Abstract
The ability of Australian native metallophytes to tolerate extreme metal concentrations in the soil and other difficult edaphic conditions is still not well understood. Copper is an essential micronutrient for plants to survive, but at high concentrations (> 20 µg g−1) in plant tissues, it can cause foliar chlorosis, stunted growth, and ultimately plant death. The Roseby Corridor in Central Queensland (near Cloncurry) is host to copper metallophytes (Polycarpaea spirostylis, Bulbostylis barbata, Tephrosia virens, Eriachne mucronata) which are able to tolerate soils with total copper concentrations up to 12,700 µg g−1. Even with these high levels of copper in the rhizosphere, the maximum copper in all species is relatively low when compared to Cu concentrations in the soil (leaf/stem: Bulbostylis barbata: 40.9 µg g−1, Eriachne mucronata: 12.3 µg g−1, Polycarpaea spirostylis: 10.9 µg g−1, Tephrosia virens: 128 µg g−1). Therefore, all of these species can be classified as copper Excluder-type metallophytes. Their affinity to copper makes these metallophytes useful indicators of copper surface mineralisation within the Roseby Corridor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gossans are heavily weathered surface outcrop expressions of metal sulphide minerals that can indicate the presence of potentially economic ore systems (Salama et al. 2016). The recognition of gossanous outcrops has been used since antiquity to aid exploration for mineral resources, and the presence of associated plant species has spurred the development of biogeochemical prospecting using indicator species (Brooks 1972; Cole 1980). Gossans developed above copper (Cu)-bearing systems, in particular, can be found in Australia, subtropical Africa, Arizona (United States), Chile, and Peru (Saad et al. 2012). Apart from mineralisation, gossans generally have poor water retention and are nutrient-depauperate, which creates a hostile environment for most plant species (Cole and Smith 1984). Gossanous surface expressions are often very limited in size and are composed of a heterogeneous mineral mosaic of contrasting metal concentrations forming distinctive micro-habitats for a few metal-tolerant plant species (Faucon et al. 2016). The metal-tolerant plants which can thrive in these environments are called ‘metallophytes' (Ernst 1974; Baker 1981, 1987). Copper is an essential micronutrient for plant growth and cell metabolism, being a structural element of metalloproteins involved in electron transport to mitochondria and chloroplasts (Yruela 2009). However, in excess, it is harmful, causing visible symptoms, such as foliar chlorosis, stunted growth, and ultimately plant death (Foy et al. 1978; Rout and Das 2009). Metal tolerance in metallophytes is the result of many, often thousands, of years of selective pressure (Shaw 1990). As plants are immobile, phenotypic plasticity allows them to respond to environmental factors and adapt their physiological function (Antonovics et al. 1971; Ernst 2006; Baker et al. 2010). So-called ‘pseudo-metallophytes’ occur on metal-enriched soils (often those created by anthropogenic activities) as well as on non-metalliferous soils while ‘obligate metallophytes’ are restricted to metalliferous soils, typically gossans or other base metal ore outcrops (Lambinon and Auquier 1963; Baker et al. 2010; Whiting et al. 2004).
Plant responses to a high exposure of metals in the soil or growth medium are categorised as ‘indicator’, ‘accumulator’ and ‘excluder’ type behavior (Baker 1981, 1987). Indicator species exhibit metal concentrations positively correlated with metal concentrations in the soil; whereas accumulator species actively absorb metals at concentrations greater than within the soil; at concentrations order of magnitudes greater in tissues, they are classified as hyperaccumulators; and finally, excluder species can tolerate high metal concentrations in the soil by resisting metal absorption into the roots, or by restricting transportation to the shoots (Baker 1981; Baker and Brooks 1989). The normal range of Cu in leaf tissue is between 5 and 20 µg g−1 and above 20 µg g−1 toxicity typically occurs (Krämer 2010). Copper hyperaccumulation was first defined as plants that have concentrations > 1000 µg g−1 foliar Cu (Malaisse et al. 1979), however, this was later revised to > 300 µg g−1 Cu (Krämer 2010; van der Ent et al. 2013).
Tolerance to Cu by Mimulus guttatus (Phrymaceae) in Copperopolis, California has been found to be dependent on the location of the population rather than the concentration of Cu in the soil (MacNair et al. 1993). This signifies that there is selection against tolerance as the ‘cost’ of tolerance towards Cu is high; and so, the location of the population acts more as an indicator towards tolerance than the Cu concentration itself. At the centre of a gossanous area tolerance in plants is expected to be highest, while on the halo Cu tolerance is less, and on non-metalliferous soils there may be no tolerance towards Cu (MacNair et al. 1993).
