Abstract
We present a case study on the tissue absorption of copper of a widely distributed moss species, Ptychostomum capillare in the polluted soil of an abandoned copper mine in central Spain. We studied the soil properties in a copper soil pollution gradient and sampled the moss tufts growing on them in four plots with contrasted soil copper levels. We determined the copper content in the soil and in the moss tissues. On these moss samples, we also performed histochemical tests and X-ray dispersive spectrometry coupled with scanning electron microscopy (SEM-EDX), both in untreated shoots and in samples where surface waxes were removed. We checked the behavior of this species using a metallophillous moss, Scopelophila cataractae, for comparative purposes. Copper contents in P. capillare seem to depend more on available, rather than total soil copper contents. Our results indicate that this moss is able to concentrate 12-fold the available soil copper in soil with low available copper content, whereas in the most polluted soil the concentration of Cu in the moss was only half those levels. Both histochemical and SEM-EDX tests show no surface copper in the mosses from the least polluted plot, whereas in samples from the soil with highest copper content, the removal of surface waxes also reduces or removes copper from the moss shoots. Our observations point at a mixed strategy in P. capillare in this copper mine, with metal accumulation behavior in the lowest Cu plot, and an exclusion mechanism involving wax-like substances acting as a barrier in the most polluted plots. These distortions impede the estimation of environmental levels and thus compromise the value of this moss in biomonitoring. We highlight the need of extending these studies to other moss species, especially those used in biomonitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pollution of soils by heavy metals poses important risks for human and ecosystem health, due to their persistence and toxicity (Giller et al. 1998; Chen et al. 2015). Among them, copper is required for life in small concentrations, because it is integrated into metalloenzymes that take part in redox processes; however, at high concentrations, it becomes toxic for organisms (Gaetke and Chow 2003; Bravo-Gómez et al. 2015; Schulten and Krämer 2017). Mining is one of the main focuses of soil contamination by heavy metals (Alloway 2013) and causes additional physical and chemical disturbances that last for a long time even after mining works end (Cooke and Johnson 2002), hindering the ecosystem regeneration. The highest levels of copper are found in mine soils affected by ore milling, concentration, and waste disposal. Copper concentrations on topsoils affected by mine wastes can reach several thousand mg kg−1 (Cornu et al. 2017).
The most important features concerning copper toxicity in vascular plants are relatively well known. The estimated copper toxicity levels that plants can allow in their tissues are around 20–30 µg g−1 (Pais and Jones 1997). The excess of copper in the cells induces a decrease of growth and development in plants and even death in the majority of organisms due to the interaction with sulfhydric groups that inhibit the enzymes (Shakya et al. 2008). High copper concentrations also can damage the plasmatic membrane, which leads to the exit of potassium cations from the cell (Brown and Wells 1990), and cause a reduction of the chlorophyll content in the plastids (Guschina and Harwood 2002; Shakya et al. 2008).
Vascular plants have developed a range of strategies to mitigate the negative effects of metal pollution (Chardonnens et al. 1999; Broadhurst et al. 2004; Psaras et al. 2000). Among them, plants frequently resort to an avoidance strategy that consists of preventing or hindering the entrance of the heavy metals to the tissues and cells (Hall 2002). Mycorrhizas and chelating substances seem to have a key role in this regard (Hall 2002; Cheng 2003) and mucilage barriers (Colzi et al. 2015). Additionally, vascular plants have evolved mechanisms that diminish cellular damage once the metal has entered the cells (i.e., tolerance sensu; Levitt 1972). These mechanisms are in general based on a rapid translocation of the metal to vacuoles of their precipitation in the cell wall (Cheng 2003; Ernst 2006).
Much less is known about copper tolerance and resistance in bryophytes. Several species of bryophytes are metallophilous, that is, they require high metal levels to develop, and thus they can uptake and concentrate these metals in their tissues. Well-known examples of metallophillous species are the genera Scopelophila and Mielichhoferia (Mårtensson and Berggren 1954; Persson 1956). Other species—not necessarily metallophilous—grow on soils with high copper concentrations accumulating copper in the cell wall and immobilizing the cations with its negative charge (Satake et al. 1988; Chettri et al. 1997; Shakya et al. 2008; Satake 2013). In species with metal-tolerant populations, there is a genetic variation that is greater among populations than within them (Shaw et al. 1987; Jules and Shaw 1994; Shaw and Schneider 1995). The development of different ecotypes depends on the intensity of adaptive pressure as heavy metal concentration or soil type (Shaw 1993; Jules and Shaw 1994), which suggests high phenotypic plasticity. In terms of reproductive expression, photosynthetic rates, and vegetative growth, mosses show a higher tolerance to heavy metal contamination when they are native from contaminated soils (Shaw et al. 1987; Jules and Shaw 1994).
However, other resistance mechanisms in bryophytes have been poorly studied. This is partly because bryophytes were frequently assumed to lack efficient avoidance mechanisms, as their passive water and nutrient uptake strategy makes them uptake heavy metals throughout their whole surface. Recent evidence has shown that some species can develop a mucilage barrier that effectively excludes some heavy metals from the cells (Cogolludo et al. 2017), but whether this is a common mechanism in mosses is unknown.
