Abstract
Fish and aquatic amphibians possess neuromasts on the surface of their body that constitute the lateral line, a sensory system used to detect water displacement. Copper is known to inactivate the neuromast organs of this system. Copper-induced neuromast loss in African clawed frogs, Xenopus laevis, was examined by exposing Nieuwkoop-Faber stage 54–55 larvae to copper concentrations of 0, 100, 200, 300, and 400 µg/L for 96 h, followed by an examination of neuromast counts, staining intensity, and behavioral responses. Neuromasts were counted using a novel imaging method across four different body regions: the whole body, partial body, head, and tail. Neuromast counts showed a decreasing, but nonsignificant, trend across increasing levels of copper exposure. Intensity of neuromast staining showed a stronger concentration-dependent decrease in all four body regions. The decrease in staining intensity, but not neuromast number, may indicate that although neuromasts are still functioning, they have a decreased number of viable hair cells. Potential loss of responsiveness related to neuromast damage was examined via sensitivity to puffs of air at varying distances. We detected little to no difference in response to the air puff stimulus between control tadpoles and tadpoles exposed to 400 µg/L of copper. Neuromasts of X. laevis may be more resistant to copper than those of North American tadpole species, possibly suggesting greater tolerance of the lateral line to environmental stressors in species that maintain this sensory system throughout their lifespan as compared with species that only have the lateral line during the larval period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The ability of organisms to interact with their biotic and abiotic environments is essential for survival and reproduction, and various sensory systems are vital for responding to environmental stimuli. Vertebrates living in aquatic environments use many of the same sensory systems as terrestrial animals, including vision, somatasense, olfaction, and auditory (Collin and Marshall 2008). Some aquatic species possess electroreception (Collin and Marshall 2008). Additionally, fish, larval amphibians, and some adult aquatic amphibians have a mechanosensory lateral line system (Russell 1976) comprised of an array of neuromasts distributed across the body (Bleckmann and Zelick 2009). In fish, neuromasts can be located on the skin (superficial neuromasts) or within canals that channel water over the neuromasts, whereas amphibians only have superficial neuromasts (Russell 1976). Lateral lines enable animals to sense water displacement and pressure changes (Bleckmann and Zelick 2009). By sensing water displacement, the lateral line system helps fish and amphibians perform various behaviors, including escaping predators (Stewart et al. 2013), capturing prey (Junges et al. 2010), schooling (Partridge and Pitcher 1980), and navigating their surroundings (Burt de Perera 2004).
Neuromasts contain hair cells covered by a cupula that provides some protection from mechanical damage to the cells (Bleckmann and Zelick 2009). The hair bundle is the part of the cell responsible for signal transduction (Schwander et al. 2010) and is composed of stereocilia of varying length (Bleckmann and Zelick 2009). Mechanotransduction is the conversion of a mechanical stimulus to an electrochemical signal and occurs at the apices of the stereocilia (Hudspeth and Corey 1977; Chou et al. 2017). This process occurs when water displacement bends the hair bundle towards the longest stereocilia, thereby opening mechanically gated ion channels located on the apices and allowing cations to move into these channels.
Sublethal concentrations of certain chemicals can temporarily or permanently disable neuromasts (Faucher et al. 2006; Olivari et al. 2008). Temporary disabling of neuromasts may occur by toxicants competing with calcium ions for fixation sites, preventing the ion fluxes necessary for signal transduction (Hudspeth 1983; Faucher et al. 2006). Permanent damage is more likely due to oxidative stress and the accompanying necrosis or apoptosis may be responsible for killing hair cells of neuromasts (Forge and Schacht 2000; Olivari et al. 2008). For example, aminoglycosides can disable neuromasts via blockage of transduction channels (Hudspeth 1983; Kroese et al. 1989) or destruction of hair cells by free radicals (Forge and Schacht 2000). In addition, various divalent metal cations including cobalt, cadmium, and copper can cause hair cell death in fish neuromasts, damaging these organs and impairing lateral line function (Hernandez et al. 2006; Faucher et al. 2006; Olivari et al 2008). Although concentrations of cadmium (Faucher et al. 2006) and cobalt (Karlsen and Sand 1987) associated with neuromast loss are unlikely to be encountered in nature, environmentally relevant levels of copper cause neuromast damage (Linbo et al. 2006).
Copper is an essential trace element, although excess amounts can cause toxicity, particularly for aquatic organisms where copper can have lethal and sublethal impacts at concentrations less than 100 µg/L (Howarth and Spague 1977; Perwak et al. 1980; Irwin et al. 1997). Sublethal effects of copper exposure in fish have been examined extensively, including reproduction (Johnson et al. 2007; Sonnack et al. 2015), body size and heart rate (Johnson et al. 2007), and olfaction (Hara et al. 1976). Copper also disrupts the lateral line system of fish by killing hair cells within the neuromasts at concentrations as low as 25 µg/L (Linbo et al. 2006). Neuromast damage is not necessarily permanent, as cells can regenerate after removing fish from the copper exposure (Hernandez et al. 2006). Sustained neuromast damage likely requires constant exposure to copper as shown by Linbo et al. (2006) wherein damaged neuromasts did not recover for zebrafish larvae in an experiment in which a 50-µg/L copper solution was renewed every 24 h. However, after 5 h of copper exposure and a 72-h recovery period, zebrafish neuromasts were able to regenerate. Additionally, neuromast loss without regeneration occurred when Johnson et al. (2007) exposed zebrafish embryos to copper concentrations of 68 µg/L and 244 µg/L for 120 h, renewing 50% of the test solution every 24 h.