The currently proposed mechanisms of Cu tolerance on a cellular level are either: storage in the areas where Cu cannot interfere with essential processes, such as in the vacuole; chelation with metallothioneins, phytochelatins and organic acids; or, enhances Cu ion pumping over the plasma membrane (Krämer and Clemens 2006; Yruela 2009). Other passive and active mechanisms on a plant level include a reduction in uptake due to mycorrhizal associations with the roots; precipitation of Cu in the soil caused by root exudates; and, accumulation into the roots but restriction into the shoots (Lange et al. 2017). The genes which determine tolerance appear to differ between species, including the composition of genes that determine tolerance. A single major gene determines whether a population of M. guttatus (from Copperopolis) is tolerant at any level with several modifier genes that are hypostatic to the major gene for tolerance (MacNair and Christie 1983). However, in different species and populations, it is suggested that different levels of tolerance to a metal can be attributed to different tolerance mechanisms which are determined by different genes, including within the same species, but also for the same mechanism at different tolerance capacities (MacNair and Christie 1983; Schat et al. 1993). For example, within Silene vulgaris (Caryophyllaceae), two major genes control Cu tolerance (Schat et al. 1993). One gene segregates in crosses to non-tolerant populations, but not between tolerant populations occurring in different populations. The second gene only segregates in crosses from the same high-tolerant population. However, all of the genes involved in controlling the same exclusion mechanisms operating at the plasmalemma (Schat et al. 1993). Meaning that tolerance can evolve multiple times to tolerate different levels of Cu for the same tolerance mechanism.
Approximately 70% of known hyperaccumulators are Ni hyperaccumulators and only 7% of known hyperaccumulators are Cu hyperaccumulators (Reeves et al. 2018a). The evolutionary reasons of hyperaccumulation are still poorly understood, however, the cost/benefit of hyperaccumulating a more toxic metal, such as Cu, may be greater than other metals if it is to fulfil a herbivory protection role (Boyd and Martens 1998). The majority of Cu metallophyte research has been undertaken in the Copper Hills of Central Africa (DR Congo and Zambia) where more than 95% of the Cu–Co hyperaccumulators have been identified, the region hosts the aptly named “Copper Flora” (Morrison et al. 1979; Brooks et al. 1985; Reeves et al. 2018a; van der Ent et al. 2019). This region alone has over 700 metallophyte species distributed across hundreds of Cu–Co ‘geochemical islands’ (Brooks et al. 1980, 1986; Faucon et al. 2010; Lange et al. 2017; Malaisse et al. 1979, 2006). However, little is known about Cu metallophytes in Australia, and most of the research on true Cu metallophytes within Australia has been conducted on the native herbs from the Caryophyllaceae family (Polycarpaea breviflora, P. spirostylis, P. longiflora) (Brooks and Radford 1978). The association of P. spirostylis with Cu has been well documented in the 1960s and early 1970s (Nicolls et al. 1965; Cole et al. 1968; Grooves et al. 1972). Accounts of hyperaccumulation, such as that by Millotia myosotidifolia (Asteraceae), in South Australia have since been disproven (Reeves et al. 2018b) and the previous claim of 2400 µg g−1 is not supported (Blissett 1966). Only one other report has identified plants accumulating Cu greater than 40 µg g−1 within the Roseby Corridor (Queensland, Australia), a site now known for its Cu-bearing soils (Nicolls et al. 1965). The metallophytes identified and their respective maximum Cu concentrations in leaves/stems are: a native perennial grass, Eriachne mucronata (leaves: 56.9 µg g−1); a native erect shrub growing up to 2-m tall, Tephrosia sp. nov. (now T. virens) (leaves: 73.3 µg g−1; stem: 31.2 µg g−1); and, the previously mentioned herb, Polycarpaea glabra (synonym P. spirostylis subsp. glabra) (leaves: 30.3 µg g−1, stem: 55.9 µg g−1) (Nicolls et al. 1965). Over the previous decades, virtually no research has been conducted on metallophytes growing on metalliferous gossans in Australia, with the exception of a recent study conducted on Zn–Pb gossans in North–West Queensland (Lottermoser et al. 2008). This study identified multiple metallophyte species which were able to grow on Zn–Pb-enriched soils, including Eriachne mucronata which had lower foliar Cu concentrations (11.7 µg g−1) than reported from Roseby (Nicolls et al. 1965), but was able to transport Cd, Pb and Zn from the roots to the above-ground biomass in a linear relationship with DTPA-extractable soil metal concentrations.
This study focuses on the Cu metallophytes of the Roseby Corridor, North-West Queensland, Australia, and specifically the historical Cu-prospect locations of Turkey Creek and Green Hills, which are extensions of the Little Eva Cu deposit. These locations are of great interest as they are situated on subcropping Cu-bearing lithologies with areas of gossan; and, are located within 10 km of other significant habitats of Zn (+Pb) bearing outcrops of the Dugald River deposit (Erskine et al. 2012). As Cu metallophytes are so scarce it is of importance to further investigate the biogeochemistry of these unique species. Mining activities have since commenced within the Roseby Corridor and the metallophytes have not been investigated since Nicolls et al. (1965). The objective of this study is to further investigate the biogeochemistry of Cu metallophytes by identifying the tolerance range of species; the areas of metal accumulation within plant parts; characterise plants as excluder, indicator, and accumulator (or hyperaccumulator); and, to form a picture of plant communities in relation to soil mineralisation.
