Abstract
The concentrations, composition, sources, and risks of polycyclic aromatic hydrocarbons (PAHs) in topsoil and surface water of the Liuxi River basin, south China were analyzed in this study. The total concentrations of 16 PAHs ranged from 296.26 to 888.14 ng/g in topsoil and from 156.73 to 422.03 ng/L in surface water, indicating mild pollution. The PAHs in topsoil exhibited an even spatial distribution, suggesting that they originated primarily from dry and wet deposition of transported pollutants. The concentration of PAHs in surface water did not differ significantly geographically, but the concentrations of total, three-, and four-ring PAHs were significantly lower in the Liuxi River than in its tributaries. Three- and two-ring PAHs predominated in topsoil and surface water, respectively. A correlation analysis suggested that the total organic carbon content and pH exerted a negligible effect on the spatial distribution of PAHs in topsoil, and they may have common sources. Fossil fuel combustion (particularly vehicle emissions) and coking production were the dominant sources of PAHs in topsoil, whereas those in surface water were derived from a variety of sources. The total toxic equivalent concentrations of 16 PAHs in topsoil ranged from 3.73 to 105.66 ng/g (mean, 30.93 ng/g), suggesting that exposure to the basin’s topsoil does not pose a risk to the environment or public health according to the Canadian soil quality guidelines. A risk assessment revealed that the total PAH concentrations in surface water posed a low ecological risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polycyclic aromatic hydrocarbons (PAHs), which have at least two fused aromatic rings, are ubiquitous in the environment. PAHs are typically generated by anthropogenic activities, such as the incomplete combustion or pyrolysis of fossil fuel and biomass, vehicle emissions, and petroleum leakage (Gan et al. 2009; Yu et al. 2016; Zhang et al. 2017a), and are introduced into the environment by processes, including atmospheric deposition, wastewater discharge, and surface runoff (Hu et al. 2017a; Johnsen et al. 2005; Pheiffer et al. 2018). Due to their distinct physicochemical properties, PAHs interact to different extents with air, water, soil, sediment, and biota (Fan et al. 2019; Montuori et al. 2016; Pérez-Fernández et al. 2015). Low-molecular-weight (LMW; three or fewer rings) PAHs typically predominate in water (Zeng et al. 2018), whereas high-molecular-weight (HMW; 4 or more rings) PAHs tend to be absorbed in soil phase for a longer period of time (Chen et al. 2018; Yang et al. 2017). PAHs exerts serious harmful effects on ecosystems and public health due to their toxicity (carcinogenic, teratogenic, and mutagenic), persistence, long-distance transport, lipophilicity, and capacity for bioaccumulation (Hu et al. 2017b; Kim et al. 2013; Sarria-Villa et al. 2016; Gope et al. 2018). Thus, the United States Environmental Protection Agency (USEPA) lists 16 PAHs as priority pollutants, 7 of which are designated as potential human carcinogens (IARC 2010; Zheng et al. 2016).
The environmental fate and behavior of PAHs are of great concern. Persistent organic pollutants, including PAHs, are detected worldwide in areas of high human activity up to the remote polar regions (Doong and Lin 2004; Wania and Mackay, 1993). Therefore, PAH pollution has become a global issue. Soil is the major terrestrial sink for PAHs (Chen et al. 2004; Wang et al. 2017; Yang et al. 2012). Wild and Jones (1995) estimated that approximately 90% of environmental PAHs was stored in soil in the United Kingdom. Peng et al. (2013) reported that the concentrations of PAHs in soil were significantly correlated with the level of urbanization. Zhang and Chen (2017) analyzed > 6000 topsoil samples from China and found that the PAH concentrations in soil decreased along the urban–suburban–rural gradient. Moreover, the soil physicochemical properties also affect the distribution of PAHs (Heywood et al. 2006; Li et al. 2010; Bucheli et al. 2004). PAHs in soil can be diverted into water by flow scouring. PAHs are not stored in water but can directly enter and accumulate within aquatic organisms, particularly when solubilized (Countway et al. 2003; Zhang et al. 2017b). Humans tend to be indirectly exposed to PAHs in aquatic ecosystems upon ingesting aquatic food, which may represent health risk (Zeng et al. 2018).
Guangzhou is one of the most developed cities in China; it has undergone rapid economic development and urbanization over the last several decades, resulting in increased discharge of organic contaminants and deterioration of environment quality. The majority of the local water resources are polluted, and thus unsuitable for drinking, resulting in an increasingly serious scarcity of clean water (Peng et al. 2017); this has constrained the economic development of Guangzhou. As the longest river in Guangzhou (171 km), the Liuxi River is of great importance to the city and in fact is regarded as the “mother river” of Guangzhou. The Liuxi River basin is the major source of drinking water within the administrative area of Guangzhou and so plays an important role in the exploitation and utilization of water resources in the city. However, contaminants originating from local human activity, and from atmospheric deposition (both dry and wet), have exerted an adverse effect on the environment in the basin. According to monitoring data, nitrogen and phosphorus pollution have resulted in class III–V water quality in the lower reaches of the Liuxi River (GZEMC 2012). To date, few studies have investigated PAHs in the Liuxi River basin. Therefore, information on the species and concentrations of PAHs in the area would facilitate the prevention and control of pollution.
In this study, the main objectives are to (1) evaluate the spatial distribution and composition of PAHs in topsoil and surface water of the Liuxi River basin, (2) identify potential sources of PAHs, and (3) assess the risks posed by PAHs in topsoil and surface water to the environment and public health.
Materials and Methods
Study Area
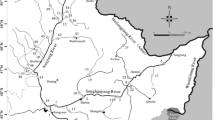
The Liuxi River basin (23°12′30″–23°57′36″N, 113°10′12–114°2′00″E) is located in the north of Guangzhou, the capital city of Guangdong Province, China (Fig. 1) and has a total area of approximately 2300 km2. The basin is elongated from northeast to southwest and has a high elevation in the northeast and low elevation in the southwest. Accordingly, the Liuxi River flows from northeast to southwest. The upper reaches of the basin are mountainous and dominated by forest; the middle reaches are hilly and encompass orchards, forests, croplands, and grasslands; and the lower reaches are plains (mainly built-up land and cropland). The area has a southern subtropical monsoon climate. The mean annual air temperature is 21.4–21.8 °C, with average maximum and minimum temperatures of 38.7 °C in July and −7 °C in January, respectively. The mean annual precipitation is 1800 mm, 81.3% of which falls between April and September. The basin spans four county-level districts (Conghua, Huadu, Huangpu, and Baiyun), with a total residential population of approximately 5.0 million.