Although most amphibians lose the lateral line system upon metamorphosis, several aquatic species, including Xenopus laevis, retain this sensory system throughout their entire lifespan, making them ideal model organisms for lateral line research (Shelton 1970; Russell 1976). Consequently, the lateral line system of Xenopus laevis has been extensively studied, indicating that the neuromasts of these animals differ in innervation, distribution, and function among different parts of the body (Shelton 1970; Mohr and Gorner 1996; Russell 1976). Neuromasts of the head region, called anterior neuromasts, are innervated by the anterior lateral line nerve, whereas trunk (posterior) neuromasts are innervated by the posterior lateral line nerve (Russell 1976). Another innervation difference for Xenopus is that groups of neuromasts, called stitches (Harris and Milne 1966), located in the anterior lateral line are more often connected to more than two fibers than in the posterior lateral line (Mohr and Gorner 1996). The posterior lateral line has more stitches than the anterior lateral line, but stitch density is greater in the head region (Mohr and Gorner 1996). Due to these differences in innervation and density, neuromasts of the two body regions have contrasting roles. Head neuromasts are more important than body neuromasts for detecting surface waves in X. laevis (Russell 1976) and in surface feeding fish (Schwartz and Hasler 1966; Bleckmann and Zelick 2009). Furthermore, although anterior lateral line neuromasts are likely more important in prey detection in Xenopus, posterior lateral line neuromasts are proposed as more important in predator avoidance (Mohr and Gorner 1996). Posterior lateral line neuromasts may be more sensitive to water disturbances, whereas anterior lateral line neuromasts have better spatial resolution due to their higher density (Mohr and Gorner 1996).
As a result of their different innervation, anterior and posterior neuromasts may differ in sensitivity to chemicals. Indeed, Hernandez et al. (2006) found differential regeneration of hair cells between neuromasts of the head and body in zebrafish, where body neuromasts were unable to regenerate at copper concentrations greater than 3.18 mg/L, but head neuromasts regenerated at copper levels up to 25.42 mg/L. Similarly, posterior neuromasts of zebrafish were more sensitive than anterior neuromasts exposed to caffeine, dichlorvos, 4-nonylphenol, and perfluorooctanesulfonic acid, as indicated by the number of hair cells stained and fluorescent intensity of mitochondria (Stengel et al. 2017). Posterior neuromasts were more affected by copper sulfate and neomycin than were anterior neuromasts after a 30-min exposure period (Stengel et al. 2017). Conversely, after 96 h of exposure, anterior neuromasts exhibited greater cellular damage (Stengel et al. 2017). The authors speculated differential regeneration or repair mechanisms may account for the contradictory results at 30 min and 96 h.
Neuromast loss can influence animal behavior, as disabling the lateral line system can reduce the ability of fish to escape predation (Stewart et al. 2013). Even low toxicant concentrations can impact anti-predator behaviors. For example, exposure to cadmium interfered with the startle response in sea bass (Faucher et al. 2006). Ablation of the lateral line system also affects predators, as when cobalt chloride reduced predation success in marbled swamp eels (Junges et al. 2010). Additionally, damage to the lateral line can decrease the swimming abilities of aquatic animals, as shown with cadmium (Baker and Montgomery 2001) and the aminoglycoside gentamicin (Coombs et al. 2001) interfering with the ability of fish to orientate to currents. Similar effects were observed with streptomycin interference in schooling of X. laevis tadpoles (Lum et al. 1982).
The purpose of this study was to examine copper toxicity on neuromasts in X. laevis, a species commonly used in studies of lateral line function and resulting links to behavior (Gorner 1973; Elepfandt 1982; Gorner et al. 1984; Claas et al. 1993; Claas and Dean 2006). Furthermore, we were interested whether amphibians would exhibit differential sensitivity of head and body neuromasts, as observed for zebrafish (Hernandez et al. 2006; Stengel et al. 2017), and whether copper exposure and its possible damage to the lateral line system would impact behavior as seen with fish and tadpoles exposed to metals and aminoglycosides (Lum et al. 1982; Baker and Montgomery 2001; Coombs et al. 2001; Faucher et al. 2006). Neuromasts were quantified by using a novel automated imaging method, which allowed the counting of neuromasts across large portions of animals’ bodies.
Methods
Animal Source and Husbandry
Nieuwkoop-Faber stage 54–55 Xenopus laevis tadpoles were obtained from Xenopus1 (Dexter, MI). Stage 54–55 tadpoles were chosen, because the lateral line system of less developed tadpoles (stage 51–54) was difficult to image using our imaging procedure (Krupa 2019). Before the beginning of copper exposures, the stage 54–55 tadpoles were communally housed in several 2.5-gallon glass tanks. The tadpoles in each tank were fed 3 mL of 60 g/L solution of Micron Growth Food by Sera (Heinsberg, Germany) twice a day.
During exposures, tadpoles were kept individually in 3-L glass jars filled with 1000 mL of carbon-filtered water and were fed 3 drops of the Sera Micron solution twice a day. Water temperature ranged between 23–24 °C, pH between 7.8 and 8.0, and photoperiod was set at 12 h light to 12 h darkness.