Materials and methods
Site description
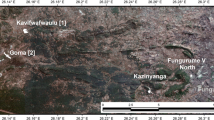
The Roseby Corridor is located in North-West Queensland, ~ 65 km North-West of Cloncurry and ~ 90 km northwest of Mount Isa (Fig. 1). The environment is dry with summer (November/December) temperatures reaching 38 °C and winter lows of 11 °C (June/July), the majority of the rainfall occurs in January–March (monthly means 79–162 mm), and the driest months April–Oct (monthly means 3.2–16.6 mm) (BOM 2020). Two sites within the Roseby Corridor were sampled, Turkey Creek (WGS84: 140.163–20.151) and Green Hills (WGS84: 140.174–20.178) which are 3 km apart. The Roseby Corridor extends for approximately 25 km × 6 km and is primarily composed of rocks of the Corella formation (~ 1740 Ma), which were originally carbonate-rich, fine clastic and evaporitic sediments (Blake 1987). These units were metamorphosed, deformed, metasomatically altered and mineralised during the Isan Orogeny (~ 1600–1540 Ma) (Betts et al. 2006). The lithologies are generally steeply dipping to the West with a North–South strike, and are fault-bounded to the East by Mount Rose Bee Fault and by the Coolullah Fault to the West. Primary (hypogene) copper mineralisation of chalcopyrite and bornite is associated with carbonate fluids (dominantly calcite) and is strongly structurally controlled with larger accumulations at fault intersections. Secondary (supergene) copper species are very common and include malachite, chrysocolla, tenorite and chalcocite. The background flora of the Roseby Corridor is composed of low tree and shrub savannah. Eucalyptus brevifolia and Triodia pungens are widely distributed over the Kanpdale quartzite range. Other species of Eucalypts, such as E. agrillacea, E. dichromophloia, E. papuana and E. terminalis, occur across the Roseby Corridor. Cleome viscosa and Acacia chisholmii form the dominant shrub layer, with A. chisholmii occurring near stream courses. Areas composed of lateralized Mesozoic sandstone host Acacia cambagei and species of Myporum and Eremophilia shrub (Nicolls et al. 1965).
a The study site is located in the Roseby Corridor in Queensland, Australia. b Turkey Creek and Green Hills are within the Roseby corridor (Esri 2020). c A surface geology map shows the study sites on Mount Roseby Schist. The abbreviations used in the map are: PLpo Mount Roseby Schist, PLkc Corella Formation, PLkc-c Corella Formation-c, PLb/s Boomarra Metamorphics/s, Qa Qa-QLD, TQW TQw-QLD (QLD GOV 2020)
Sample collection
Fieldwork took place during the main flowering season in 2017. Plant material and associated soil samples were collected using spot sampling of species of interest in the Roseby area: Polycarpaea spirostylis (Caryophyllaceae); Bulbostylis barbata (Cyperaceae); Tephrosia virens (Fabaceae); Eriachne mucronata (Poaceae). During the fieldwork, rhizosphere soil samples (~ 100 g) and plant foliar samples (~ 10 g) were collected and analysed for selected elemental concentrations. The sampling was aimed at indicating whether, at a local scale, soil Cu concentrations may be present at ostensibly phytotoxic levels, and whether plants might accumulate Cu and/or other elements.
Sample processing and analysis
Foliar samples were dried at 60 °C for 4 days until fully dry. Samples (0.3 g) were then digested with 5 mL HNO3 (70%) (Milestone S.r.l.-START D) for 1.5 h (30 min for the temperature to reach 125 °C, 125 °C for 30 min, cool down for 30 min). The digestates were then diluted to 30 mL using ultra-pure water and analysed using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) as described below.
All soil samples were dried to constant weight at room temperature and then sieved to < 2 mm. Samples (0.1 g) were then digested with 9 mL HCl (37%) and 3 mL HNO3 (70%) on a hot block (140 °C) for 3 h and then filtered and diluted to 40 mL. Potentially phytoavailable trace elements (Ni, Co, Cu, Zn, Mn, Fe) were extracted with Diethylenetriaminepentaacetic acid (DTPA) (Lindsay and Norvell 1978) using an adaptation by Becquer et al. (1995), which excludes triethanolamine, adjusted to pH 5.3 and an equilibrium time of 2 h (instead of 1 h). Free and easily exchangable metal ions in pore water were assessed using 0.01 M Sr(NO3)2 extractant in which 20 mL Sr(NO3)2 solution was mixed with 5 g soil and shaken for 2 h (method adapted from Kukier and Chaney 2001), centrifuged for 10 min at 4000 rpm and 0.1 mL HNO3 added to stabilize and avoid metal precipitation.