These mechanisms may affect a common usage of mosses. Because of their wide distribution, their ability to uptake and accumulate heavy metals throughout their whole surface, and their sensitivity, bryophytes are extensively used as bioindicators (Onianwa 2001). Some important biomonitoring programs are based on the metal contents in the tissues of selected mosses to test heavy metal deposition, such as the quinquennial programs set up by the European ICP Vegetation international research consortium in central and northern Europe to assess heavy metal pollution and dynamics (Harmens et al. 2010, 2015).
The technique has several disadvantages, including distortions of the metal content levels by environmental factors or by differences in the behavior of the diverse species used (Lepp and Salmon 1999; Aboal et al. 2010, 2017; Schröder et al. 2010, 2014). Besides, the mechanisms allowing mosses to resist metal pollution can affect the actual uptake and content of these elements in the moss samples (Aboal et al. 2010). If mosses can exclude heavy metals, the estimates based on tissue concentration would underestimate the incidence of the pollutant, whereas tolerance involving a hyper-accumulative strategy of the moss (as suggested in Vukojević et al. 2005) would cause an overestimation of the pollutant in the environment.
Thus, it is necessary to investigate the response and resistance strategy of mosses to assess their validity in biomonitoring studies. This is especially important regarding widely distributed species that are liable to be used in Mediterranean countries, where the implementation of the ICP Vegetation surveys is hindered by the scarcity of the species recommended by the ICP Vegetation team. Although pleurocarpous mosses, with larger growth forms, are usually preferred, in these drier areas even acrocarpous mosses, provided they are common enough, have been suggested for biomonitoring surveys (e.g., Izquieta-Rojano et al. 2016, putting Tortella squarrosa forward for consideration).
The small-scale copper mines (mostly abandoned) that are common in central Spain (Jordá 2008) provide a good opportunity to explore the relationship between copper content in the soil and the moss tissues, as they include variously polluted soils with almost no lithologic or climatic heterogeneity. The moss Ptychostomum capillare (Hedw.) Holyoak & N. Pedersen (= Bryum capillare Hedw.) is a common terricolous species in this copper-mine and in some areas becomes the most abundant species in the soil cover.
We selected one of these abandoned mines in which P. capillare is common as a case study, and conducted a comparative investigation of the copper content in this moss species, first, to explore the occurrence of resistance mechanisms, and, second, how these mechanisms may affect its potential use in heavy metal bioindication studies. If no distorting mechanisms are present, we should expect a predictable relationship between copper content in the moss tissues and that of the soil.
Material and Methods
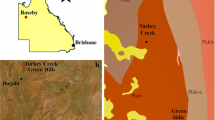
Bryophytes and soils samples used in this work were collected from the mining waste dump of a depleted Cu-W-Pb mine (Caridad-La Estrella mine, Lozoyuela, Madrid, Spain, 40º 56′ 25″ N, 3º 37′ 55″ W, Fig. 1a), at 1117 m.a.s.l. The ore vein was mainly composed of chalcopyrite (CuFeS2) hosted in augen gneiss. The mining activity between 1890 and 1965 was focused on the extraction of copper. The main vein ore contained 16–23% copper (Jordá 2008). The total area affected by the mining activities is approximately 4500 m2 of which the dump area is around 1600 m2 (Fig. 1b). Currently, the mineral wastes show a high degree of weathering, and consequently, the sulfides, mainly chalcopyrite, have given rise to powdery copper carbonates (Jordá 2008). We performed a preliminary soil survey of the area before the sampling to identify the presence of heavy metals in the wastes dump. TXRF analysis confirmed that copper is the major metal in these mine wastes while some other elements appear at lower trace concentrations: Cu (16,022 mg kg−1) ≫ Zn (182.7 mg kg−1) > As (37.8 mg kg−1) > Cr (18.7 mg kg−1) > Pb (11.1 mg kg−1) > Co (10.7 mg kg−1).
a Location of the study area. b Location of the plots at La Estrella mine. Both white and colored circles correspond with the nine plots sampled: the ones with a number and color are the four plots selected for copper tissue analyses and the white circles correspond with the other sampled plots. The color gradation in the circles represents the in situ estimated copper content gradient in the soil of each plot (later confirmed analytically). We observed maximum copper contamination in the darkest polygon and its surroundings
P. capillare was widespread across all the study area. The study materials were collected in January 2016 at different distances from the mine wastes (Fig. 1b). We established nine 1-m2 plots within which we collected all the P. capillare moss turfs growing in the soil of the plot, and samples of topsoil at a depth up to 5 cm. In each plot, we surveyed the plant cover and occurrence of other moss species. We then selected the plots with sufficient moss quantity to analyze its copper content in the tissues: four plots in total (plots 1, 2, 3, and 4; Fig. 1b).