Collection and Preparation of Samples
The levels of PAHs in topsoil and surface water may be higher between April and September due to the impact of wet deposition in the study area. Also, the month of April, as the beginning of the flood season, the dilution effect of precipitation on PAHs may be not significant. Thus, a total of 24 topsoil samples (0–20 cm) were collected using precleaned stainless-steel augers in April 2018: six from rural areas (S1–S6); ten from suburban areas (S7–S16); and eight from urban areas (S17–S24) (Fig. 1). Of the 24 samples, 16 were from agricultural land (9 from cropland and 7 from orchards) and 8 were from green spaces (4 each from forests and grasslands). At each site, five subsamples (4 corners and the center) were collected from within a 100 m2 area (10 × 10 m) and mixed thoroughly to generate a composite sample (Jia et al. 2017; Tong et al. 2018). The positions of the sampling sites were recorded using a global positioning system (GPS). The samples were placed in plastic automatic-sealing bags and immediately transported to the laboratory. The topsoil samples were lyophilized at −80 °C, passed through a 60-mesh sieve after removing stones, twigs, and other materials, and stored in dark glass bottles at −4 °C until analysis.
A total of 24 surface water samples were taken from the top layer (0–20 cm) of the waterbody in April 2018. The sampling locations were distributed along the Liuxi River (13 sites, L1–L13) and its main tributaries (11 sites, T1–T11) (Fig. 1), which were located using GPS. The surface water samples were collected into individual 2-L precleaned brown glass bottles with PTFE screw caps. After collection, the samples were immediately transported to the laboratory and stored in desiccators at 4 °C before analysis.
Reagents
The standards of the following 16 PAHs (US EPA priority control): naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chry), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (InP), dibenz[a,h]anthracene (DaA), and benzo[g,h,i]perylene (BgP), and five deuterated PAH standards (D8-naphthalene, D10-acenaphthene, D10-phenanthrene, D12-chrysene, and D12-perylene) were used, which were purchased from o2si Smart Solutions (Charleston, SC). The internal standard (hexamethylbenzene) was obtained from Aldrich Chemical (Gillingham, Dorset, UK). The organic solvents used for extraction and purification were purchased from CNW Technologies (Düsseldorf, Germany) and were of pesticide-grade purity. Anhydrous Na2SO4 (Guangzhou Chemicals Inc., Guangzhou, China) was heated at 500 °C for 5 h and stored in a sealed container. Oxides were removed from copper powder using 10% dilute hydrochloric acid, which was then washed using MeOH and n-hexane.
PAH Extraction and Analysis
The methods for topsoil sample extraction and analysis were described previously (Ping et al. 2007; Rachwał et al. 2015). To extract PAHs, 5 g of freeze-dried soil, spiked with five deuterated PAH standards, was mixed with 5 g of anhydrous sodium sulfate and 2 g of copper powder, and extracted in 15 mL of dichloromethane (DCM) at 40 °C for 25 min in an ultrasonic shaker. The extracts ware centrifuged and harvested three times. The resulting three extracts were combined and concentrated to ~ 2 mL. Subsequently, 2 mL of n-hexane was added and the samples were concentrated to 2 mL. Next, the samples were cleaned by being passed over a silica column (CNW) and eluted using 30 mL of n-hexane/DCM (7/3, v/v). The eluent was concentrated to 0.2 mL and transferred to 2-mL capped Teflon-lined vials. Then, internal standards were added and the volume was made up to 1 mL using n-hexane before analysis by gas chromatography-mass spectrometry (GC–MS).
Water samples were filtered through a 0.45-μm organic membrane. To the filtered samples was added 20 mL of methanol, followed by spiking with five deuterated PAH standards. A solid-phase extraction system was used to extract water samples. MIP-PAHs cartridges (500 mg, 6 mL; CNW) were preconditioned with 6 mL of DCM, 6 mL of methanol, and 6 mL of ultrapure water. The water samples were passed through the cartridge at a flow rate of 5 mL/min and eluted using 12 mL of DCM. Next, water was removed from the eluent, which was concentrated to 0.2 mL and transferred to 2-mL, capped Teflon-lined vials. Next, the internal standards were added, and the volume was made up to 1 mL using n-hexane before analysis by GC–MS.
The concentrations of 16 PAHs were quantified using a model 7890 gas chromatograph with a 5975 mass-selective detector (MSD; Agilent Technologies, Santa Clara, CA) equipped with an HP-5MS capillary column (30 m × 0.32 mm × 0.25 μm) and helium carrier gas (1 mL/min). The GC oven temperature program was 50 °C for 1 min, followed by heating at 20 °C/min to 200 °C, and at 10 °C/min to 290 °C, which was maintained for 15 min. The injector temperature was 280 °C. Sample (1 µL) was injected in spitless mode. The MSD was operated in the electron impact mode at 70 eV; the ion source temperature was 300 °C and mass spectra were recorded in selected ion-monitoring mode. PAHs were identified based on their retention times and selection ions and quantified by using internal standards. The concentrations of PAHs were determined at the Instrumental Analysis and Research Center of Sun Yat-sen University.
Determination of Total Organic Carbon Content and pH
The total organic carbon (TOC) content of topsoil was determined using a TOC analyzer (Vario TOC; Elementar, Germany). Topsoil pH was measured in a 2.5: 1 (v/w) water: soil slurry using a Compact pH Meter (Horiba, Kyoto, Japan).
Quality Assurance and Control
A procedural blank, spiked blank, spiked samples, and sample duplicates were analyzed for quality assurance and control. Internal calibration based on a five-point calibration curve was used to quantify the concentrations of the 16 PAHs. Procedural blanks were analyzed after every five samples; no PAH was detected in any of the blanks. The average recovery of 15 standard PAHs was 75.6–93.7% (except for NaP; 57.3%) in spiked water blanks (n = 5) and 78.3–99.2% (except for NaP; 62.4%) in spiked soil blanks (n = 5). The mean recoveries of the five deuterated PAH standards were 58.1–91.1% and 64.9–96.3% in water and soil samples, respectively. The detection limits of the 16 PAHs were 0.15–0.5 ng/L and 0.20–1.2 ng/g in water and soil samples, respectively.