All acid digests and extracts were analysed with ICP-AES using a Thermo Scientific iCAP 7400 instrument for macro-elements (Na, Mg, Al, P, S, K, Ca), trace elements (Cr, Mn, Fe, Co, Ni, Cu, Zn) and ultra-trace elements (As, Se, Pb, Cd, Tl) in radial and axial modes depending on the element and expected analyte concentration. All elements were calibrated with a 4-point curve covering analyte ranges in the samples. In-line internal addition standardization using yttrium was used to compensate for matrix-based effects. Quality controls included matrix blanks, certified reference material (Sigma-Aldrich Periodic table mix 1 for ICP TraceCERT®, 33 elements, 10 mg L−1 in HNO3), Standard Reference Material (NIST Apple 1515 digested with HNO3), and internal reference materials.
Data processing
Survey data are coded using an alphanumeric system and transcribed into Microsoft Office Excel (2016). ICP-AES results for DTPA extraction are received and interpreted in Microsoft Office Excel (2016). Statistical analysis is conducted using R 3.4.2. Box plots are used to show the accumulation of metals within plant parts and the tolerance range of each species. A test of difference (Paired Student’s t test or Wilcoxon rank-sum test) is used to determine whether there is a difference in metal accumulation between plant parts. Another test of difference (Kruskal–Wallis H test or One-way ANOVA) is used to identify whether there is a difference in metal uptake between species. A test of correlation (Pearson product-moment correlation or Spearman’s rank correlation rho) is conducted between soil and plant parts to identify potential indicator species. Relationships are identified using tests of correlation (Pearson product-moment correlation or Spearman’s rank correlation rho). Soil contamination of ICP-AES results is assessed by identifying relationships of high metal concentrations with elements not usually found in plant samples (e.g. Cr) (Reeves and Kruckeberg 2018).
Results
Mineralogy and geochemistry
All soil samples analysed are rhizosphere samples from the sampled plant species. The concentrations of total soil digests are reported in Table 1. All metals of interest are low or below the limit of detection (LOD) apart from Cu which varies greatly (Co range: 8.79–129 µg g−1, mean 17.4 µg g−1; Zn range: 10.4–85.8 µg g−1, mean 27.9 µg g−1; Cd range: < LOD–20.8 µg g−1; Pb range: < LOD–9.49 µg g−1, Cu range: 393–12,700 µg g−1, mean 3500 µg g−1). Compared to Green Hills, Turkey Creek has significantly greater total concentrations of metals in soil for Cu, Co, Zn, Cd (Wilcoxon rank-sum test Turkey Creek > Green Hills Total Digests—Cu: W = 533, p ≤ 0.001, Co: W = 174, p ≤ 0.001, Zn: W = 487, p ≤ 0.001, Cd: W = 266, p ≤ 0.001). All soil samples analysed are rhizosphere samples that were subjected to a DTPA extraction experiment to determine the bioavailability of metals in rhizosphere samples from sampled plants which are shown in Table 2. Compared to the total digests, the inverse is true for (bio)available (DTPA and Sr(NO3)2) Cu and Zn concentrations which are significantly higher in Green Hills (Wilcoxon rank-sum test Turkey Creek < Green Hills DTPA—Cu: W = 2446, p = 0.00141; Sr(NO3)2-Cu: W = 2269, p = 0.0233, Zn: W = 2763, p ≤ 0.001). For DTPA-extractable concentrations, Cu concentrations are high with high variability (range: 49.2–1600 µg g−1, mean: 528 µg g−1) whilst other metals have low concentrations (Zn range: 0.22–171 µg g−1, mean: 3.17 µg g−1; Co range < LOD–33.9 µg g−1, Cd < LOD–0.128 µg g−1, Pb: < LOD–2.24 µg g−1). For Sr(NO3)2 extractable concentrations, only Cu and Zn have reportable values (Cu range: 0.165–144 µg g−1, mean: 12.2 µg g−1; Zn range: < LOD–0.965 µg g−1); whilst Co, Cd, and Pb values were below the limit of detection.
