and emerging processing methods. Environ Sci Pollut Res 29:40342–40357. https://doi.org/10.1007/s11356-022-19294-9
Niu Z, Zhang Z, Li J, He J, Zhang Y (2019) Threats of organophosphate esters (OPEs) in surface water to ecological system in Haihe River of China based on species sensitivity distribution model and assessment factor model. Environ Sci Pollut Res 26:10854–10866. https://doi.org/10.1007/s11356-019-04461-2
Petropoulou SSE, Petreas M, Park JS (2016) Analytical methodology using ion-pair liquid chromatography–tandem mass spectrometry for the determination of four di-ester metabolites of organophosphate flame retardants in California human urine. J Chromatogr A 1434:70–80. https://doi.org/10.1016/j.chroma.2016.01.020
Phung DT, Connell D, Miller G et al (2012) Biological monitoring of chlorpyrifos exposure to rice farmers in Vietnam. Chemosphere 87:294–300. https://doi.org/10.1016/j.chemosphere.2011.11.075
Preston EV, McClean MD, Henn BC et al (2017) Associations between urinary diphenyl phosphate and thyroid function. Environ Int 101:158–164. https://doi.org/10.1016/j.envint.2017.01.020
Pundir C, Malik AJB (2019) Bio-sensing of organophosphorus pesticides: a review. Biosens Bioelectron 140:111348. https://doi.org/10.1016/j.bios.2019.111348
Salamova A, Hermanson MH, Hites RA (2014) Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site. Environ Sci Technol 48:6133–6140. https://doi.org/10.1021/es500911d
Schecter A, Kincaid J, Quynh HT, Lanceta J, Tran HTT, Crandall R, Shropshire W, Birnbaum LS (2018) Biomonitoring of metals, polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in Vietnamese female electronic waste recyclers. J Occup Environ Med 60:191–197. https://doi.org/10.1097/JOM.0000000000001200
Shi Y, Gao L, Li W, Wang Y, Liu J, Cai Y (2015) Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ Pollut 209:1–10. https://doi.org/10.1016/j.envpol.2015.11.008
Słowik-Borowiec M, Szpyrka E, Podbielska M, Kurdziel A, Matyaszek M (2012) Pesticide residues in root vegetables and potatoes in South-Eastern Poland (2009–2011). Pol J Agron 11:47–51
Sun Y, Gong X, Lin W, Liu Y, Wang Y, Wu M, Kannan K, Ma J (2018) Metabolites of organophosphate ester flame retardants in urine from Shanghai, China. Environ Res 164:507–515. https://doi.org/10.1016/j.envres.2018.03.031
Suzuki T, Sasaki K, Takeda M, Uchiyama MJ (1984) Metabolism of tributyl phosphate in male rats. J Agric Food Chem 32:603–610. https://doi.org/10.1021/jf00123a046
Tao Y, Shang Y, Li J, Feng J, He Z, Covaci A, Wang P, Luo J, Mao X, Shi B (2018) Exposure to organophosphate flame retardants of hotel room attendants in Wuhan City, China. Environ Pollut 236:626–633. https://doi.org/10.1016/j.envpol.2018.01.079
Thai PK, Li Z, Sjödin A, Fox A, Diep NB, Binh TT, Mueller JF (2015) Biomonitoring of polycyclic aromatic hydrocarbons exposure in small groups of residents in Brisbane, Australia and Hanoi, Vietnam, and those travelling between the two cities. Chemosphere 139:358–364. https://doi.org/10.1016/j.chemosphere.2015.07.004
Thomas M, Stapleton H, Dills R, Violette H, Christakis D, Sathyanarayana S (2017) Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere 185:918–925. https://doi.org/10.1016/j.chemosphere.2017.07.015
Truong DA, Trinh HT, Le GT et al (2023) Occurrence and ecological risk assessment of organophosphate esters in surface water from rivers and lakes in urban Hanoi, Vietnam. Chemosphere 331:138805. https://doi.org/10.1016/j.chemosphere.2023.138805
Van Cong N, Giao NT, Hang BTBJE, Safety E (2022) Sensitivity of cholinesterase activity in juvenile giant freshwater prawn (Macrobrachium rosenbergii de Man, 1879) to organophosphate diazinon. Ecotoxicol Environ Saf 238:113578. https://doi.org/10.1016/j.ecoenv.2022.113578
Van den Eede N, Dirtu AC, Neels H, Covaci A (2011) Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37:454–461. https://doi.org/10.1016/j.envint.2010.11.010
Van den Eede N, Heffernan AL, Aylward LL et al (2015) Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int 74:1–8. https://doi.org/10.1016/j.envint.2014.09.005