Neuromast Study Exposure
Copper sulfate pentahydrate (Sigma-Aldrich, St. Louis, MO) was used to prepare a stock solution (1000 µg/mL Cu) which was then used to spike exposure jars. Tadpoles were divided between 5 treatment groups with 16 tadpoles each. Treatments included a control (0 µg/L Cu) and concentrations of 100, 200, 300, and 400 µg/L copper. These concentrations were chosen based on preliminary trials to be low enough to avoid mortality. The copper concentrations used in our study are higher than those commonly observed in freshwater in the United States (1–100 µg/L) but are comparable to concentrations found in polluted sites (Perwak et al. 1980). Tadpoles were exposed to copper for 96 h in a nonrenewal exposure. Exposure was split evenly between 2 days to account for the amount of time needed for the staining and imaging process. Half of the tadpoles were exposed the day after arrival; the others were dosed 2 days after arrival. One individual from each treatment was grouped into a block (16 total) and members of a block were randomly positioned adjacent to each other on shelves.
Water samples (10 mL) were collected from each treatment group at approximately 24-h intervals to monitor copper concentrations during exposure. Samples were acidified with 1 mL of 20% nitric acid. Four samples from each treatment group collected at 0 and 96 h were analyzed via inductively coupled plasma optical emission spectrometry (ICP-OES; iCAP 7400, Thermo Fisher Scientific, Waltham, MA) to verify concentrations of copper and other metals. A multielement standard (CPI International, Santa Rosa, CA) and internal yttrium standard (Peak Performance Inorganic Trace Metal Yt Standard, CPI International) were used as calibration standards. The limit of quantitation for copper was approximately 25 µg/L. The ICP-OES indicated calcium concentrations of approximately 30 mg/L and magnesium concentrations of 4 mg/L.
Neuromast Study Staining and Imaging Procedure
Tadpoles were stained and photographed by block (5 tadpoles at a time) at the end of the 96-h exposure period. The staining procedure consisted of placing a tadpole in a solution of 6.34 µM 4-(4-Diethylaminostyryl)-1-methylpyridinium iodide (DiAsp, from Sigma-Aldrich) for 8 min and then rinsing with carbon-filtered water for 2 min. DiAsp is used to selectively label neuromasts as the dye enters live hair cells through mechanotransduction channels located on the stereocilia (Faucherre et al. 2009). Immediately after rinsing, tadpoles were euthanized in a 500 mg/L tricaine mesylate (MS-222) solution for at least 5 min. Tadpoles were always kept in separate containers, which were covered with aluminum foil to prevent photobleaching.
Immediately after euthanasia, tadpoles were placed one to a well in a six-well plate (Greiner Bio-One, Kremsmünster, Austria). Each tadpole was positioned on its side with the head oriented to the left and the tail to the right. Then, tadpoles were individually photographed by the Cytation 5 Imaging Reader (BioTek Instruments, Winooski, VT) with Gen5 software, using the red fluorescent protein (RFP) filter, 4 × objective, and LED intensity of 10. Images (TIFF) were saved for further analysis. A protocol file was created in the Gen5 software to image the tadpoles using the laser autofocus feature. Using laser autofocus decreased the amount of time needed to capture images and also was a consistent and efficient method to ensure that neuromasts were in focus, rather than manually adjusting the focus settings for each tadpole to account for the slight differences in size and position. Exposure settings for each block were adjusted by focusing on the upper lateral line (Shelton 1970) or head neuromasts of the tadpole in the 100 µg/L treatment and held constant for all others. These neuromasts were chosen because they incorporated stain well and could be located quickly and easily. Ten larvae (blocks 1 and 2) were excluded from data analysis since they were unintentionally photographed at a different LED intensity.
Neuromast Study Quantification
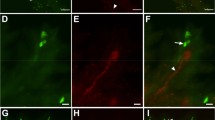
Image files of tadpoles were opened in manual mode of the Gen5 software and the measure line tool was used to verify tadpole stage according to foot and leg dimensions (Nieuwkoop and Faber 1994). Number of neuromasts was quantified using the cellular analysis tool to count neuromasts at different background intensity thresholds optimized to detect separate objects (Held and Banks 2013). Thresholds that are too low produce artificially high counts by quantifying nontarget (i.e., nonneuromast) fluorescing objects and often incorrectly count multiple neuromasts as a single object. Setting thresholds too high increases the risk of excluding target neuromasts. Thus, each image was tested at various thresholds to find an optimal balance between minimal background interference and neuromast detectability (see supplementary information for a detailed explanation of how the optimal threshold was chosen). To determine this optimal threshold, accuracy of neuromast labeling was examined at thresholds of 5000, 7000, 10,000, and 15,000 relative intensity units (RIU). If higher thresholds were needed, 20,000, 25,000, and 30,000 RIU were examined. Once the optimal threshold was determined, the neuromast count and the mean intensity of the enumerated objects from each image was recorded (Fig. 1).