Plant distribution and foliar chemistry
Three plant species (P. spirostylis, B. barbata and T. virens) were found on both Turkey Creek and Green Hills, whilst E. mucronata was only found on Green Hills; Green Hills has higher concentrations of bioavailable Cu compared to Turkey Creek which would indicate that E. mucronata has higher tolerance towards and a potential preference towards bioavailable Cu (Cu Mean: Green Hills-567 µg g−1, Turkey Creek-477 µg g−1). There is no significant difference in Cu accumulation in P. spirostylis, T. virens and B. barbata between sites despite Green Hills having greater total and extractable Cu (Wilcoxon rank-sum test for Cu in P. spirostylis: W = 1019, p = 0.300, T. virens: W = 297, p = 0.576, B. barbata: W = 454, p = 0.936). The results for total metal concentrations in plant tissue are reported in Table 3. None of the species accumulated Cu concentrations above the hyperaccumulator threshold of 300 µg g−1 (leaf or stem Cu maxima’s, B. barbata: 40.9 µg g−1, E. mucronata: 12.3 µg g−1, P. spirostylis: 10.9 µg g−1, T. virens: 128 µg g−1; Fig. 2). There is a significant difference in Cu accumulation between the species (Kruskal–Wallis: X2 = 91.3, df = 3, p ≤ 0.001). The highest accumulator of Cu is T. virens within its leaves (range: 6.74–128 µg g−1, mean: 44.8 µg g−1) and the lowest is P. spirostylis in its stem/leaves (range: 1.52–10.9 µg g−1, mean: 5.63 µg g−1). Significant concentrations of Cu are accumulated into the roots of all plants. Bulbostylis barbata was able to accumulate the highest concentrations of Cu in its roots compared to the other species and had the greatest range (B. barbata: 95.6–2020 µg g−1, E. mucronata: 345–848 µg g−1, P. spirostylis: 83.2–434 µg g−1, T. virens: 144). There is a weak significant difference of total Cu in the plant rhizosphere between the species, E. mucronata has the least tolerance to higher total metals (Kruskal–Wallis: X2 = 8.195, df = 3, p = 0.0422). Bioavailable Cu in rhizosphere soil is analysed for differences between species, and T. virens is the least tolerant species despite accumulating the most Cu in plant tissue (Kruskal–Wallis: X2 = 15.6, df = 3, p = 0.00137). Only B. barbata exhibits a strong positive correlation between plant Cu content and extractable Cu in the soil (B. barbata Cu DTPA—stem: S = 848, rs = 0.791, p ≤ 0.001, Sr(NO3)2-stem: S = 1016, rs = 0.750, p ≤ 0.001; Fig. 3). A test of difference has also shown that there is no significant difference in Cu concentrations between roots and bioavailable concentrations (Kruskal–Wallis rank-sum test Cu B. barbata roots—DTPA: X2 = 0.0270, df = 1, p = 0.870).
Discussion
Four metallophytes were sampled at Turkey Creek and Green Hills (P. spirostylis, B. barbata, T. virens, E. mucronata; Fig. 4), all of which have been reported in Nicolls et al. (1965) but only T. virens had been sampled at Turkey Creek. The reported values in this study match the concentrations reported in Nicolls et al. (1965). Tephrosia virens has shown to accumulate greater concentrations (75%, 73.3 µg g−1) of Cu than previously reported. Bulbostylis barbata and P. spirostylis were both shown to accumulate less Cu. This study reports P. spirostylis, B. barbata and E. mucronata sampled on Cu mineralization as Cu excluders for the first time. Both B. barbata and E. mucronata had foliar Cu concentrations similar to those reported in another study with the same species growing on a Zn–Pb gossan that had relatively low concentrations of Cu in the soil (Lottermoser et al. 2008). The concentrations are comparable with foliar Cu concentrations on the higher end reported previously in the Roseby Corridor (Nicolls et al. 1965). However, Cu concentrations in E. mucronata roots were found to be much higher on the Cu gossan (596 µg g−1) than on the Zn-Pb gossan (37 µg g−1), this further supports E. mucronata being categorised as a Cu excluder type metallophyte. Only T. virens (maximum: 128 µg g−1, mean: 44.8 µg g−1) consistently exceeded the previous 40 µg g−1 maxima in plant leaf material for Cu in Australia. However, P. spirostylis exceeded 40 µg g−1 Cu in flower material (maximum: 59.9 µg g−1, mean: 7.27 µg g−1), but due to it being a strong outlier this may be caused by contamination; and, B. barbata exceeded 40 µg g−1 Cu in flower and stem material (flower maximum: 48 µg g−1, mean: 23.8 µg g−1; stem maximum: 40.9 µg g−1, mean: 11.4 µg g−1).
All plants accumulated low Cu concentrations relative to concentrations in the soil. Only B. barbata had a strong positive relationship with extractable Cu in the soil which would suggest that it is an indicator type metallophyte. The rate of accumulation in above-ground plant tissue is reflective of bioavailable Cu by 1:50 (stem:DTPA); although there is a significant correlation between the stem and bioavailable Cu, the concentrations in the stem are not directly reflective of what is in the soil and B. barbata is able to control Cu uptake into the stem. Greater concentrations of Cu are accumulated and stored in the root at concentrations more reflective of bioavailable Cu concentrations. This suggests that Cu is freely accumulated into the roots and transport to the shoots is restricted, categorising B. barbata as a Cu-excluder-type metallophyte. All other species would be classified as Cu excluder-type metallophytes and none exceeded the 300 µg g−1 hyperaccumulator threshold and no relationship is exhibited with Cu in the soil. Further analysis of root uptake was not possible due to the small sample size. However, the Cu concentrations in the roots would suggest that E. mucronata also freely accumulates bioavailable Cu into the roots and P. spirostylis and T. virens have stronger mechanisms of reducing the uptake of Cu into the roots. This behaviour corresponds to what other studies have found for other Cu metallophytes in Australia (Cole et al. 1968; Grooves et al. 1972; Lange et al. 2017).