Data Analyses
The concentrations of PAHs were assessed by one-way analysis of variance (ANOVA) and the least-significant difference test; a value of P < 0.05 was taken to indicate statistical significance. Spearman’s correlation analysis was performed to assess the relationships between pairs of variables. Principal component analysis (PCA) was used to identify the major sources of PAHs. Statistical analysis was carried out using SPSS for Windows software (ver. 17.0; SPSS Inc., Chicago, IL) and figures were generated using Origin 9.0 (Origin Lab, Northampton, MA) and ArcGIS 10.1 (Environmental Systems Research Institute, Inc., Redlands, CA) software.
Results and Discussion
Concentrations of PAHs
The levels of 16 PAHs in topsoil and surface water of the Liuxi River basin are listed in Table 1. The detection rates of the 16 PAHs in topsoil were 100.00%, with the exception of BbF (95.83%), BkF (95.83%), DaA (91.67%), and BgP (95.83%). Therefore, PAHs were ubiquitous in topsoil in the study area. The coefficients of variation of BbF and BgP were 100.59% and 100.76%; those of the other 14 PAHs were < 100% (mean, 53.51%), indicating little variation in their concentrations in the topsoil samples. BbF, InP, and BgP were detected in 8.33% of the surface water samples, whereas DaA, BaP, BbF, Chry, and BaA were detected in 29.17–66.67%. The other eight PAHs were detected in all of the surface water samples.
The total concentration of the 16 PAHs (∑16PAH) in topsoil of the Liuxi River basin ranged from 296.26 to 888.14 ng/g, with a mean of 490.68 ng/g. The concentrations of the seven carcinogenic PAHs (∑7CarPAH) (BaA, Chry, BbF, BkF, BaP, InP, and DaA) ranged from 1.81 to 359.20 ng/g, with a mean of 92.05 ng/g (1.81–40.44% of the ∑16PAH concentration [mean, 18.76%]), indicating that ∑7CarPAH accounts for a small proportion of ∑16PAH. Compared with topsoil in other areas, the mean ∑16PAH concentration in topsoil of the Liuxi River basin was comparable to or higher than that in agricultural or rural topsoil in South Korea (236 ng/g; Nam et al. 2003), Norway (150 ng/g; Nam et al. 2008), and the Huanghuai plain (129.5 ng/g; Yang et al. 2012), Chongming Island (192.8 ng/g; Lu et al. 2012), Taizhou (466.5 ng/g; Tang et al. 2010), and Shangdong (407.4 ng/g; Chai et al. 2017), China. However, the ∑16PAH concentration was markedly lower than that in urban topsoil from London, United Kingdom (18,000 ng/g; Vane et al. 2014), Erbil city, Iraq (2296.1 ng/g; Amjadian et al. 2016), Dhanbad, India (3488 ng/g; Suman et al. 2016), and Ji’nan (23,250 ng/g; Dai et al. 2008), Shanghai (3290 ng/g; Jiang et al. 2009), Beijing (1228 ng/g; Peng et al. 2011), Chengdu (3234 ng/g; Xing et al. 2011), Nanjing (3330 ng/g; Wang et al. 2015), and Lanzhou (2360 ng/g; Jiang et al. 2016), China. Moreover, compared with the ∑16PAH concentrations in more than 6000 topsoil samples from China (Zhang and Chen 2017), those in topsoil were lower than the mean in China (730 ng/g) but higher than that in South China (349 ng/g). The levels of PAHs in soil are not regulated in China (Chen et al. 2018); therefore, to assay the level of contamination, the contamination classification proposed by Maliszewska-Kordybach (1996) was used. This showed that 87.50% of the topsoil samples were slightly contaminated (200–600 ng/g), 12.50% were contaminated (600–1000 ng/g); no samples were uncontaminated (< 200 ng/g) or heavily contaminated (> 1000 ng/g).
The ∑16PAH concentration in surface water ranged from 156.73 to 422.03 ng/L, with a mean of 268.83 ng/L, whereas the ∑7CarPAH concentrations ranged from 1.30 to 70.76 ng/L (not detected in L13), with a mean of 12.16 ng/L (0.55–18.98% of ∑16PAH [mean, 4.16%]). The ∑16PAH concentrations in surface water were compared to those in other areas worldwide, which vary considerably. The mean ∑16PAH concentration in this study was significantly lower than that in surface water from the Gomti River, India (10,330 ng/L; Malik et al. 2011), the Almendares River, Cuba (2512 ng/L; Santana et al. 2015), the Nile River, Egypt (1877.5 ng/L; Mohamed and Mohamed 2010), and the Humen River mouth (652.54 ng/L; Liu et al. 2014), Wuhan section of the Yangtze River (2095.2 ng/L; Feng et al. 2007), Lanzhou reach of the Yellow River (1375 ng/L; Zhao et al. 2015), Taizi River (1818 ng/L; Song et al. 2013), Daliao River estuary (748.76 ng/L; Zheng et al. 2016), and Songhua River (934.62 ng/L; Zhao et al. 2014b), China. In contrast, they were higher than those in the Tiber River and estuary, Italy (90.5 ng/L; Montuori et al. 2016), the Danube River, Hungary (122.6 ng/L, Nagy et al. 2014), and the Yellow River estuary (52.49 ng/L; Li and Li 2017) and rivers in Shanghai (112.92 ng/L; Liu et al. 2016), China. The PAH concentrations in surface water were therefore lower than most values reported previously.
Spatial Distribution of PAHs
The spatial distribution of PAHs in topsoil of the Liuxi River basin is presented in Fig. 2. The rural, suburban, and urban areas were selected, which approximately corresponded to the upper, middle, and lower reaches, respectively, of the river basin. The mean ∑16PAH concentration was 624.78, 427.80, and 468.71 ng/g in the topsoil of the rural, suburban, and urban areas, respectively. The concentrations of ∑16PAH or two- to six-ring PAHs did not significantly vary among the three zones (ANOVA, P > 0.05), indicating that PAHs in topsoil are not influenced by urban activities. To evaluate the effect of agricultural activities on the PAH concentrations in topsoil, the sampling sites were divided into agricultural land (vegetable fields and fruit gardens) and green space (grassland and woodland). No significant difference in the concentrations of ∑16PAH or two- to six-ring PAHs was found between the two land types (ANOVA, P > 0.05), indicating that the PAH concentrations in topsoil are little affected by agricultural activities: e.g., use of pesticides, chemical fertilizers, and membranes. Also, the 16 PAHs in topsoil were evenly distributed, suggesting that the PAHs in the Liuxi River basin are derived mainly from dry and wet deposition of transported pollutants. This result is in agreement with the circumstances in the study area. Because the basin is a crucial source of drinking water, there is at present no point source of pollution. Zhang and Chen (2017) found that the PAH concentrations in China decreased along the urban-suburban-rural gradient, and the mean ∑16PAH concentrations therein were 1243, 525, and 278 ng/g, respectively. Our results were not in agreement with previous reports of the distribution of PAHs in topsoil, possibly due to the absence of serious point sources of pollution in the Liuxi River basin.