Visualization of how neuromasts were quantified. a Image of a tadpole as captured by the Cytation5 cell reader using the RFP filter. b Same image opened in the Gen5 software for neuromast quantification. This image has been cropped according to the partial body method. The bright yellow circles indicate objects (neuromasts and a few instances of nonspecific label) enumerated by the software; these are automatically quantified to provide the “neuromast count.” The intensity of each counted object is averaged by the software to compute the mean “neuromast intensity” of the image
Number and fluorescent intensity of neuromasts were quantified for four different regions of the body. First, neuromasts with an object size restricted to 20–150 µm were counted over the whole body (no cropping of the image). Next, neuromasts were counted for the “partial body,” which involved cropping out areas commonly possessing high levels of nonspecific stain: the bottom of the head, the legs, and the posterior end of the tail (supplemental information; Krupa 2019). Cropping these areas reduced background interference, allowing the use of a larger and thus more sensitive object size range (20–200 µm). For a few tadpoles, areas of concentrated nonspecific stain remained, particularly on the tail, after the images were cropped. These areas of nonspecific stain were removed if they could be cropped without excluding neuromasts. After this, neuromasts of the head were counted within a region ventral to the nostrils, dorsal to the eye, and anterior to the stirnorgan (supplemental information; Krupa 2019). This excluded the bottom of the head, which usually contained significant amounts of nonspecific staining. This method also used an object size of 20–200 µm. Lastly, neuromasts were counted on a position of the tail 20,000-µm long by 2000-µm wide (supplemental information; Krupa 2019) using an object size of 20–200 µm. These different approaches to quantifying neuromasts were done to reduce artificially high neuromast counts by excluding areas of nonspecific staining and to compare neuromast loss between the anterior and posterior regions of the body.
Images were excluded from analysis if none of the examined thresholds balanced neuromast detectability with excluding interference from nontarget objects (see supplemental information). This typically occurred if areas of nonspecific stain could not be excluded and had a greater intensity than neuromasts. Five images were excluded from the whole body method, four from the partial body method, two from the head method, and six from the tail method.
Behavioral Assay
Stage 54–55 tadpoles were exposed to either 0 µg/L (n = 10) or 400 µg/L (n = 10) copper for 96 h. We chose to test a control group and the highest concentration from the neuromast quantification study (400 µg/L copper), so we could potentially associate altered behavior with a reduction of neuromast number or intensity. We did not test additional copper concentrations, because we assumed if altered behavior was not observed for tadpoles in the 400-µg/L copper treatment group, behavioral differences in animals exposed to lower copper concentrations would be unlikely. During the exposure period, tadpoles were housed individually in 3-L glass jars filled with 1000 mL of carbon-filtered water. At 96 h, we quantified the response to surface wave stimuli using a modification of Claas and Dean (2006). Adult African clawed frogs orient to air puff stimuli as a predatory behavior (Claas and Dean 2006). However, our initial efforts indicated that tadpoles exhibited an antipredator startle response rather than orientation toward the stimulus. Briefly, an air puff was delivered to the center of a 60-mm Petri dish filled to capacity with carbon filtered water and holding the tadpole (see supplemental information for a photograph of the apparatus and further details). Tadpoles would typically orient themselves with the head toward the outside of the petri dish and the tail near the center, so that the air puff would be delivered above the posterior end of the tail. Each tadpole was allowed a 5-min acclimation period after being placed in the petri dish before delivery of air puffs. Air puffs were delivered 2, 4, 6, 8, 10, 12, and 14 cm above the surface of the water. A maximum height of 14 cm was selected, because although the air puff stimulus still visibly disturbed the water, tadpoles in preliminary trials rarely reacted to the stimulus at this height. The order of heights was randomized for each tadpole, and the initial stimulus at that height was given 20 s after the height adjustment. Furthermore, stimuli were delivered five times at each height, and each stimulus was delivered only after the tadpole had remained motionless for 10 s. To ensure that the tadpoles were responding to the air puff and not to hand movement, the observer would hold the pipette bulb for at least 5 s before delivering the stimulus. Reaction of the tadpole to the stimulus also had to be scored before the observer removed their hand.
The response of tadpoles to each stimulus was scored on a scale of zero to four. Zero indicates no discernible response to the stimulus. A score of one denotes one of the following slight movements: increased tail movements, a more rapid rate of tail vibration, or flaring out of the feet. It also includes a slight forward drift accompanied by no twitching or active swimming movement; this forward drift had to be less than one-fourth of the tadpole’s snout-vent length. A score of two denotes movement less than one snout-vent length in distance. This includes swimming forward, a full-body twitch, or a side-to-side rocking movement. A score of three denotes normal reactions in which tadpoles swam greater than one snout-vent length, but remained within two quadrants of the dish (delineated beforehand by drawing lines on the Petri dish). Delayed reactions that involved the tadpole swimming across three or more quadrants were also placed in this category. A score of four denotes the tadpole swam across three or more quadrants, without showing a delayed reaction. Before data collection for the behavioral assay, the observer practiced scoring behavioral responses with tadpoles not used in the experiment.
Statistical Methods
For ICP-OES results, the mean and standard deviation were calculated for samples from each copper concentration at the initial and final time-points. Neuromast counts and intensity data were analyzed using the general linear mixed models procedure in SAS 9.4. Because camera exposure settings were adjusted for each of the 16 blocks, these were treated as a random variable. Neuromast counts were modeled with a normal distribution and intensity values were analyzed with an exponential distribution because of their greater variance and departures from normality. The “random residuals” statement and “group = effect” option also were used to account for heterogeneity of variances. Tukey–Kramer pairwise comparisons were performed when the treatment effect was significant. All tests were done at the α = 0.05 level.