In each plot, we took a sample consisting of a composite of five subsamples combined and homogenized in each plot. After collection, two replicates of each soil sample were weighed, then oven-dried at 105 ºC for 72 h, left to cool at room temperature and weighed again to determine the moisture. For chemical analysis, soil samples were air-dried and sieved (2 mm). Mineralogical characterization of soil was performed by X-ray diffraction XRD, powder method using a Siemens D-5000 equipment with monochromated CuKα X-radiation. Soil texture was determined via particle size analysis using the Boyoucos hydrometer method. The soil pH was measured in deionized water in a 1:2.5 soil-solution ratio with a glass electrode pH-meter. Soil organic carbon (SOC) was determined using the Walkley–Black wet oxidation method (Nelson and Sommers 1982). Total nitrogen was determined by the Kjeldahl digestion procedure (Bremner and Mulvaney 1982). The pseudo-total concentration of Cu in soils was determined after digestion with aqua regia according to ISO11466 procedure (1995). Available copper and phosphorous were determined by the Mehlich-3 method (Mehlich 1984); P was determined colorimetrically by the molybdenum-blue method (Murphy and Riley 1962) with a Photoanalizer D-105 Dinko. Pseudo-total and available copper contents were determined by absorption atomic spectrophotometry (AAS) in a Perkin-Elmer Analyst 800 equip. All analyses were performed in duplicate. The accuracy of the analytical procedure for the pseudo-total digestion was checked with the analysis of the soil standard reference material, CRM Canmet SO-3 (Canada Centre for Mineral and Energy Technology). Replicate analysis of this CRM standard showed good accuracy of experimental results with certified values with a recovery percentage of 95%.
Dried P. capillare samples from four selected plots with contrasted soil copper concentrations (plots 1, 2, 3, and 4; Fig. 1b) were digested with aqua regia on a hot plate. Before analysis, moss shoots were manually separated and carefully cleaned individually to remove all soil particles and debris visible with the stereoscope. Smaller particles were not removed; we did not wash the shoots in order not to lose any soluble copper on the plant surface or in the tissues. Cu concentration in digested solutions was determined by AAS.
For histochemical tests and SEM-EDX (scanning electron microscopy with energy-dispersive X-ray spectroscopy), we selected the P. capillare samples from the two plots with the most contrasted copper content in soil (plots 1 and 4 of Fig. 1b) and also samples of the reference metallophilous species, S. cataractae. All of these tests were performed on material washed 15 min in chloroform (to remove waxes, as a possible surface barrier; Proctor 1979) and on unwashed samples.
Histochemical analyses were made in the samples to identify the copper presence and its location in the tissues (rubeanic acid test), and the possible presence of waxes acting as barriers (Sudan Black B test). The rubeanic acid test follows Bancroft and Stevens (1982), slightly modified. We prepared 0.005% rubeanic acid (Aldrich 379387) in 5% ethanol containing 10% sodium acetate. Moss samples were incubated ca. 72 h at 37 ºC in darkness. Rubeanic acid reacts with copper forming dark depositions (Fig. 2). Sudan Black B test was after Jensen (1962): samples were briefly placed in 50% ethanol, then in a saturated and filtered solution of Sudan Black B (Fluka 86015) in 70% ethanol, 5 min, and differentiated in 50% ethanol. Lipophilic substances, including waxes, stain black.
For SEM-EDX microanalyses, both unwashed and chloroform-washed samples of P. capillare (plots 1 and 4) and S. cataractae were air-dried, gold-sputtered, and observed with a scanning electron microscope (Hitachi S-3000 N) operating at 20 kV, with an environmental secondary electron detector coupled to an X-ray dispersive energy analyzer (Oxford Instruments, NCAx-sight). In each sample, we observed separately apical parts of the moss shoots (with living tissues) and basal, senescent regions (mostly dead cells).
For comparative purposes, we performed the AAS, histochemical and SEM-EDX analysis also on a sample of a well-known metallophilous moss Scopelophila cataractae (Mitt.) Broth, collected in copper-rich soils in Hiraizumi, Iwate, Japan (August 23, 2015) and deposited in MA-UAM (Herbarium of Universidad Autónoma, Madrid).
Results
Soil Properties
The general properties of selected soils in the study area are shown in Table 1. The soils were slightly acidic to neutral (pH range 6.08–7.16). The soils most affected by the mining wastes (plots 3 and 4) presented more poor physicochemical properties compared to the less affected soils (plots 1 and 2): lower organic carbon, total nitrogen, and available phosphorus contents, higher percentage of sand fraction, lower water holding capacity and much higher pseudo-total, and available copper concentrations. Pseudo-total soil copper contents (1651 and 8756 mg kg−1, plots 3 and 4 respectively) were far above the threshold value for agricultural and forestry use in the regional regulation (BOCM 2006). These contents surpass largely the range for copper in the soil (60–125 mg kg−1) that would be toxic to plants (Kabata-Pendias and Pendias 1984). The available copper contents of these soils (615 and 1968 mg kg−1, plots 3 and 4, respectively) also far exceed 50 mg kg−1, which represents the threshold Mehlich III Cu levels above which the agronomic crop production is harmed (Morse et al. 2016).