Tephrosia virens accumulates the highest Cu concentrations in its tissue and some of the lowest Cu concentrations in its roots, which would suggest that it is not as strong of a Cu excluder as the other species and it exhibits weak Cu accumulator behaviour. Tephrosia virens also has the ostensibly lowest Cu tolerance to bioavailable Cu compared to the other species. Two other species of Tephrosia, T. longipes in Botswana (Mogopodi et al. 2008) and T. villosa in Sri Lanka (Rajakaruna and Bohm 2002), have been identified as Cu metallophytes, this suggest species of Tephrosia are Cu-tolerant and may have shared genetic traits for tolerance between species within the family, such as T. longipes and T. villosa, developing additional tolerance mechanisms for greater Cu concentrations. Eriachne mucronata only grows on one of the sites, where soils have greater concentrations of bioavailable Cu. The data show that E. mucronata is the least tolerant of the species towards total Cu concentrations. This may be due to low tolerance towards competition with other species and/or bioavailable Cu concentrations being a greater indicator of species composition than total Cu in the soil (Fig. 5). Interestingly, one of the most studied metallophytes in Australia, P. spirostylis the famous ‘copper plant' is the weakest Cu accumulator of the metallophytes observed. Few species reach the hyperaccumulation threshold, which is one of the reasons to why the hyperaccumulation threshold was reduced from 1000 to 300 µg g−1 foliar Cu (Krämer 2010; van der Ent et al. 2013). However, 300 µg g−1 may still be too exclusionary when taking into account the percentage of Cu hyperaccumulators identified, apart from the metallophyte research being conducted on the Cu-rich soils outside of the Central African Copper Belt, Cu hyperaccumulation is very rare (Shirong et al. 1997; Rajakaruna and Bohm 2002; van der Ent and Reeves 2015).
Different microhabitats can be identified within the Roseby Corridor. The images show clustering of metallophytes which are reflective of underlying Cu concentrations. a The dashed line highlights a cluster of B. barbata by a creek bed. b B. barbata is often found with P. spirostylis which can be seen in the dashed circle. c Shows a sharp transition between E. mucronata and Triodia pungens. d and e P. spirostylis following along strike of mineralised Cu lithologies
Conclusion
None of the metallophytes identified accumulated Cu concentrations greater than the hyperaccumulation threshold (300 µg g−1 foliar Cu). This study classifies B. barbata, P. spirostylis, E. mucronata, as Cu-excluder-type metallophytes which is representative of previous findings of Cu metallophytes in Australia. Tephrosia virens can be classified as a weak Cu accumulator as it accumulates somewhat higher Cu concentrations in its leaves relative to Cu concentrations in its roots. The most studied of the species, P. spirostylis (the well-known ‘copper flower’), is the weakest Cu accumulator of the species investigated here. Bioavailable (Cu extractable by DTPA solution) appears to have the strongest relationship with the metallophytes and is the best indicator of their distribution. As previously mentioned, the geospatial location relative to the lode may the best indicator of tolerance, and so it is recommended that a geospatial assessment of the bioavailable metal distribution and its relation to metallophyte species composition is made. Quantitative mapping of elemental distribution in leaves and roots of the metallophytes on the Roseby Corridor may provide better insight into the tolerance mechanisms of different metallophytes towards Cu, most notably between accumulators, such as T. virens, and excluders, such as B. barbata, and how this may differ on other sites nearby with the same species composition but different metalliferous soils, such as the Dugald River Lode, which is highly enriched in Zn–Pb–Cu.