The behavioral responses of control tadpoles and those exposed to 400 µg/L of copper were compared over changes in height. For each tadpole, the five behavioral response scores at each height were averaged for the mean response of the tadpole at that height. The mean response scores for each tadpole at each height were then pooled and analyzed using the general linear mixed models procedure in SAS 9.4. A normal distribution was used, and individual tadpoles were treated as random effects. Tukey–Kramer pairwise comparisons were used when the mixed models indicated a significant treatment effect upon behavior. To calculate the mean response score of a tadpole, the mean reaction at each of the seven heights for that tadpole was averaged together. This also was analyzed in SAS 9.4 using the general linear mixed models, normal distribution, and least square means with the Tukey–Kramer adjustment.
Results
Measured copper concentrations of each treatment group, with one exception, were within 12% of the nominal concentration immediately after dosing (Table 1). One of the 200-µg/L treatment samples had a copper concentration equivalent to that of the controls (< 25 µg/L); the other 200-µg/L samples averaged 194 µg/L. Copper concentrations remained within 30% overall of the nominal values after 96 h. Copper concentrations in the control group were below the 25 µg/L limit of quantitation.
At the end of the neuromast study, tadpole stage averaged Nieuwkoop-Faber 55 across all treatments, slightly less than those in the behavioral assay, which averaged stage 57–58 (Table 2). No mortality or adverse symptoms were observed in any of the tadpoles.
Counts of neuromasts did not differ (p > 0.05) among copper treatments for any of the body regions despite a trend of dose related differences (Table 3). For example, neuromast counts for the partial body (F4,18 = 0.65, p = 0.63) and tail (F4,20 = 1.20, p = 0.34) generally decreased with increasing copper concentration and were 87% and 78% as numerous in the 400-µg/L group than the controls, respectively. Conversely, neuromast counts for whole body increased (F4,20 = 1.81, p = 0.17) and neuromast counts for the head (F4,23 = 1.06, p = 0.40) remained relatively stable across treatment groups.
Despite the lack of significant effect on the number of neuromasts, fluorescent intensity of neuromasts decreased with increasing copper concentration for all body regions (Table 4). Intensity values for the whole body for tadpoles exposed to 300 and 400 µg/L Cu were 20–30% less than those in the control group (F4,47 = 4.75, p = 0.003). Average intensity for the partial body was 22–37% lower in the highest treatments than the control (F4,48 = 3.74, p = 0.01). A significant effect of copper concentration on neuromast intensity also was observed for the head (F4,50 = 5.66, p < 0.001), in which the average intensity of the 400 µg/L group was 30% lower than the control. Last, the average intensity of tail neuromasts was 40% lower for the 400 µg/L treatment than the control group. Despite the overall treatment effect for the tail (F4,46 = 3.00, p = 0.03), Tukey–Kramer pairwise comparisons did not detect any differences in intensity between concentrations (Table 4).
In the behavior assay, overall stimulus response did not differ between tadpoles exposed to 400 µg/L copper or controls (F1,18 = 2.15, p = 0.16). Both groups scored ≥ 3 at 2 cm, with decreasing responses over increasing heights (Fig. 2). Results indicate a slight divergence in response at 12 cm (F1,9 = 5.71, p = 0.04), but that effect is lost at 14 cm.
Discussion
Given previous studies on copper effects in lateral lines of fish (Hernandez et al. 2006; Linbo et al. 2006; Johnson et al. 2007), we expected to see dose dependent effects in neuromasts culminating in an effect on a simple behavior response. However, neuromast loss in X. laevis tadpoles exposed to copper was not evident for any of the four body regions. The similarity of neuromast numbers among treatment groups was likely not due to nonspecific labeling or software error with enumerating neuromasts. Counts of fluorescing objects were performed using the best threshold for each image and the majority of the counted objects were neuromasts. Whole body counts did contain some nonspecific labeling, especially near the bottom of the head and the limbs, rendering these counts less accurate than for the other body regions. Regardless, our methodology resulted in reliable counts overall. Although it is possible the lack of observed neuromast loss in our study was a consequence of an initial neuromast loss followed by neuromast regeneration, this seems unlikely because neuromast loss or damage without regeneration has been observed in both zebrafish (Stengel et al. 2017) and Great Plains toad larvae (Vazquez 2016) exposed to copper for respective 96- and 168-h time periods. Additionally, neuromast damage in zebrafish larvae has occurred after a 30-min exposure to copper (Stengel et al. 2017), so it is unlikely that the exposure period used in our study was not long enough for neuromast loss to occur.
Although we observed no decrease in the number of counted neuromasts, some portion of the individual hair cells within a neuromast could be destroyed despite the continued viability of the remaining hair cells (Hernandez et al. 2006; Buck et al. 2012). In addition, hair cell integrity could be partially compromised without complete destruction of the cell (Stengel et al. 2017). Indeed, neuromasts contain multiple hair cells. For example, a neuromast in a juvenile zebrafish has 20–30 hair cells (Olt et al. 2014), and larval X. laevis neuromasts contain 8–28 hair cells (Lannoo 1987). Thus, if some hair cells within a neuromast are lost or disabled from copper exposure, that neuromast could still be counted if remaining hair cells are viable and can take up some stain. If this partial loss of individual cells is occurring, we would expect a corresponding effect on fluorescent intensity across dose groups. Indeed, despite the lack of effects on neuromast counts, the intensity results support the prediction that neuromasts were affected by copper. Neuromast intensity decreased with increasing copper concentration for the four body regions. As mentioned, these lower intensity values indicate reduced uptake of DiAsp by some or all hair cells, perhaps via closed mechanotransduction channels (Faucherre et al. 2009) or simply a reduction in the number of hair cells capable of incorporating any amount of dye.