Van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Wang G, Du Z, Chen H, Su Y, Gao S, Mao L (2016) Tissue-specific accumulation, depuration, and transformation of triphenyl phosphate (TPHP) in adult zebrafish (Danio rerio). Environ Sci Technol 50:13555–13564. https://doi.org/10.1021/acs.est.6b04697
Wang X, He Y, Lin L, Zeng F, Luan T (2014) Application of fully automatic hollow fiber liquid phase microextraction to assess the distribution of organophosphate esters in the Pearl River Estuaries. Sci Total Environ 470–471:263–269. https://doi.org/10.1016/j.scitotenv.2013.09.069
Wang Y, Li W, Martínez-Moral MP, Sun H, Kannan K (2019a) Metabolites of organophosphate esters in urine from the United States: concentrations, temporal variability, and exposure assessment. Environ Int 122:213–221. https://doi.org/10.1016/j.envint.2018.11.007
Wang Y, Yao Y, Han X, Li W, Zhu H, Wang L, Sun H, Kannan K (2020) Organophosphate di-and tri-esters in indoor and outdoor dust from China and its implications for human exposure. Sci Total Environ 700:134502. https://doi.org/10.1016/j.scitotenv.2019.134502
Wang Y, Yao Y, Li W, Zhu H, Wang L, Sun H, Kannan K (2019b) A nationwide survey of 19 organophosphate esters in soils from China: spatial distribution and hazard assessment. Sci Total Environ 671:528–535. https://doi.org/10.1016/j.scitotenv.2019.03.335
WHO (2006) Principles for evaluating health risks in children associated with exposure to chemicals. Environmental health criteria 237, World Health Organization, Geneva
Xu L, Zhang B, Hu Q, Liu Y, Shang T, Zeng X, Yu Z (2021) Occurrence and spatio-seasonal distribution of organophosphate tri- and di-esters in surface water from Dongting Lake and their potential biological risk. Environ Pollut 282:117031. https://doi.org/10.1016/j.envpol.2021.117031
Yang F, Li J, Pang G, Ren F, Fang B (2019) Effects of diethyl phosphate, a non-specific metabolite of organophosphorus pesticides, on serum lipid, hormones, inflammation, and gut microbiota. Molecules 24:2003. https://doi.org/10.3390/molecules24102003
Yao C, Yang H, Li Y (2021) A review on organophosphate flame retardants in the environment: occurrence, accumulation, metabolism and toxicity. Sci Total Environ 795(15):148837. https://doi.org/10.1016/j.scitotenv.2021.148837
Ye L, Xing L, Li J, Kong M, Su G (2022) Recognition of organophosphate esters (OPEs) in the sediment of 12 Lakes in the Taihu Lake Basin of China. ACS EST Water 2:2450–2459. https://doi.org/10.1021/acsestwater.2c00298
Zhang Q, Wang Y, Zhang C, Yao Y, Wang L, Sun H (2022a) A review of organophosphate esters in soil: Implications for the potential source, transfer, and transformation mechanism. Environ Res 204:112122. https://doi.org/10.1016/j.envres.2021.112122
Zhang W, Giesy JP, Wang PJ (2022b) Organophosphate esters in agro-foods: occurrence, sources and emerging challenges. Sci Total Environ 827:154271. https://doi.org/10.1016/j.scitotenv.2022.154271
Zhang W, Wang P, Li Y et al (2019) Spatial and temporal distribution of organophosphate esters in the atmosphere of the Beijing-Tianjin-Hebei region, China. Environ Pollut 244:182–189. https://doi.org/10.1016/j.envpol.2018.09.131
Zheng G, Schreder E, Dempsey JC et al (2021) Organophosphate esters and their metabolites in breast milk from the United States: breastfeeding is an important exposure pathway for infants. Environ Sci Technol Lett 8:224–230. https://doi.org/10.1021/acs.estlett.0c00916
Acknowledgements
The Vietnam Academy of Science and Technology provided funding for this study under Grant No. "TĐPCCC.05/21-23".
Author information
Authors and Affiliations
Contributions
HTT, DAT: sampling, writing manuscript, supervision and reviewing; HTD: sample preparation, data treatment, reviewing and editing; TMB and NTTN: Analytical samples, data treatment, reviewing and editing. PTTN: sample preparation, reviewing and editing; The remaining authors have reviewed and edited.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trinh, H.T., Truong, D.A., Duong, H.T. et al. Investigation of Urinary Metabolites of Organophosphate Esters in Hanoi, Vietnam: Assessment Exposure and Estimated Daily Intake. Arch Environ Contam Toxicol 86, 335–345 (2024). https://doi.org/10.1007/s00244-024-01065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-024-01065-x