Five- and six-ring PAHs were not detected in some surface water samples. The mean ∑16PAH concentration was 281.18, 278.97, and 253.52 ng/L in the surface water in rural, suburban, and urban areas, respectively (Fig. 3). The concentrations of ∑16 PAH and two- to four-ring PAHs in the surface water did not differ significantly among the three zones (ANOVA, P > 0.05), similar to the distribution of PAHs in topsoil. No significant difference in the concentration of two-ring PAHs was observed between the water of the Liuxi River and its tributaries (ANOVA, P > 0.05). The concentrations of three- and four-ring PAHs in the water of the tributaries were significantly higher than those in Liuxi River water (ANOVA, P < 0.05). The ∑16PAH concentration was higher in the water of the tributaries (ANOVA, P < 0.05). The differences in PAH concentrations between the water of tributaries and the main stream may be due to the physicochemical properties of HMW PAHs, which are preferentially adsorbed by particulates and sediments in aquatic systems, hampering their migration (Zhu et al. 2008; Adeniji et al. 2019). Thus, the concentrations of two-ring PAHs (lowest MW) were similar in surface water from the tributaries to the main stream of the Liuxi River, but the concentrations of three- and four-ring PAHs in the main stream were lower than those in the tributaries.
Mean concentrations of PAHs in surface water of the Liuxi River basin in different functional zones (a) and in surface water of the Liuxi River and its tributaries (b). (Same alphabet means no significant differences among concentrations of PAHs from different locations according to ANOVA test, P > 0.05)
Proportions of individual PAHs
In topsoil, three-ring PAHs accounted for 59.68% (39.39–78.94%) of the total amount of PAHs, followed by two-, four-, and five-ring PAHs (13.00% [5.88–22.49%], 10.51% [6.56–16.55%], and 10.43% [1.06–26.15%], respectively) (Fig. 4). Six-ring PAHs, which accounted for 4.61% (0.36–17.76%) of all PAHs, were the least prevalent. Regarding individual PAHs, Phe predominated (36.76% [25.23–47.52%]), followed by Nap (13.00% [5.88–22.49%]) and Ant (12.13% [7.73–16.45%]). Overall, compared with HMW (4 or more rings) PAHs (mean, 27.32%), the proportions of LMW (3 or fewer rings) PAHs (mean, 72.68%) in topsoil were higher. The contribution of LMW PAHs was similar to that in Xinzhou (60%, Zhao et al. 2014a), but different from that in Beijing (30–34%, Ma et al. 2005), Hangzhou (≤ 20%, Yu et al. 2014), and Nanjing (15%, Wang et al. 2015), China. LMW PAHs in the vapor phase can be transported long distances due to their high saturated vapor pressure. Correspondingly, HMW PAHs, particularly five-ring and six-ring PAHs, which have lower saturated vapor pressures, readily bind to particles and can be transported only a short distance (Zhang and Chen 2017). The PAHs in topsoil originate mainly from atmospheric deposition after distant transport, which explains the higher proportions of LMW compared with HMW PAHs.
An occupation analysis of PAHs in surface water demonstrated that the proportions of PAHs decreased in the following order: two rings (36.59–75.81%; mean, 55.07%), three rings (19.49–54.81%; mean, 35.31%), four rings (3.30–16.51%; mean, 6.62%), five rings (0–18.34%; mean, 2.75%), and six rings (0–3.42%; mean, 0.25%) (Fig. 5). Moreover, Nap predominated (55.07% [36.59–75.81%]), followed by Phe and Flu (19.26% [9.60–37.86%] and 9.21% [5.45–14.20%], respectively). Therefore, LMW PAHs accounted for ~ 90.38% (74.53–96.25%) of the 16 PAHs; this is consistent with the values reported for the Tiber River and estuary, Italy (Montuori et al. 2016), the Danube River and its tributaries, Hungary (Nagy et al. 2014), the Gomti River, India (Malik et al. 2011), the Chenab River, Pakistan (Farooq et al. 2011), the Almendares River, Cuba (Santana et al. 2015), and the Songhua River Basin (Hu et al. 2017a), Liaohe River Basin (Bai et al. 2014), Henan reach of the Yellow River (Sun et al. 2009), Songhua River (Zhao et al. 2014b), drinking water source in Henan Province (Ma et al. 2008), Hun River (Zhang et al. 2013), and Poyang Lake (Zhi et al. 2015), China, likely due to the higher vapor pressure and solubility in water of LMW compared to HMW PAHs (Lan et al. 2016).
Correlation Analysis
Generally, after PAHs enter the topsoil, a variety of factors modulate their sorption, sequestration, and level, among which soil organic matter (SOM) is one of the most important (Duan et al. 2015). The concentrations of PAHs are positively correlated with the TOC of soil (Tang et al. 2005; Jiang et al. 2011; Li et al. 2014; Jiang et al. 2016; Cai et al. 2017). Also, no correlation will be established until an equilibrium is reached by continuous input of fresh PAHs (Nam et al. 2008). In this study, the topsoil TOC ranged from 0.17% to 2.59% with a mean of 1.11%. The topsoil TOC was not correlated with the concentrations of PAHs or ∑16PAH (Table 2), suggesting that TOC is not an important determinant of the spatial distribution of PAHs in topsoil. An increase in the soil pH enhances the polarity of SOM, hampering adsorption of PAHs (Li et al. 2014). A decrease in soil pH influences microbial degradation and may affect the levels of PAHs (Bucheli et al. 2004). The concentrations of LMW PAHs are negatively correlated with the soil pH (Chai et al. 2017; Schlautman and Morgan 1993). In this study, topsoil pH ranged from 4.64 to 7.18, with a mean of 6.26, and was not significantly correlated with the concentrations of two- to six-ring PAHs or ∑16PAH; this indicated that pH did not influence the PAH distribution in topsoil.