References
Antonovics J, Bradshaw AD, Turner RG (1971) Heavy metal tolerance in plants. Adv Ecol Res 7:1–85
Baker AJM (1981) Accumulators and excluders -strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1(2):81–126
Baker AJM, Ernst WH, van der Ent A, Malaisse F, Ginocchio R (2010) Metallophytes: the unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. In: Batty LC, Hallberg KB (eds) Ecology of industrial pollution. Cambridge University, Cambridge, pp 7–40
Becquer T, Bourdon E, Pétard J (1995) Disponibilité du nickel le long d'une toposéquence de sols développés sur roches ultramafiques de Nouvelle-Calédonie. C R Acad Sci 321(7):585–592
Betts P, Giles D, Mark G, Lister G, Goleby B, Ailleres L (2006) Synthesis of the proterozoic evolution of the Mt Isa Inlier. Aust J Earth Sci 53(1):187–211
Blake D (1987) Geology of the Mt Isa Inlier and environs. Bur Min Res Bull 225
Blissett AH (1966) Copper tolerant plants from the Ukaparinga copper mine, Williamstown. Q Geol Notes Geol Surv S Aust 18:1–3
BOM (2020) Monthly Mean Maximum Temperatures- Cloncurry Airport. https://www.bom.gov.au/jsp/ncc/cdio/wData/wdata?p_nccObsCode=36&p_display_type=dataFile&p_stn_num=029141. Accessed 24 Mar 2020
Boyd RS, Martens SN (1998) Nickel hyperaccumulation by Thlaspi montanum var. montanum (Brassicaceae): a constitutive trait. Am J Bot 85(2):259–265
Brooks RR (1972) Geobotany and biogeochemistry in mineral exploration.Croneis Series, Volume 1 of Harper's geoscience series, Harper & Row, The University of Michigan, p 290
Brooks RR, Radford C (1978) An evaluation of background and anomalous copper and zinc concentrations in the ‘copper plant’ Polycarpaea spirostylis and other Australian species of the genus. Proc Australas Inst Min Metall 268:33–37
Brooks RR, Reeves RD, Morrison RS, Malaisse F (1980) Hyperaccumulation of copper and cobalt – a review. Bull Soc R Bot 113:166–172
Brooks RR, Malaisse F, Empain A (1985) The heavy metal tolerant flora of Southcentral 595 Africa: a multidisciplinary approach. A.A. Balkema, Rotterdam
Brooks RR, Naidu SM, Malaisse F, Lee J (1986) The elemental content of metallophytes from the copper/cobalt deposits of Central Africa. Bull Soc R Bot 119:179–191
Cole MM (1980) Geobotanical expression of orebodies. Trans Inst Min Metall Sect B 89:B73–B91
Cole MM, Smith RF (1984) Vegetation as indicator of environmental pollution. Trans Inst Br Geogr 9:477–493
Cole M, Provan D, Tooms J (1968) Geobotany, biogeochemistry and geochemistry in the Bulman-Waimuna springs area, Northern territory, Australia. Trans Inst Min Metall Sect B Appl Earth Sci 77:81–104
Ernst WHO (1974) Heavy metal vegetation of the world (in German). Geobotanica Selecta, Band V. Gustav Fischer Verlag, Stuttgart
Ernst WH (2006) Evolution of metal tolerance in higher plants. For Snow Landsc Res 80(3):251–274
Erskine P, Van der Ent A, Fletcher A (2012) Sustaining metal-loving plants in mining regions. Science 337(6099):1172–1173
Esri (2020) “Imagery” [basemap]. Scale Not Given. “World Imagery Map”. Accessed 18 Mar 2020
Faucon MP, Meersseman A, Shutcha MN, Mahy G, Ngongo LM, Malaisse F, Meerts P (2010) Copper endemism in the Congolese flora: a database of copper affinity and conservational value of cuprophytes. Plant Ecol Evol 143:5–18
Faucon MP, Le Stradic S, Boisson S, wa Ilunga EI, Séleck M, Lange B, Guillaume D, Shutcha MN, Pourret O, Meerts P, Mahy G (2016) Implication of plant-soil relationships for conservation and restoration of copper-cobalt ecosystems. Plant Soil 403(1–2):153–165
Foy CD, Chaney RT, White MC (1978) The physiology of metal toxicity in plants. Ann Rev Plant Physiol 29(1):511–566
Grooves RW, Stevenson BG, Stevenson EA, Taylor RG (1972) Geochemical and geobotanical studies in the Emuford district of the Herberton tin field, North Queensland, Australia. Trans Inst Min Metall Sec B Appl Earth Sci 81:127–137
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Krämer U, Clemens S (2006) Functions and homeostasis of zinc, copper, and nickel in plants. Molecular biology of metal homeostasis and detoxification from microbes to man. Springer, Berlin, pp 214–272
Kukier U, Chaney RL (2001) Amelioration of nickel phytotoxicity in muck and mineral soils. J Environ Qual 30(6):1949–1960
Lambinon J, Auquier P (1963) Flora and vegetation of calaminarian soils in the northern Walloon region and the western Rhineland. Chorological types and ecological groups (in French). NaturaMosana 16:113–130
Lange B, van der Ent A, Baker AJM, Echevarria G, Mahy G, Malaisse F, Meerts P, Pourret O, Verbruggen N, Faucon MP (2017) Copper and cobalt accumulation in plants: a critical assessment of the current state of knowledge. New Phytol 213:537–551
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42(3):421–428