Other studies have observed similar decreases in the intensity of fluorescent staining in neuromasts after exposure to substances known to damage hair cells. For example, intensity of staining within zebrafish neuromasts labeled with the dye DASPEI decreased after exposure to copper (Linbo et al. 2006) and a variety of different ototoxic compounds, including copper sulfate (Buck et al. 2012). Likewise, the intensity of FM-143 dye used to label the hair cells of neuromasts in X. laevis larvae decreased with increasing concentrations of antibiotics (Nishikawa and Sasaki 1996). Additionally, scoring fluorescent intensity of hair cells on numeric scales has been used to evaluate damage to neuromasts (Harris et al. 2003; Stengel et al. 2017).
Although beyond the scope of this study, individual neuromast organs could be photographed in addition to imaging the entire tadpole to help to verify whether the intensity reduction observed in this study was due to decreased hair cell viability. For example, confocal microscopy has been used to photograph hair cells (Hernandez et al. 2006). By using similar techniques to examine individual neuromasts, we can better understand how copper impacts hair cells, particularly whether the observed copper concentrations physically damage or destroy the cells. While this study used a single dye (DiAsp) to label functional hair cells, acridine orange stain, which labels dying cells, could additionally be used to verify whether increasing copper concentrations increases hair cell death (Hernandez et al. 2006).
We also predicted that a loss of neuromasts would result in an altered behavioral response to a mechanosensory stimulus. This prediction is viable given the copper related effects on fluorescent intensity. However, differential responses to air puff stimuli were not observed for tadpoles exposed to 0 and 400 µg/L Cu, despite the 30–40% reduction in neuromast intensity across the four body regions in the copper exposed group. This suggests that despite hair cell damage, other hair cells remain fully or partially functional and any damage was insufficient to render cells unresponsive or the animal incapable of responding to the stimulus. The goal of using surface waves as a stimulus was rooted in its simplicity and ability to be used in any lab. However, response to surface waves may not be a particularly sensitive endpoint even with significant damage to the lateral line system. For example, despite the destruction of all neuromasts, adult clawed frogs still orient to surface waves, but less accurately (Gorner et al. 1984). Similarly, after exposure to various ototoxins resulted in complete loss of labeled neuromasts, a startle response was still observed in zebrafish larvae (Buck et al. 2012). The presence of a response despite inactivation of the lateral line suggests that other sensory systems, such as the vestibular system or touch, may help the animals respond to surface waves (Claas et al. 1993). Because adult frogs and larval zebrafish can still detect and respond to stimuli despite extensive damage to the lateral line system, it seems probable that tadpoles would exhibit normal responses to stimuli after the lesser degree of damage observed in this study. Despite the lack of sensitivity to surface waves, a decrease in viable hair cells could still negatively impact other lateral-line mediated behaviors. For instance, treatment of X. laevis tadpoles with cobalt chloride and streptomycin resulted in less precise rheotaxis (Simmons et al. 2003) and for streptomycin only, altered schooling behavior (Lum et al. 1982).
In previous studies, neuromasts of the head and tail of larval zebrafish show differential sensitivity (Stengel et al. 2017) and regeneration (Hernandez et al. 2006). Most relevant to our study, a greater sensitivity of head neuromasts compared with tail neuromasts was observed in a similar 96-h exposure of zebrafish to copper sulfate (Stengel et al. 2017). Depending upon copper concentration tested, anterior neuromasts were approximately 10–50% more sensitive than posterior neuromasts, but this difference was never significant (Stengel et al. 2017). However, the results of this study suggest that the tail neuromasts may be more sensitive to copper than head neuromasts in X. laevis larvae. Tail neuromast intensity decreased by 40% in tadpoles exposed to 400 µg/L compared with the controls versus a 30% decrease in head neuromast intensity between the 400 µg/L group and the controls. Due to the greater role of head neuromasts compared with tail neuromasts in prey localization and spatial resolution (Mohr and Gorner 1996), which are important for the survival of predatory post-metamorphic frogs, there is a possibility that the anterior lateral line (i.e., head) may have evolved greater resistance to environmental stressors than the posterior lateral line. Perhaps X. laevis also may have a greater regenerative ability in anterior neuromasts than in posterior neuromasts, similar to that observed in zebrafish (Hernandez et al. 2006), to further protect their ability to detect and capture prey.
Neuromasts were quantified by using a novel automated imaging method. As far as the authors are aware, such automated software has not been used before to count the number of neuromasts across large portions of animals’ bodies. Other studies looking at neuromast loss typically involve manually counting the organs or examining a few predefined neuromasts (Hernandez et al. 2006; Johnson et al. 2007; Linbo et al. 2009; Vazquez 2016). Automatic quantification methods similar to those in this study could be used in future research to estimate large numbers of neuromasts without the possible bias or human error associated with manual neuromast counts.