P. capillare was the dominant moss at the plots more affected by the mining wastes. P. capillare covered approximately 15% and 30% of the areas of plots 4 and 3, which suggests a higher copper tolerance of P. capillare respect to the rest of the mosses identified in the surroundings of the mine that had a very low abundance or were absent from the most contaminated plots.
Copper contents in the selected mosses are shown in Table 1. The metal concentration in the moss samples increased with soil copper concentration, although the only significant correlations were obtained between soil Cu extractable and Cu moss (r = 0.94, p = 0.05). In fact, the copper content in the mosses grown in plots 2 and 3 (46.9 and 655.3 µg g−1, respectively) was similar to the extractable copper content of these soils (Table 1). In mosses growing in the least polluted soil (plot 1), the copper content exceeded 1.6- and 11.9-fold the pseudo-total and extractable copper contents in the soil, respectively. In mosses from the most polluted soil (plot 4), the copper content of the moss tissue (974 µg g−1) only represented half of the extractable soil copper content. Because the moss samples were not washed before measuring copper content, the results cannot be attributed exclusively to the copper absorbed in the tissues, as our methodology does not preclude the presence of minute soil particles on the surface of the moss plants, but, as we will discuss later, these particles do not account for the moss accumulation pattern.
Histochemical Analysis and SEM-EDX
The rubeanic acid test on P. capillare samples from plot 4, with the highest Cu content, revealed mainly extracellular dark particles on the leaf surface (Fig. 4a, arrowheads). Only occasionally, also the walls of the leaf nerve and marginal cells stained dark. In dead tissues and old leaves, the staining was very irregular (not shown). In contrast, samples from the least contaminated plot (plot 1) showed very little or no reaction with the rubeanic acid. This contrasts with the pattern shown in S. cataractae (Fig. 2b), in this bioaccumulator species the rubeanic acid reacted strongly and consistently with the walls of the leaf cells, both in young, apical parts of the shoots and in senescent, basal regions.
Sudan Black B detected the presence of lipophilic substances, probably waxes, as a thin, extracellular dark film on the leaves of P. capillare (Fig. 4b, arrowheads), especially in plants from the most contaminated plot (plot 4). In chloroform-washed samples, we get neither staining with Sudan Black B, nor detection of any extracellular, dark particles, with rubeanic acid, suggesting the role of waxes as a barrier to copper.
We did not detect copper in any sample of P. capillare collected in the plot with the lowest Cu levels (Table 2). In the samples of P. capillare from the most contaminated plot (4), the copper content was highly variable. In basal areas of the shoot, especially in the senescent leaf nerve and margins, it was more abundant (Fig. 5a, b; Table 2) than in juvenile ones. In the apical parts of the shoots, Cu often was undetected (Fig. 5d, e), and, when detected, it was present only in low percentages of atomic weight. Chloroform-washed samples presented much lower levels of copper both in apical and basal parts (Fig. 5b, d, e; Table 2). The SEM-EDX observations (Figs. 4c–f, 5b, d, e; Table 2) confirm that the chloroform treatment is apparently effective in washing away some surface lipophilic substances obscuring the cell outline, thus supporting their wax-like nature.
As for the metallophilous moss S. cataractae, although the analyses also yielded a high variability, copper was detected much more consistently in the samples (Fig. 5f, g; Table 2), and even the apical parts presented high copper contents (although lower than the basal parts). The chloroform treatment had little effect (Table 2), perhaps only removing soil particles from the basal parts, showing that most of the metal had been absorbed into the cell walls (as described by Satake et al. 1988 and Konno et al. 2010).
Discussion
The moss species P. capillare is a cosmopolitan species, quite common in Europe, and shows ample resistance to contamination (Dierßen 2001). This resistance also is confirmed more specifically for Cu, not only from previous research (Vukojević et al. 2005) but also from the Cu levels in the soils here analyzed, as in the plots with highest contents they are well over the environmentally acceptable levels established in the local legislation (BOCM 2006).
Given the results, P. capillare in this mine would present a mixed strategy. In the least polluted plot, it would behave as an accumulator of the bioavailable soil copper fractions. For instance, in plot 1, the samples are able to concentrate up to 12 times the level of available copper from soil (Table 1). In more polluted soils, the moss would behave as an excluder. The copper content in the tissues of P. capillare sampled in the more contaminated plots, as detected in the atomic absorbance analyses, remains in comparatively low levels and does not parallel the increase of copper (both pseudo-total and extractable values) in plot soils (Table 1; Fig. 3). Thus, in plot 4, with available copper levels in the soil of 1,968 mg kg−1, P. capillare tissues concentrate only half of these levels (974.2 mg kg−1).
The comparison of P. capillare with the metallophilous S. cataractae (Fig. 3; Table 1), with a well-known ability to accumulate copper in its tissues (Satake et al. 1988, 1990; Konno et al. 2010), puts forward the different behavior of both species in copper-rich environments. A true metallophilous moss such as S. cataractae can concentrate copper from the environment from a hundred- to million-fold. This suggests the existence of an exclusion mechanism in P. capillare, involving surface barriers preventing copper entrance into the tissues, and thus direct contact of the cells with excessive levels of this metal, which could explain, at least partially, the resistance of this species to copper contamination in the soil.