Lottermoser BG, Ashley PM, Munksgaard NC (2008) Biogeochemistry of Pb–Zn gossans, northwest Queensland, Australia: implications for mineral exploration and mine site rehabilitation. Appl Geochem 23(4):723–742
MacNair MR, Christie P (1983) Reproductive isolation as a pleiotropic effect of copper tolerance in Mimulus guttatus? Heredity 50(3):295–302
MacNair MR, Smith SE, Cumbes QJ (1993) Heritability and distribution of variation in degree of copper tolerance in Mimulus guttatus at Copperopolis, California. Heredity 71(5):445–455
Malaisse F, Grégoire J, Morrison RS, Brooks RR, Reeves RD (1979) Copper and cobalt in vegetation of Fungurume, Shaba Province, Zaïre. Oikos 1:472–478
Malaisse F, Schaijes M, D’Outreligne C (2006) Copper/cobalt flora of upper Katanga copperbelt. Field Guide, Les Presses agronomiques de Gembloux
Mogopodi D, Mosetlha K, Torto N, Wibetoe G (2008) Accumulation patterns of Cu and Ni for Indigofera melanadenia and Tephrosia longipes plant species growing in Cu–Ni mining area in Botswana. J Geochem Explor 97(1):21–28
Morrison RS, Brooks RR, Reeves RD, Malaisse F (1979) Copper and cobalt uptake by 592 metallophytes from Zaire. Plant Soil 53:535–539
Nicolls OW, Provan DMJ, Cole MM, Tooms JS (1965) Geobotany and geochemistry in mineral exploration in the Dugald River area, Cloncurry district Australia. Trans Inst Min Metall Sect B Appl Earth Sci 74:695–799
QLD GOV (2020) Queensland Globe. Detailed Surface Geology [layer]. https://qldglobe.information.qld.gov.au. Accessed 18 Mar 2020
Rajakaruna N, Bohm BA (2002) Serpentine and its vegetation: a preliminary study from Sri Lanka. J Appl Bot 76:20–28
Reeves RD, Kruckeberg AR (2018) Re-examination of the elemental composition of some Caryophyllaceae on North American ultramafic soils. Ecol Res 33(4):715–722
Reeves RD, Baker AJ, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018a) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol 218(2):407–411
Reeves RD, van der Ent A, Baker AJM (2018b) Global distribution and ecology of hyperaccumulator plants. Agromining: farming for metals. Springer, Cham, pp 75–92
Rout GR, Das P (2009) Effect of metal toxicity on plant growth and metabolism: I. Zinc. Sustainable agriculture. Springer, Dordrecht, pp 873–884
Saad L, Parmentier I, Colinet G, Malaiss F, Faucon MP, Meerts P, Mahy G (2012) Investigating the vegetation–soil relationships on the copper–cobalt rock outcrops of Katanga (DR Congo), an essential step in a biodiversity conservation plan. Restor Ecol 20(3):405–415
Salama W, Gazley MF, Bonnett LC (2016) Geochemical exploration for supergene copper oxide deposits, Mount Isa Inlier, NW Queensland, Australia. J Geochem Explor 168:72–102
Schat H, Kuiper E, Ten Bookum WM, Vooijs R (1993) A general model for the genetic control of copper tolerance in Silene vulgaris: evidence from crosses between plants from different tolerant populations. Heredity 70(2):142–147
Shaw AJ (1990) Heavy metal tolerance in plants: evolutionary aspects. CRC Press, Boca Raton
Shirong T, Changyong H, Zuxiang Z (1997) Commelina communis L.: Copper hyperaccumulator found in Anhui Province of China. Pedosphere 7(3):207–210
van der Ent A, Reeves RD (2015) Foliar metal accumulation in plants from copper-rich ultramafic outcrops: case studies from Malaysia and Brazil. Plant Soil 389:401–418
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362(1–2):319–334
van der Ent A, Malaisse F, Erskine PD, Mesjasz-Przybyłowicz J, Przybyłowicz WJ, Barnabas AD, Sośnicka M, Harris HH (2019) Abnormal concentrations of Cu-Co in Haumaniastrum katangense, Haumaniastrum robertii and Aeolanthus biformifolius: contamination or hyperaccumulation? Metallomics 11(3):586–596
Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, Paton A, Smith JAC, Angle JS, Chaney RL, Ginocchio R (2004) Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor Ecol 12(1):106–116
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Acknowledgements
Roger Tang is the recipient of a UQ Graduate School Scholarship (UQGSS) from The University of Queensland. The authors would like to thank Mount Isa Mines Resource Development, Trevor Shaw, Mark Jobling and Gavin Maher for assistance with field work and logistics. Copper Mountain Mining Corporation (formerly Altona Mining Limited) and George Ross are thanked for their assistance with site access.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marko Rohlfs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, R.H., Erskine, P.D., Lilly, R. et al. The biogeochemistry of copper metallophytes in the Roseby Corridor (North-West Queensland, Australia). Chemoecology 31, 19–30 (2021). https://doi.org/10.1007/s00049-020-00325-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00325-1