The correlation coefficients of the PAHs in topsoil are listed in Table 2. The concentrations of three- to six-ring PAHs were strongly positively correlated. However, there was no correlation between the concentration of two-ring PAHs and those of other PAHs. The reduced concentration of two-ring PAHs in different topsoil sites over time was likely difference, because it is the most degradable, volatile, and soluble in water among the 16 PAHs. Therefore, the PAHs detected in topsoil could have common sources.
Identification of the Sources of PAHs
Because of their different physiochemical properties, the concentrations of PAHs change after emission, but the ratios of PAHs of similar molecular weight are relatively stable and can be used to identify sources thereof (Yunker et al. 1996; Ping et al. 2007; Lv et al. 2014). The Ant/(Ant + Phe) and Flu/(Flu + Pyr) concentration ratios are used to distinguish combustion and petroleum sources (Montuori et al. 2016; Yunker et al. 2002). An Ant/(Ant + Phe) ratio of < 0.1 indicates a petroleum source, whereas a ratio of > 0.1 indicates combustion. A Flu/(Flu + Pyr) ratio of < 0.4 is indicative of petroleum, a ratio of 0.4–0.5 implies petroleum combustion, and a ratio of > 0.5 indicates combustion of coal or biomass. The Ant/(Phe + Ant) ratios in 83.33% of the topsoil and surface water samples were > 0.1 (Fig. 6). In addition, the Fla/(Fla + Pyr) ratios in all of the topsoil samples, and all but four of the surface water samples, were > 0.5 (0.4–0.5 in four surface water samples). Therefore, combustion of coal and biomass was the major source of PAHs in topsoil, and emission from combustion and petroleum was the major source in surface water.
The BaA/(BaA + Chry) and BgP/(BgP + InP) ratios are used to identify the sources of PAHs in soil (Devi et al. 2016; Thiombane et al. 2018). A BaA/(BaA + Chry) ratios of < 0.2 indicate petroleum sources; ratios of 0.2–0.35 imply petroleum or combustion, and ratios of > 0.35 indicate combustion. An InP/(InP + BgP) ratio of < 0.2 is indicative of petroleum; ratios of > 0.5 indicate combustion of coal or biomass, and ratios of 0.2–0.5 are markers of petroleum combustion. The two topsoil samples had BaA/(BaA + Chry) ratios of 0.2–0.35, while those of the other topsoil samples were > 0.35. With a single exception, the InP/(InP + BgP) ratios of the other topsoil samples were > 0.5 (Fig. 6), suggesting that the major source of the PAHs was combustion of coal and biomass.
PCA, a type of multivariate analysis, reduces the number of input variables and extracts a small number of latent factors to analyze relationships among variables (Yuan et al. 2014). To explore the sources of PAHs in topsoil, PCA of the PAH concentrations was performed. PC1, PC2, and PC3 (eigenvalues > 1) were extracted by varimax normalized rotation and explained 91.26% of the total variance in the dataset (Table 3). PC1, which accounted for 45.02% of the total variance, was characterized by high loadings of five- and six-ring PAHs (InP, BbF, BkF, BaP, DaA, and BgP), and moderately high loadings of four-ring PAHs (BaA, Fla, Pyr, and Chry). Among these, InP, DaA, and BgP are associated with traffic emissions (Fraser et al. 1998; Devi et al. 2016; Valavanidis et al. 2006). BbF and BkF are mostly generated by combustion of fossil fuels (Kavouras et al. 2001; Rogge et al. 1993) and BaP by coal combustion and traffic emissions (Li et al. 2017; Wilckel 2000). BaA and Chry are produced during incomplete combustion of diesel and gasoline (Bi et al. 2016; Lee et al. 2005). Fla and Pyr are generally generated by coal combustion (Liu et al. 2018; Luo et al. 2016). Hence, the PAHs in PC1 mainly originated from fossil fuel combustion, particularly vehicular emissions. PC2 accounted for 25.69% of the total variance and had high loadings of Flu, Ace, Phe, and Ant, which are markers of coking sources (Jiang et al. 2016; Simcik et al. 1999; Yuan et al. 2014). PC3 contributed 20.55% of the total variance and had high loading of Acy and moderately high loadings of Chry, BaA, Nap, and Pyr. Acy is indicative of wood combustion (Khalili et al. 1995). Nap is related to incomplete combustion or petroleum sources (Chen et al. 2016; Simcik et al. 1999). Thus, PC3 was identified as a mixed combustion source.
Risk Assessment
The carcinogenicity of the PAHs relative to BaP was quantified by estimating the BaP-equivalent concentration (BaPeq). The toxic equivalency factors (TEFs) (Nisbet and LaGoy 1992) were used to estimate the BaPeq of PAHs in topsoil. BaPeq was calculated as BaPeq = Ci × TEFi, where Ci is the concentration of a certain PAH and TEFi is its TEF value. The BaPeqs of PAHs in the topsoil of the Liuxi River basin are shown in Table 4. The total BaPeq of the 16 PAHs was 3.73–105.66 ng/g, with a mean of 30.93 ng/g. The BaPeq value of the seven carcinogenic PAHs was 3.30–104.39 ng/g, with a mean of 30.22 ng/g (97.70% of the total), suggesting that these carcinogenic PAHs are the major contributors to the carcinogenic potential. Among the PAHs, BaP and DaA were the predominant contributors, accounting for 48.39% and 29.75% of the total BaPeq, respectively, followed by BbF (8.17%) and InP (6.88%). The Canadian Soil Quality Guidelines for the protection of the environment and human health suggest a safe BaPeq value of 600 ng/g in soil (CCME, 2010). In this study, the BaPeq value of all of the topsoil samples was < 600 ng/g, indicating that the topsoil does not pose a risk to the environment or public health.
Kalf et al. (1997) devised the risk quotient (RQ) to estimate the environmental risk posed by PAHs in aquatic ecosystems, as well as the negligible concentrations (NCs) and maximum permissible concentrations (MPCs) of PAHs. The risks posed by PAHs were quantified by calculating the RQs. The RQ(NCs) and RQ(MPCs) values were calculated as follows:
where CPAHs is the concentration of a certain PAH in surface water and CQV(NCs) and CQV(MPCs) are its NC and MPC, respectively. The RQ of Kalf et al. (1997) can be used to assess the ecological risks posed by only 10 individual PAHs; thus, to evaluate the ecological risk of the 16 individual PAHs listed by the USEPA, Cao et al. (2010) inferred the NCs and MPCs of Acy, Ace, Flu, Pyr, BbF, and DaA according to the TEFs (Nisbet and LaGoy 1992) reported previously (Wang et al. 2016; Yan et al. 2016; Zhang et al. 2017a). The quality values of the 16 individual PAHs in surface water are listed in Table 5.
The RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) values were defined as follows (Cao et al. 2010):
The RQ(NCs) and RQ(MPCs) of 16 PAHs with values of ≥ 1 were summed to calculate the RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) values of total PAHs. The ecosystem risk posed by the PAHs is shown in Table 6.
In surface water, the mean RQ(NCs) values of 13 PAHs were > 1.0 (except for Chry, BkF, and InP), and those of Nap, Ace, Flu, Phe, Ant, BaA, and BaP were > 10.0 (Table 5). Also, the RQ(MPCs) values of the PAHs were < 1.0, except for BaA in a water sample from T2 (RQ(MPCs) = 1.73), and the mean RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) values in surface water were 158.35 and 0.07, respectively. Therefore, PAHs in surface water of the Liuxi River basin posed a relatively low ecological risk.
Conclusions
The occurrence, sources, and risk levels of 16 PAHs in the topsoil and surface water of the Liuxi River basin were evaluated in this study. The ∑16PAH concentration was 296.26–888.14 ng/g in topsoil and 156.73–422.03 ng/L in surface water, indicating a low level of pollution. The seven carcinogenic PAHs comprised 1.81–40.44% of all PAHs in topsoil and 0.55–18.98% of all PAHs in surface water (not detected in L13). The PAH concentrations in topsoil did not significantly vary and were not influenced by urban or agricultural activities. The spatial distribution of PAHs in surface water was similar to that in topsoil, but the concentrations of three- and four-ring PAHs, and of ∑16PAH, differed significantly between the Liuxi River and its tributaries. LMW PAHs, particularly three-ring PAHs (Phe, Nap, and Ant) predominated in topsoil, whereas Nap, Phe, and Flu predominated in surface water. There was no significant relationship between PAHs and the TOC or pH of topsoil, but strong positive correlations were exhibited in three- to six-ring PAHs. Pyrogenic sources were the major sources of PAHs in topsoil, whereas PAHs in surface water originated from a variety of sources. The PAHs in topsoil were derived primarily from fossil fuel combustion (especially vehicular emissions) and coking. The total BaPeq of the 16 PAHs in topsoil was 3.73–105.66 (mean 30.93) ng/g, indicating a low carcinogenic risk. The RQs indicated that the total PAHs in surface water posed a low ecological risk, while some of the individual PAHs were associated with a moderate level of risk. Therefore, Some concerns and control measures should be conducted to the contamination sources of PAHs of the Liuxi River basin.
References
Adeniji AO et al (2019) Levels of polycyclic aromatic hydrocarbons in the water and sediment of buffalo river estuary, South Africa and their health risk assessment. Arch Environ Contam Toxicol 76:657–669
Amjadian K et al (2016) Heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) in soils of different land uses in Erbil metropolis, Kurdistan Region, Iraq. Environ Monitor Assess 188:605
Bai YW et al (2014) Occurrence, distribution, environmental risk assessment and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in water and sediments of the Liaohe River Basin, China. Bull Environ Contam Toxicol 93:744–751
Bi X et al (2016) Polycyclic aromatic hydrocarbons in soils from the Central-Himalaya region: distribution, sources, and risks to humans and wildlife. Sci Total Environ 556:12–22
Bucheli TD et al (2004) Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland. Chemosphere 56:1061–1076
Cai CY et al (2017) Spatial distribution, emission source and health risk of parent PAHs and derivatives in surface soils from the Yangtze River Delta, eastern China. Chemosphere 178:301–308
Cao ZG et al (2010) Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology 19:827–837
CCME (Canadian Council of Ministers of the Environment) (2010) Polycyclic aromatic hydrocarbons. Canadian soil quality guidelines for protection of environmental and human health. Canadian soil quality guidelines. http://ceqg-rcqe.ccme.ca/
Chai C et al (2017) Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in the soils of vegetable greenhouses in Shandong, China. Ecotoxicol Environ Saf 142:181–188
Chen B et al (2004) Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res 38:3558–3568
Chen M et al (2016) Polycyclic aromatic hydrocarbons in soils from Urumqi, China: distribution, source contributions, and potential health risks. Environ Monitor Assess 185:5639–5651
Chen YN et al (2018) Contamination and health risk assessment of PAHs in farmland soils of the Yinma River Basin, China. Ecotoxicol Environ Saf 156:383–390
Countway RE et al (2003) Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary. Org Geochem 34:209–224
Dai JL et al (2008) Distributions, sources and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in topsoil at Ji’nan city, China. Environ Monitor Assess 147:317–326
Devi NL et al (2016) Environmental carcinogenic polycyclic aromatic hydrocarbons in soil from Himalayas, India: implications for spatial distribution, sources apportionment and risk assessment. Chemosphere 144:493–502
Doong RA, Lin YT (2004) Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-Ping River, Taiwan. Water Res 38:1733–1744
Duan YH et al (2015) Characteristics of polycyclic aromatic hydrocarbons in agricultural soils at a typical coke production base in Shanxi, China. Chemosphere 127:64–69
Fan XL et al (2019) Atmospheric PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Guiyang City, Southwest China: concentration, seasonal variation, sources and health risk assessment. Arch Environ Contam Toxicol 76:102–113
Farooq S et al (2011) Occurrence, finger printing and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the Chenab River, Pakistan. J Environ Monitor 13:3207–3215
Feng CL et al (2007) Distribution and sources of polycyclic aromatic hydrocarbons in Wuhan section of the Yangtze River, China. Environ Monitor Assess 133:447–458
Fraser MP et al (1998) Air quality model evaluation data for organics. 5. C6–C22 nonpolar and semipolar aromatic compounds. Environ Sci Technol 32:1760–1770
Gan S et al (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172:532–549
Gope M et al (2018) Exposure and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the street dust of Asansol city, India. Sustain Cities Soc 38:616–626
GZEMC (Guangzhou Environmental Monitoring Center) (2012) Water Quality Assessment Report for the Liuxi River Watershed. GZEMC, Guangzhou (in Chinese)
Heywood E et al (2006) Factors influencing the national distribution of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in British soils. Environ Sci Technol 40:7629–7635
Hu J et al (2017a) Characteristics, source, and potential ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the Songhua River Basin, Northeast China. Environ Sci Pollut Res 24:17090–17102