We must take into account the presence of dust particles in the P. capillare samples (visible, for instance, in Figs. 4a–c, e, and 5a, c). In the moss shoots from the least polluted plot 1, the high copper content detected in the tissues cannot be an artifact caused by this debris. Not only does the surrounding soil have much lower copper levels, but also all 17 SEM_EDX mosses analyzed from this plot (8 analyses on unwashed samples, 9 on washed shoots; see Table 2) render no detectable copper on the moss surface. Thus, the copper detected in the moss must have been absorbed and accumulated into the tissues.
Microanalysis of P. capillare samples collected in plot 4, the most contaminated. a Rubeanic acid test for Cu on an apical leaf, showing some dark, extracellular particles near the leaf nerve (arrowheads). b Sudan Black B test on a leaf, showing a thin, dark film. Arrowheads highlight the areas showing most visible dark staining. c SEM image of an unwashed shoot, with ill-defined cell outlines. d SEM image of a chloroform-washed shoot, showing clearly defined cell outlines after removing the lipophilic surface substances masking them. e SEM-EDX analysis on an unwashed basal leaf, showing a clear Cu signal. f SEM-EDX analysis on a chloroform-washed basal leaf. In this case, all the copper also has been washed away (see values of percentage of atomic weight on several washed and unwashed samples in Table 2)
SEM-EDX analyses of senescent and apical parts of shoots of P. capillare samples collected in plot 4 (the most contaminated) and of the metallophilous S. cataractae, used for comparison. a–eP. capillare samples. a, b Senescent, basal parts of the shoots: a unwashed, with some Cu detectable; b Chloroform-washed, with some scarce quantities of Cu detectable. c–e Apical parts of the shoots: c unwashed, with some Cu detectable; d, e Chloroform-washed, two large areas of the same leaf, no Cu detected. f, g Chloroform-washed S. cataractae samples, both showing detectable, comparatively high Cu content. f Senescent, basal leaf. g Apical leaf (see values of percentage of atomic weight on several washed and unwashed samples in Table 2)
In turn, in the most polluted plot (plot 4), the presence of copper from soil dust on the moss surface is visible in all the analyses. It is detected in unwashed samples both using rubeanic acid (Fig. 4a, b) and SEM-EDX analysis (Figs. 4c, e, 5a, c). This means that our detected copper content in the moss tissues is an overestimation. Still, if anything, this confirms the exclusion strategy of this moss in this heavily polluted mine site. The actual levels inside the tissues must be lower, more clearly differing from those in the soil.
The results of the histochemical tests and the SEM-EDX analyses support the presence of an exclusion strategy involving the production of extracellular, chloroform-soluble substances, likely surface waxes, which prevent the copper from being absorbed by the cell walls, or entering the cytoplasm, and thus constituting an effective avoidance mechanism of metal stress. This is observable comparing the SEM-EDX analyses in washed and unwashed moss shoots. The effect of chloroform washing results in the removal of a remarkable proportion of the copper on the plant surface, especially in the basal parts of the moss shoots (Figs. 4, 5; Table 2). Thus, our moss cleaning methods, omitting any washing of the samples before AAS, SEM-EDX and histochemical analyses, make it impossible to quantify accurately the copper levels in the tissues but allow the exploration of accumulation or exclusion strategies in this case study.
Our observations contradict those of the results of Vukojević et al. (2005), who found tissue concentrations up to 5250 µg g−1 in P. capillare from substrata five times less contaminated than ours, leading them to classify this species as hyper-accumulative. This discrepancy can be due to substrata differences in the two studies. Vukojević et al. (2005) studied plants growing on ashes, and the extractable copper content (not reported in their paper) could be higher than in the soils of our copper mine waste dumps. In any case, the diverse strategy of this species in different substrata, and the distortions involved in each (whether hyper-accumulative or presenting exclusion mechanisms) would yield little consistency to any study beyond local scopes. It also illustrates our lack of knowledge on survival strategies of mosses, even considering a single species, in metal-polluted environments.
This is the first time that wax-like substances are shown to be involved in an exclusion mechanism of toxic metals in mosses. Traditionally, waxes in mosses are considered to appear only in endohydric mosses, with highly developed internal conductive systems (Buch 1945; Proctor 1979). Although P. capillare is not included in this group of mosses, the presence of waxes in a wide range of bryophytes is increasingly recognized (e.g., see the revision of Glime 2015). To date, waxes in bryophytes were assumed to be a mechanism of protection from desiccation, but our study suggests that some chloroform-soluble, wax-like substances might play some other roles that should be studied in a wide taxonomic spectrum in this plant group.
Remarkably, all the moss samples analyzed in this study exceed 20 µg g−1, the toxic threshold usually considered for copper in plants (Borkert et al. 1998). This indicates some metal accumulation trend for this moss, probably involving physiological adaptations leading to copper tolerance when the available copper levels in the soil are below the threshold, in addition to exclusion mechanisms. Despite the ability of P. capillare to concentrate Cu as an accumulator, the comparatively low levels in its tissue, and the reversion of the strategy in most polluted plots would impede the use of this species for phytoremediation of mine soils.