Overall, gross counts of neuromasts of stage 54–55 X. laevis larvae were not particularly sensitive exposure to copper up to 400 ug/L. However, neuromast intensity was sensitive to increasing copper concentration, suggesting damage to the neuromast organs. The lack of behavioral response suggests some redundancy in the means by which the larvae can perceive their environment (Claas et al. 1993). Imaging individual neuromasts in X. laevis could verify this link between intensity and the loss of hair cells. Although environmentally relevant copper concentrations do not appear particularly harmful to the lateral line system of X. laevis, this may not be true for all amphibians. For example, two North American anuran species that lose their lateral line system upon metamorphosis were more sensitive than X. laevis in terms of neuromast counts, because neuromast loss occurred at environmentally relevant concentrations of 50 µg/L copper or less (Vazquez 2016). Presumably, these species also would be more sensitive than X. laevis regarding neuromast intensity, so we can predict that intensity would be decreased at copper concentrations less than 50 µg/L. Future studies could examine how other metals and aminoglycosides affect X. laevis neuromasts to investigate whether the lateral line of X. laevis is specifically resistant to copper or to toxicants in general. Perhaps the lack of sensitivity of neuromasts exposed to copper is simply due to the species being relatively resistant to copper in general (Fingal and Kaplan 1963; Fort and Stover 1996) and not due to any specific property of the lateral line system. Alternatively, it is possible that as an aquatic species that retains the lateral life throughout its life, Xenopus laevis has evolved a more robust lateral line system that is better adapted to tolerate environmental stressors compared with species that only possess the lateral line during a short larval stage.
Code Availability
The SAS codes used in data analysis are available from the corresponding author on request.
Availability of Data and Material
The datasets from the current study are available from the corresponding author on request.
References
Baker CF, Montgomery JC (2001) Sensory deficits induced by cadmium in banded kokopu, Galaxias fasciatus, juveniles. Environ Biol Fishes 62:455–464
Bleckmann H, Zelick R (2009) Lateral line system of fish. Integrative Zool 4:13–25
Buck LMJ, Winter MJ, Redfern WS, Whitfield TT (2012) Ototoxin-induced cellular damage in neuromasts disrupts lateral line function. Hear Res 284:67–81
Burt de Perera T (2004) Spatial parameters encoded in the spatial map of the blind Mexican cave fish, Astyanax fasciatus. Anim Behav 68(2):291–295
Chou SW, Chen Z, Zhu S, Davis RW, Hu J, Liu L, Fernando CA, Kindig K, Brown WC, Stepanyan R, McDermott BM (2017) A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish. Nat Commun 8:2234
Claas B, Dean J (2006) Prey-capture in the African clawed toad (Xenopus laevis): comparison of turning to visual and lateral line stimuli. J Comp Physiol A 192:1021–1036
Claas B, Munz H, Gorner P (1993) Reaction to surface waves by Xenopus laevis Daudin: are sensory systems other than the lateral line involved? J Comp Physiol A 172:759–765
Collin SP, Marshall NJ (2008) Sensory processing in aquatic environments. Springer, Berlin
Coombs S, Braun CB, Donovan B (2001) The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. J Exp Biol 204:337–348
Elepfandt A (1982) Accuracy of taxis response to water waves in the clawed toad (Xenopus laevis Daudin) with intact or with lesioned lateral line system. J Comp Physiol 148:535–545
Faucher K, Fichet D, Miramand P, Lagardére JP (2006) Impact of acute cadmium exposure on the trunk lateral line neuromasts and consequences on the “C-start” response behaviour of the sea bass (Dicentrarchus labrax L.; Teleostei, Moronidae). Aquat Toxicol 76:278–294
Faucherre A, Pujol-Martí J, Kawakami K, López-Schier H (2009) Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS ONE. https://doi.org/10.1371/journal.pone.0004477
Fingal W (1963) Kaplan HM (1963) Susceptibility of Xenopus laevis to copper sulfate. Copeia 1:155–156
Forge A, Schacht J (2000) Aminoglycoside antibiotics. Audiol Neurotol 5:3–22
Fort DJ, Stover EL (1996) Effect of low-level copper and pentachlorophenol exposure on various early life stages of Xenopus laevis. In Henshel D, Bengtson D (eds) Environmental toxicology and risk assessment: biomarkers and risk assessment: 5th vol. ASTM International, West Conshohocken; pp. 188–203
Gorner P (1973) The importance of the lateral line system for the perception of surface waves in the claw toad, Xenopus laevis, Daudin. Experientia 29:295–296
Gorner P, Moller P, Weber W (1984) Lateral-line input and stimulus localization in the African clawed toad Xenopus sp. J Exp Biol 108:315–328
Hara TJ, Law YMC, MacDonald S (1976) Effects of mercury and copper on the olfactory response in rainbow trout, Salmo gairdneri. J Fish Res Board Can 33(7):1568–1573
Harris GG, Milne DC (1966) Input-output characteristics of the lateral-line sense organs of Xenopus laevis. J Acoust Soc Am 40:32–42
Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW (2003) Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol 4:219–234
Held P, Banks P (2013) Analysis of nuclear stained cells using the Cytation™3 Cell Imaging Multi-Mode Microplate Reader with DAPI-stained cells. Resources: application note. Applications Department, BioTek Instruments Inc., Winooski
Hernandez PP, Moreno V, Olivari FA, Allende ML (2006) Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Hear Res 213:1–10
Howarth RS, Sprague JB (1977) Copper lethality to rainbow trout in waters of various hardness and pH. Water Res 12:455–462