Hu TP et al (2017b) Status, source and health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soil from the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Geochem Explor 172:20–28
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In: IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer, vol 92, p 1
Jia JP et al (2017) Characteristics, identification, and potential risk of polycyclic aromatic hydrocarbons in road dusts and agricultural soils from industrial sites in Shanghai, China. Environ Sci Pollut Res 24:605–615
Jiang YF et al (2009) Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 75:1112–1118
Jiang YF et al (2011) Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in agricultural soil of Shanghai, China. Environ Monitor Assess 183:139–150
Jiang Y et al (2016) Distribution, compositional pattern and sources of polycyclic aromatic hydrocarbons in urban soils of an industrial city, Lanzhou, China. Ecotoxicol Environ Saf 126:154–162
Johnsen AR et al (2005) Principles of microbial PAH-degradation in soil. Environ Pollut 133:71–84
Kalf DF et al (1997) Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol Environ Saf 36:89–97
Kavouras I et al (2001) Source apportionment of urban particulate aliphatic and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods. environmental science and technology. 35:2288-2294
Khalili NR et al (1995) PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ 29:533–542
Kim KH et al (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Lan JC et al (2016) Polycyclic aromatic hydrocarbon contamination in a highly vulnerable underground river system in Chongqing, Southwest China. J Geochem Explor 168:65–71
Lee RG et al (2005) Emission factors and importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the domestic burning of coal and wood in the UK. Environ Sci Technol 39:1436–1447
Li J, Li FD (2017) Polycyclic aromatic hydrocarbons in the Yellow River estuary: levels, sources and toxic potency assessment. Mar Pollut Bull 116:479–487
Li H et al (2010) Distribution of polycyclic aromatic hydrocarbons in different size fractions of soil from a coke oven plant and its relationship to organic carbon content. J Hazard Mater 176:729–734
Li JL et al (2014) The source apportionment of polycyclic aromatic hydrocarbons (PAHs) in the topsoil in Xiaodian sewage irrigation area, North of China. Ecotoxicology 23:1943–1950
Li J et al (2017) PAHs behavior in surface water and groundwater of the Yellow River estuary: evidence from isotopes and hydrochemistry. Chemosphere 178:143–153
Liu F et al (2014) Distribution and transportation of polycyclic aromatic hydrocarbons (PAHs) at the Humen river mouth in the Pearl River delta and their influencing factors. Mar Pollut Bull 84:401–410
Liu S et al (2016) Levels, sources and risk assessment of PAHs in multi-phases from urbanized river network system in Shanghai. Environ Pollut 219:555–567
Liu H et al (2018) Occurrence, characteristics and sources of polycyclic aromatic hydrocarbons in arable soils of Beijing, China. Ecotoxicol Environ Saf 159:120–126
Lu JG et al (2012) Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in agricultural soil of the Chongming Island in Shanghai. Environ Sci 33:4270–4275 (in Chinese with English abstract)
Luo W et al (2016) Identification of sources of polycyclic aromatic hydrocarbons based on concentrations in soils from two sides of the Himalayas between China and Nepal. Environ Pollut 212:424–432
Lv JP et al (2014) Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in surface water from Liaohe River Basin, northeast China. Environ Sci Pollut Res 21:7088–7096
Ma LL et al (2005) Polycyclic aromatic hydrocarbons in the surface soils from outskirts of Beijing, China. Chemosphere 58:1355–1363
Ma YG et al (2008) Distribution, sources, and potential risk of polycyclic aromatic hydrocarbons (PAHs) in drinking water resources from Henan Province in middle of China. Environ Monitor Assess 146:127–138
Malik A et al (2011) Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environ Monitor Assess 172:529–545
Maliszewska-Kordybach B (1996) Polycyclic aromatic hydrocarbons in agricultural soils in Poland: preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochem 11:121–127
Mohamed IB, Mohamed AE (2010) Distribution of polycyclic aromatic hydrocarbons in drinking water in Egypt. Desalination 251:34–40
Montuori P et al (2016) Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber River and estuary, Italy. Sci Total Environ 566–567:1254–1267
Nagy AS et al (2014) Occurrence and distribution of polycyclic aromatic hydrocarbons in surface water and sediments of the Danube River and its tributaries, Hungary. J Environ Sci Health Part A 49:1134–1141
Nam JJ et al (2003) Distribution of polycyclic aromatic hydrocarbons in agricultural soils in South Korea. Chemosphere 50:1281–1289
Nam JJ et al (2008) PAHs in background soils from Western Europe: influence of atmospheric deposition and soil organic matter. Chemosphere 70:1596–1602
Nisbet ICT, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Peng C et al (2011) Polycyclic aromatic hydrocarbons in urban soils of Beijing: status, sources, distribution and potential risk. Environ Pollut 159:802–808
Peng C et al (2013) Assessing the combined risks of PAHs and metals in urban soils by urbanization indicators. Environ Pollut 178:426–432
Peng FJ et al (2017) Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in China. Sci Total Environ 589:46–55
Pérez-Fernández B et al (2015) PAHs in the Ría de Arousa (NW Spain): a consideration of PAHs sources and abundance. Mar Pollut Bull 95:155–165
Pheiffer W et al (2018) Polycyclic aromatic hydrocarbons (PAHs) in sediments from a typical urban impacted river: application of a comprehensive risk assessment. Ecotoxicology 27:336–351
Ping LF et al (2007) Distribution of polycyclic aromatic hydrocarbons in thirty typical soil profiles in the Yangtze River Delta region, east China. Environ Pollut 147:358–365
Rachwał M et al (2015) Coke industry and steel metallurgy as the source of soil contamination by technogenic magnetic particles, heavy metals and polycyclic aromatic hydrocarbons. Chemosphere 138:863–873
Rogge WF et al (1993) Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ Sci Technol 27:636–651
Santana JL et al (2015) Occurrence and source appraisal of polycyclic aromatic hydrocarbons (PAHs) in surface waters of the Almendares River, Cuba. Arch Environ Contam Toxicol 69:143–152
Sarria-Villa R et al (2016) Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Sci Total Environ 540:455–465
Schlautman MA, Morgan JJ (1993) Effects of aqueous chemistry on the binding of PAHs by dissolved humic materials. Environ Sci Technol 27:961–969
Simcik MF et al (1999) Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos Environ 33:5071–5079