The value of this species in biomonitoring studies also would be compromised, because we could not find a direct relationship between the environmental copper and the content of this element in the tissues (Table 1; Fig. 3), both in plots with the low copper levels and in plots with the highest copper pollution. Our microanalytical observations (Fig. 5a–e; Table 2) also show considerable variability between apical, young parts (with less copper content) and senescent, basal areas of the moss shoots (with higher levels), as it is to be expected in an active exclusion strategy: living tissues would be able to expend more energy in resistance mechanisms, whereas senescent tissues would show more passive absorption. We also have detected high internal variability even in similar samples from the same plot. Again, this would represent another source of noise in quantitative estimations of environmental copper.
Overall, the mixed strategy of this moss, and especially the presence of an exclusion mechanism allowing a high resistance to metal, does not support the use of P. capillare for quantitative estimations of copper levels in the soil. Some authors have warned against the use of mosses for direct estimations of pollutants (Aboal et al. 2010, 2017; Schröder et al. 2010). A similar caution note must be taken into account in studies concerning the atmospheric deposition of these and other metals, because it is possible that the resistance strategy found here is more widespread than previously suspected. We thus recommend a careful examination of the species selected for biomonitoring before their use in wide, systematic biomonitoring programs.
Conclusions
-
The ability of Ptychostomum capillare to grow in heavily polluted soil at this mine confirms its resistance to high environmental Cu levels.
-
The resistance strategy of this moss to Cu seems to be mixed; at our mine, it is able to concentrate Cu when the soil levels are not too high, whereas in the most polluted plot, it shows an exclusion mechanism mediated by lipophilic substances, presumably waxes, which would act as a barrier preventing the entry of the metal in the cells.
-
This case study compromises the use of Ptychostomum capillare in biomonitoring heavy metal pollution, as the behavior observed would distort the direct estimation of environmental Cu through the levels detected in its tissues. Caution should be extended to other common mosses that could be considered as potential candidates for biomonitoring programs.
References
Aboal JR, Fernández JA, Boquete T, Carballeira A (2010) Is it possible to estimate atmospheric deposition of heavy metals by analysis of terrestrial mosses? Sci Total Environ 408(24):6291–6297. https://doi.org/10.1016/j.scitotenv.2010.09.013
Aboal JR, Boquete MT, Carballeira A, Casanova A, Debén S, Fernández JA (2017) Quantification of the overall measurement uncertainty associated with the passive moss biomonitoring technique: sample collection and processing. Environ Pollut 224:235–242. https://doi.org/10.1016/j.envpol.2017.01.084
Alloway BJ (ed) (2013) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer, Dordrecht
Bancroft JD, Stevens A (1982) Theory and practice of histological techniques, 2nd edn. Churchill Livingstone, London
BOCM (2006) Order 2770/2006, 11 Aug, Regional Ministry of Environment and Territory of the Government of Madrid, establishing generic reference levels of heavy metals and other trace elements in contaminated soils of the Community of Madrid BOCM 204 (28 Aug 2006), pp 29–30 (in Spanish)
Borkert CM, Cox FR, Tucker MR (1998) Zinc and copper toxicity in peanut, soybean, rice and corn in soil mixtures. Commun Soil Sci Plat Anal 29(192):2991–3005. https://doi.org/10.1080/00103629809370171
Bravo-Gómez ME, Espinoza-Guillén A, Castillo S, Barba N (2015) Metalochaperonas: escoltas personales en el tráfico intracelular de iones metálicos. Educación Química 26(1):26–37. https://doi.org/10.1016/S0187-893X(15)72095-1
Bremner JM, Mulvaney CS (1982) Nitrogen-Total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, Wisconsin, pp 595–624
Broadhurst CL, Chaney RL, Angle JS, Erbe EF, Maugel TK (2004) Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil 265(1):225–242. https://doi.org/10.1007/s11104-005-0974-8
Brown DH, Wells JM (1990) Physiological effects of heavy metals on the moss Rhytidiadelphus squarrosus. Ann Bot 66(6):641–647. https://doi.org/10.1093/oxfordjournals.aob.a088078
Buch H (1945) Über die Wasser- und Mineralstoffversorgung der Moose (I). Comm Biol Soc Sci Fenn 9(16):1–44