Hudspeth AJ (1983) Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci 6:187–215
Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74:2407–2411
Irwin RJ, VanMouwerik M, Stevens L, Seese MD, Basham W (1997) Environmental contaminants encyclopedia. National Park Service, Water Resources Division, Fort Collins
Johnson A, Carew E, Sloman K (2007) The effects of copper on the morphological and functional development of zebrafish embryos. Aquat Toxicol 84:431–438
Junges CM, Lajmanovich RC, Peltzer PM, Attademo AM, Bassó A (2010) Predator–prey interactions between Synbranchus marmoratus (Teleostei: Synbranchidae) and Hypsiboas pulchellus tadpoles (Amphibia: Hylidae): importance of lateral line in nocturnal predation and effects of fenitrothion exposure. Chemosphere 81:1233–1238
Karlsen SE, Sand O (1987) Selective and reversible blocking of the lateral line in freshwater fish. J Exp Biol 133:249–262
Kroese ABA, Das A, Hudspeth AJ (1989) Blockage of the transduction channels of hair cells in the bullfrog’s sacculus by aminoglycoside antibiotics. Hear Res 37:203–218
Krupa PM (2019) Effects of copper upon lateral line neuromasts in Xenopus laevis. Thesis, Oklahoma State University
Lannoo MJ (1987) Neuromast topography in anuran amphibians. J Morphol 191:115–129
Linbo TL, Baldwin DH, McIntyre JK, Scholz NL (2009) Effects of water hardness, alkalinity, and dissolved organic carbon on the toxicity of copper to the lateral line of developing fish. Environ Toxicol 28:1455–1461
Linbo TL, Stehr CM, Incardona JP, Scholz NL (2006) Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem 25:597–603
Lum AM, Wassersug RJ, Potel MJ, Lerner SA (1982) Schooling behavior of tadpoles: a potential indicator of ototoxicity. Pharmacol Biochem Behav 17:363–366
Mohr C, Gorner P (1996) Innervation patterns of the lateral line stitches of the clawed frog, Xenopus laevis, and their reorganization during metamorphosis. Brain Behav Evol 48(2):55–69
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). Garland Publishing Inc., New York
Nishikawa S, Sasaki F (1996) Internalization of styryl dye FM1-43 in the hair cells of lateral line organs in Xenopus larvae. J Histochem Cytochem 44(7):733–741
Olivari FA, Hernández PP, Allende ML (2008) Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res 1244:1–12
Olt J, Johnson SL, Marcotti W (2014) In vivo and in vitro biophysical properties of hair cells from the lateral line and inner ear of developing and adult zebrafish. J Physiol 592:2041–2058
Partridge BL, Pitcher TJ (1980) The sensory basis of fish schools: relative roles of lateral line and vision. J Physiol 135(4):315–325
Perwak J, Bysshe S, Goyer M., Nelken L, Scow K, Walker P, Wallace D (1980) An exposure and risk assessment for copper. EPA, Washington DC. EPA-440/4-81-015
Russell IJ (1976) Amphibian lateral line receptors. In: Llinás R, Precht W (eds) Frog neurobiology. Springer-Verlag, Berlin, pp 513–550
Schwander M, Bechara K, Müller U (2010) The cell biology of hearing. J Cell Biol 190(1):9–20
Schwartz E, Hasler AD (1966) Perception of surface waves by the blackstripe topminnow, Fundulus notatus. J Fish Res Board Can 23:1331–1352
Shelton PMJ (1970) The lateral line system at metamorphosis in Xenopus laevis (Daudin). J Embryol Exp Morphol 24:511–524
Simmons AM, Costa LM, Gerstein HB (2004) Lateral line-mediated rheotactic behavior in tadpoles of the African clawed frog (Xenopus laevis). J Comp Physiol A 190:747–758
Sonnack L, Kampe S, Muth-Köhne E, Erdinger L, Henny N, Hollert H, Schafers C, Fenske M (2015) Effects of metal exposure on motor neuron development, neuromasts and the escape response of zebrafish embryos. Neurotoxicol Teratol 50:33–42
Stengel D, Zindler F, Braunbeck T (2017) An optimized method to assess ototoxic effects in the lateral line of zebrafish (Danio rerio) embryos. Comp Biochem Physiol C 193:18–29
Stewart WJ, Cardenas GS, McHenry MJ (2013) Zebrafish larvae evade predators by sensing water flow. J Exp Biol 216:388–398
Vazquez A (2016) Kin recognition and copper toxicity to lateral lines in Great Plains toads and southern leopard frog tadpoles. Thesis, Oklahoma State University
Acknowledgements
The authors thank the Oklahoma State University Department of Integrative Biology for their financial support. They also thank Dr. Doug Fort of Fort Environmental Laboratories for donating tadpole food and larvae for preliminary trials, as well as his help and advice regarding animal husbandry; Emily Glotfelty for helping design the behavioral assay; Dr. Carla Goad for advice on statistics; and Dr. Ryan Sherman and Joel Hickey for assistance with the ICP-OES.
Funding
Funding was provided by the Oklahoma State University Department of Integrative Biology.
Author information
Authors and Affiliations
Contributions
PK: conceptualization, methodology, formal analysis, investigation, writing: original draft, visualization, project administration. SM: Supervision, writing: review and editing, resources, funding acquisition. MM: methodology (neuromast imaging and quantification), resources, supervision, writing: review and editing. JB: resources, supervision, writing: review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed under an approved Animal Care and Use Protocol through the Institutional Animal Care and Use Committee, Oklahoma State University (ACUP AS-18-3).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krupa, P.M., McMurry, S.T., Minghetti, M. et al. Effects of Copper on the Neuromasts of Xenopus Laevis. Arch Environ Contam Toxicol 80, 769–778 (2021). https://doi.org/10.1007/s00244-020-00778-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00778-z