Song XY et al (2013) Distribution and sources of polycyclic aromatic hydrocarbons in the surface water of Taizi River, Northeast of China. Environ Monitor Assess 185:8375–8382
Suman S et al (2016) Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Sci Total Environ 545–546:353–360
Sun JH et al (2009) Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol Environ Saf 72:1614–1624
Tang L et al (2005) Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environ Int 31:822–828
Tang X et al (2010) Levels and distributions of polycyclic aromatic hydrocarbons in agricultural soils in an emerging e-waste recycling town in Taizhou area, China. J Environ Sci Health Part A 45:1076–1084
Thiombane M et al (2018) Source patterns and contamination level of polycyclic aromatic hydrocarbons (PAHs) in urban and rural areas of Southern Italian soils. Environ Geochem Health 41:507–528
Tong R et al (2018) Levels, sources and probabilistic health risks of polycyclic aromatic hydrocarbons in the agricultural soils from sites neighboring suburban industries in Shanghai. Sci Total Environ 616–617:1365–1373
Valavanidis A et al (2006) Characterization of atmospheric particulates, particle-bound transition metals and polycyclic aromatic hydrocarbons of urban air in the centre of Athens (Greece). Chemosphere 65:760–768
Vane CH et al (2014) Polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in urban soils of Greater London, UK. Appl Geochem 51:303–314
Wang CH et al (2015) Polycyclic aromatic hydrocarbons in soils from urban to rural areas in Nanjing: concentration, source, spatial distribution, and potential human health risk. Sci Total Environ 527–528:375–383
Wang CL et al (2016) Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in the water and suspended sediments from the middle and lower reaches of the Yangtze River, China. Environ Sci Pollut Res 23:17158–17170
Wang C et al (2017) Characteristics and source identification of polycyclic aromatic hydrocarbons (PAHs) in urban soils: a review. Pedosphere 27:17–26
Wania F, Mackay D (1993) Modelling the global distribution of toxaphene: a discussion of feasibility and desirability. Chemosphere 27:2079–2094
Wilckel W (2000) Polycyclic aromatic hydrocarbons (PAHs) in soil: a review. J Plant Nutr Soil Sci 163:229–248
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the United Kingdom environment: a preliminary source inventory and budget. Environ Pollut 88:91–108
Xing XL et al (2011) Spatial distribution and source diagnosis of polycyclic aromatic hydrocarbons in soils from Chengdu Economic Region, Sichuan Province, western China. J Geochem Explor 110:146–154
Yan JX et al (2016) Polycyclic aromatic hydrocarbons (PAHs) in water from three estuaries of China: distribution, seasonal variations and ecological risk assessment. Mar Pollut Bull 109:471–479
Yang B et al (2012) Risk assessment and sources of polycyclic aromatic hydrocarbons in agricultural soils of Huanghuai plain, China. Ecotoxicol Environ Saf 84:304–310
Yang JY et al (2017) Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. J Soils Sediments 17:1116–1125
Yu GG et al (2014) Polycyclic aromatic hydrocarbons in urban soils of Hangzhou: status, distribution, sources, and potential risk. Environ Monitor Assess 186:2775–2784
Yu WW et al (2016) Identifications and seasonal variations of sources of polycyclic aromatic hydrocarbons (PAHs) in the Yangtze River Estuary, China. Mar Pollut Bull 104:347–354
Yuan HG et al (2014) Distribution, sources and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in surface soils of the Yellow River Delta, China. Mar Pollut Bull 83:258–264
Yunker MB et al (1996) Polycyclic aromatic hydrocarbon composition and potential sources for sediment samples from the Beaufort and Barents Seas. Environ Sci Technol 30:1310–1320
Yunker MB et al (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochem 33:489–515
Zeng QF et al (2018) Distribution, fate and risk assessment of PAHs in water and sediments from an aquaculture- and shipping-impacted subtropical lake, China. Chemosphere 201:612–620
Zhang P, Chen Y (2017) Polycyclic aromatic hydrocarbons contamination in surface soil of China: a review. Sci Total Environ 605–606:1011–1020
Zhang HL et al (2013) Spatial distribution and seasonal variation of polycyclic aromatic hydrocarbons (PAHs) contaminations in surface water from the Hun River, Northeast China. Environ Monitor Assess 185:1451–1462
Zhang JM et al (2017a) Polycyclic aromatic hydrocarbons in the water-SPM-sediment system from the middle reaches of Huai River, China: distribution, partitioning, origin tracing and ecological risk assessment. Environ Pollut 230:61–71
Zhang D et al (2017b) Spatial-temporal and multi-media variations of polycyclic aromatic hydrocarbons in a highly urbanized river from South China. Sci Total Environ 581–582:621–628
Zhao L et al (2014a) Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicol Environ Saf 108:120–128
Zhao XS et al (2014b) Spatial distribution and temporal trends of polycyclic aromatic hydrocarbons (PAHs) in water and sediment from Songhua River, China. Environ Geochem Health 36:131–143
Zhao X et al (2015) Distribution of polycyclic aromatic hydrocarbons in surface water from the upper reach of the Yellow River, Northwestern China. Environ Sci Pollut Res 22:6950–6956
Zheng BH et al (2016) Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River estuary and the adjacent area, China. Chemosphere 149:91–100
Zhi H et al (2015) The fate of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in water from Poyang Lake, the largest freshwater lake in China. Chemosphere 119:1134–1140
Zhu LZ et al (2008) Distribution of polycyclic aromatic hydrocarbons in water, sediment and soil in drinking water resource of Zhejiang Province, China. J Hazard Mater 150:308–316
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41771027, 41701585, 41961144027, 41907179 and 41471020), the Fundamental Research Fund for the Central Universities of China (17lgpy40), the Natural Science Foundation of Guangdong, China (2017A030310309), Provincial Special Fund for Economic Development (Marine Economic Development) (GDME2018E005), and the Scientific and Technological Innovation Project of the Water Sciences Department of Guangdong Province (2018–2021) and CRRP2019-09MY-Onodera from APN. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/7053vP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xie, Z., Gao, L., Liang, Z. et al. Characteristics, Sources, and Risks of Polycyclic Aromatic Hydrocarbons in Topsoil and Surface Water from the Liuxi River Basin, South China. Arch Environ Contam Toxicol 78, 401–415 (2020). https://doi.org/10.1007/s00244-020-00711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00711-4