Chardonnens AN, Wilma M, Vellinga S, Schat H, Verkleij JA, Ernst WH (1999) Allocation patterns of zinc and cadmium in heavy metal tolerant and sensitive Silene vulgaris. J Plant Physiol 155(6):778–787. https://doi.org/10.1016/S0176-1617(99)80096-0
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153. https://doi.org/10.1016/j.scitotenv.2015.01.025
Cheng S (2003) Effects of heavy metals on plants and resistance mechanisms. Environ Sci Pollut Res 10(4):256–264. https://doi.org/10.1065/espr2002.11.141.2
Chettri MK, Sawidis T, Zachariadis GA, Stratis JA (1997) Uptake of heavy metals by living and dead Cladonia thalli. Environ Exp Bot 37(1):39–52. https://doi.org/10.1016/S0098-8472(96)01023-4
Cogolludo J, Estébanez B, Medina NG (2017) The effects of experimentally supplied lead nitrate on three common Mediterranean moss species. Environ Sci Pollut Res 24:26194–26205. https://doi.org/10.1007/s11356-017-9220-1
Colzi I, Pignattelli S, Giorni E, Papini A, Gonnelli C (2015) Linking root traits to copper exclusion mechanisms in Silene paradoxa L. (Caryophyllaceae). Plant Soil 390:1–15. https://doi.org/10.1007/s11104-014-2375-3
Cooke JA, Johnson MS (2002) Ecological restoration of land with particular reference to the mining of metals and industrial minerals: a review of theory and practice. Environ Rev 10:41–71. https://doi.org/10.1139/a01-014
Cornu J-Y, Huguenot D, Jezéquel K, Lollier M, Lebau T (2017) Bioremediation of copper-contaminated soils by bacteria. World J Microbiol Biotechnol 33:26. https://doi.org/10.1007/s11274-016-2191-4
Dierßen K (2001) Distribution, ecological amplitude and phytosociological characterization of European bryophytes. Cramer in der Gebr, Berlin
Ernst WH (2006) Evolution of metal tolerance in higher plants. For Snow Landsc Res 80(3):251–274
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1):147–163. https://doi.org/10.1016/S0300-483X(03)00159-8
Giller KE, Witter E, Mcgrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30(10–11):1389–1414. https://doi.org/10.1016/S0038-0717(97)00270-8
Glime JM (2015) Water relations: leaf strategies—cuticles and waxes. In: Glime JM (ed) Bryophyte ecology, vol 1: Physiological ecology. Michigan Technological University and the International Association of Bryologists, ebook, Ch. 7–4b
Guschina IA, Harwood JL (2002) Lipid metabolism in the moss Rhytidiadelphus squarrosus (Hedw.) Warnst. from lead-contaminated and non-contaminated populations. J Exp Bot 53(368):455–463. https://doi.org/10.1093/jexbot/53.368.455
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11. https://doi.org/10.1093/jexbot/53.366.1
Harmens H, Norris DA, Steinnes E, Kubin E, Piispanen J, Alber R, Aleksiayenak Y, Blum O, Coskun M, Dam M, De Temmerman L, Fernandez JA, Frolova M, Frontasyeva M, Gonzalez-Miqueo L, Grodzinska K, Jeran Z, Korzekwa S, Krmar M, Kvietkus K, Leblond S, Liiv S, Magnusson SH, Mankovska B, Pesch R, Ruhling Ä, Santamaria JM, Schroder W, Spiric Z, Suchara I, Thöni L, Urumov V, Yurukova L, Zechmeister HG (2010) Mosses as biomonitors of atmospheric heavy metal deposition: spatial patterns and temporal trends in Europe. Environ Pollut 158(10):3144–3156. https://doi.org/10.1016/j.envpol.2010.06.039
Harmens H, Norris DA, Sharps K, Mills G, Alber R, Aleksiayenak YB, Cucu-Man SM, Dam M, De Temmerman L, Ene A, Fernández JA, Martínez-Abaigar J, Frontasyeva M, Godzik B, Jeran Z, Lazo P, Leblond S, Liiv S, Magnússon SH, Maňkovská B, Pihl Karlsson G, Piispanen J, Poikolainen J, Santamaria JM, Skudnik M, Spiric Z, Stafilov T, Steinnes E, Stihi C, Suchara I, Thöni L, Todoran R, Yurukova L, Zechmeister HG (2015) Heavy metal and nitrogen concentrations in mosses are declining across Europe whilst some “hotspots” remain in 2010. Environ Pollut 200:93–104. https://doi.org/10.1016/j.envpol.2015.01.036
ISO (1995) Soil quality. Extraction of trace elements soluble in aqua regia. ISO11466. International Organization for Standardization, confirmed in 2016
Izquieta-Rojano S, Elustondo D, Ederra A, Lasheras E, Santamaría C, Santamaría JM (2016) Pleurochaete squarrosa (Brid.) Lindb. as an alternative moss species for biomonitoring surveys of heavy metal, nitrogen deposition and δ 15 N signatures in a Mediterranean area. Ecol Indic 60:1221–1228. https://doi.org/10.1016/j.ecolind.2015.09.023
Jensen WA (1962) Botanical histochemistry. Principles and practice. Freeman, San Francisco
Jordá L (2008) Metal mining in Madrid province: mining heritage and promotion of the underground space. PhD Thesis. Universidad Politécnica de Madrid, Madrid (in Spanish)
Jules ES, Shaw AJ (1994) Adaptation to metal-contaminated soils in populations of the moss, Ceratodon purpureus: vegetative growth and reproductive expression. Am J Bot 81(6):791–797
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and plants. CRC Press, Florida
Konno H, Nakashima S, Katoh K (2010) Metal-tolerant moss Scopelophila cataractae accumulates copper in the cell wall pectin of the protonema. J Plant Physiol 167(5):358–364. https://doi.org/10.1016/j.jplph.2009.09.011
Lepp NW, Salmon D (1999) A field study of the ecotoxicology of copper to bryophytes. Environ Pollut 106(2):153–156. https://doi.org/10.1016/S0269-7491(99)00080-9
Levitt J (1972) Responses of plants to environmental stresses. Academic Press, New York
Mårtensson O, Berggren A (1954) Some notes on the ecology of the “copper mosses”. Oikos 5:99–100
Mehlich A (1984) Mehlich-3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Morse N, Walter MT, Osmond D, Hunt W (2016) Roadside soils show low plant available zinc and copper concentrations. Environ Pollut 209:30–37. https://doi.org/10.1016/j.envpol.2015.11.011
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2: Chemical and microbiological properties. American Society of Agronomy Soil Science Society of America, Madison, pp 539–579
Onianwa PC (2001) Monitoring atmospheric metal pollution: a review of the use of mosses as indicators. Environ Monit Assess 71:13–50
Pais I, Jones B Jr (1997) The handbook of trace elements. St. Lucie Press, Boca Raton
Persson H (1956) Studies in "copper mosses.". J Hattori Bot Lab 17:1–18
Proctor MCF (1979) Surface wax on the leaves of some mosses. J Bryol 10(4):531–538. https://doi.org/10.1179/jbr.1979.10.4.531
Psaras GK, Constantinidis TH, Cotsopoulos B, Manetas Y (2000) Relative abundance of nickel in the leaf epidermis of eight hyperaccumulators: evidence that the metal is excluded from both guard cells and trichomes. Ann Bot 86(1):73–78. https://doi.org/10.1006/anbo.2000.1161
Satake K (2013) The mystery of copper bryophytes (in Japanese). Iseb, Tsukuba
Satake K, Shibata K, Nishikawa M, Fuwa K (1988) Copper accumulation and location in the moss Scopelophila cataractae. J Bryol 15(2):353–376. https://doi.org/10.1179/jbr.1988.15.2.353
Satake K, Mishikawa M, Shibata (1990) A copper-rich protonemaI colony of the moss Scopelophila cataractae. J Bryol 16(1):109–116. https://doi.org/10.1179/jbr.1990.16.1.109
Schröder W, Holy M, Pesch R, Harmens H, Ilyin I, Steinnes E, Alber R, Aleksiayenak Y, Blum O, Coşkun M, Dam M, De Temmerman L, Frolova M, Frontasyeva M, Gonzalez Miqueo L, Grodzińska K, Jeran Z, Korzekwa S, Krmar M, Kubin E, Kvietkus K, Leblond S, Liiv S, Magnússon S, Maňkovská B, Piispanen J, Rühling A, Santamaria J, Spiric Z, Suchara I, Thöni L, Urumov V, Yurukova L, Zechmeister HG (2010) Are cadmium, lead and mercury concentrations in mosses across Europe primarily determined by atmospheric deposition of these metals? J Soils Sediment 10(8):1572–1584. https://doi.org/10.1007/s11368-010-0254-y
Schröder W, Pesch R, Schönrock S, Harmens H, Mills G, Fagerli H (2014) Mapping correlations between nitrogen concentrations in atmospheric deposition and mosses for natural landscapes in Europe. Ecol Indic 36:563–571. https://doi.org/10.1016/j.ecolind.2013.09.013
Schulten A, Krämer U (2017) Interactions between copper homeostasis and metabolism in plants. In: Cánovas F, Lüttge U, Matyssek R (eds) Progress in Botany. Springer, Cham, pp 111–146
Shakya K, Chettri MK, Sawadis T (2008) Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch Environ Contam Toxicol 54(3):412–442. https://doi.org/10.1007/s00244-007-9060-y
Shaw AJ (1993) Population biology of the rare copper moss, Scopelophila cataractae. Am J Bot 80(9):1034–1041
Shaw AJ, Schneider RE (1995) Genetic biogeography of the rare “copper moss”, Mielichhoferia elongata (Bryaceae). Am J Bot 82(1):8–17
Shaw J, Antonovics J, Anderson LE (1987) Inter-and intraspecific variation of mosses in tolerance to copper and zinc. Evolution 41(6):1312–1325
Vukojević V, Sabovljević M, Jovanović S (2005) Mosses accumulate heavy metals from the substrata of coal ash. Arch Biol Sci 57(2):101–106. https://doi.org/10.2298/ABS0502101V
Acknowledgements
The authors want to thank Esperanza Salvador and Enrique Rodríguez (SEM-EDX Laboratory, SIdI, UAM) and Dr. Carlos García-Delgado (AAS Laboratory, Department of Agricultural Chemistry, UAM) for their helpfulness and kind assistance. They also thank three anonymous reviewers for their careful revision and their insights, which have helped significantly to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elvira, N.J., Medina, N.G., Leo, M. et al. Copper Content and Resistance Mechanisms in the Terrestrial Moss Ptychostomum capillare: A Case Study in an Abandoned Copper Mine in Central Spain. Arch Environ Contam Toxicol 79, 49–59 (2020). https://doi.org/10.1007/s00244-020-00739-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00739-6