Abstract
Toxicity caused by exposure to pollutants from marine sediments is a consequence of the interaction between biota and xenobiotics most frequently released by anthropogenic activities. The present work intended to characterize the toxicity of natural sediments putatively impacted by distinct human activities, collected at several sites located in the south of the Gulf of Gabes, Zarzis area, Tunisia. The selected toxicity criteria were analysed following ecologically relevant test conditions. Organisms of the polychaete species Hediste diversicolor were chronically exposed (28 days) to the mentioned sediments. Toxicity endpoints were biomarkers involved in the toxic response to common anthropogenic chemicals, namely neurotoxic (acetylcholinesterase), anti-oxidant (catalase, glutathione peroxidase), metabolic (glutathione S-transferases) enzymatic activities, and oxidative damage (lipid peroxidation, TBARS assay). The chemical characterization of sediments showed that the samples collected from the site near an aquaculture facility were highly contaminated by heavy metals (Cd, Cu, Cr, Hg, Pb, and Zn) and polycyclic aromatic hydrocarbons (fluorene, phenanthrene, anthracene, fluoranthene and pyrene). H. diversicolor individuals exposed to the sediments from this specific site showed the highest values among all tested biomarkers, suggesting that these organisms were possibly under a pro-oxidative stress condition potentially promoted by anthropogenic pollution. Moreover, it was possible to conclude that individuals of the polychaete species H. diversicolor responded to the chronic exposure to potentially contaminated sediments from the southeast coast of Tunisia, eliciting adaptive responses of significant biological meaning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Environmental marine pollution is a major public and scientific concern. Severe environmental restrictions can be imposed to organisms living in marine areas, especially in terms of adaptation to extreme conditions and unpredictable biological and ecological consequences (Matthiessen and Law 2002; Amado et al. 2006). These assumptions are particularly important for coastal areas and highly impacted zones under the influence of distinct environmental stressors (Coll et al. 2010). The Mediterranean Sea, a semienclosed basin where waters are slowly renewed (15 years for deep waters) (Zaghden et al.2016), can be more impacted than larger oceans, being more vulnerable to environmental pollution pressures (Zaghden et al. 2014, 2016). In the past few decades, the progressive economic development along the Mediterranean coastline has resulted in substantial environmental changes (Barhoumi et al. 2014). The bioaccumulative chemicals resulting from various urbanization and industrialisation processes have been discharged into this Sea via coastal outfalls, rivers, and atmospheric deposition (Saliot 2004; Zaghden et al. 2014). Due to these growing anthropogenic activities, the Mediterranean coastal environments are increasingly exposed to various contaminants, particularly from the heavy metal pollution (Rabaoui et al. 2014; Akcil et al. 2015). Heavy metals are among the most common pollutants responsible for severe deterioration of the aquatic ecosystems (Ruilian et al. 2008). They can be found in urbanized and nonurbanized areas, as well in the water column and sediments where they persist, being ultimately absorbed by marine species (Rabaoui et al. 2014). If they exceed specific levels, they could be considered as a potential environmental threat due to their toxicity, persistence, and bioaccumulation profiles (Vieira et al. 2011; Wang et al. 2015). This specific class of pollutants is considered as a threat, not only to marine biodiversity, but also to humans, through the consumption of heavy metal contaminated seafood (Rabaoui et al. 2014). An additional important type of contamination in this area is the hydrocarbon release, including of polycyclic aromatic hydrocarbons (PAHs). PAHs concentrations in specific areas of the Mediterranean (namely, the Gulf of Gabes in Tunisia) indicate the presence of a hydrocarbon profile of petrogenic and pyrolytic origin. Anthropogenic hydrocarbon inputs are obvious at sites associated with industrial discharges, shipping activities, and sewage outfalls (Zaghden et al. 2014). This contamination profile is a direct consequence of the industrial activities established at Gabes city (Zaghden et al. 2014), mainly because of the chemical industries hereby (Gabes Ghannouch industrial complex) (Louati et al. 2001; Zaghden et al. 2005). Contamination in this area includes the release of untreated phosphogypsum wastes containing high heavy metal loads into the marine environment (Kharroubi et al. 2012; El Zrelli et al. 2015). However, some southern sites in Tunisia, namely Djerba Island and Zarzis in particular, were found to be as polluted as the site of Gabes, which is considered as the origin of pollution (Rabaoui et al. 2014). Taking into account the potential presence of such specific contaminants, it is possible to pinpoint heavy metals and PAHs in particular as potential threats to marine biodiversity due to their putative bioaccumulation trend in the tissues of marine species (Rabaoui et al. 2014).
To assess the degree and the putative consequences of aquatic pollution, the alteration in key biological responses (biomarkers) of exposed organisms has been widely used over the past decades in environmental analysis (Lionetto et al. 2012). Annelid species, such as Tubifex, have been successfully used in toxicological studies (Lucan-Bouché et al. 1999). Hediste diversicolor (common ragworm) has been extensively used in research and was recommended for sediment sublethal toxicity studies (Maranho et al. 2014). Among faunal organisms, this particular species is often chosen as a sediment quality bioindicator (Catalano et al. 2012). H. diversicolor is a widespread endo-benthic marine annelid species that occurs in brackish water environments, especially in muddy to sandy habitats (Bouraoui et al. 2009). It was found along the Tunisian coasts, and across all the Mediterranean Sea, but also in the North East and West of the Atlantic Ocean and in the North Sea (Cognetti and Maltagliati 2000). H. diversicolor individuals are tolerant to variations of environmental parameters, such as temperature and salinity. These characteristics make this a suitable species to be used in environmental studies under a broad sets of abiotic conditions (Ait Alla et al. 2006). This polychaete species is regarded as highly sensitive towards metals (Moreira et al. 2006; Kalman et al. 2009) and organic contamination (Bouraoui et al. 2009) due to its close interaction with the environment (Scaps 2002). Consequently, H. diversicolor has been recommended in environmental biomonitoring and ecosystem management programs given its responsiveness to pollutants (Durou et al. 2005) but also considering its robustness towards the influence of confounding factors (Kalman et al. 2010).
Considering the putative presence of heavy metals and PAHs, a chronic exposure to this combination of chemical classes is likely to result in oxidative stress, metabolic alterations, and changes in the cholinergic neurotransmission mechanisms (Oliva et al. 2012). Therefore, the present study assessed the environmental effects of contaminated sediments from southern Tunisian coast using a biomarker approach. Biomarkers associated with oxidative stress, metabolism, and lipid peroxidation (activities of glutathione-S-transferases: GSTs; glutathione peroxidase activity: GPx; activity of Catalase: CAT; levels of thiobarbituric acid reactive substances: TBARS) and neurotoxicity (activity of acetylcholinesterase: AChE) were evaluated in specimens tissues of the polychaete H. diversicolor chronically exposed to marine sediments originating in different locations of the Tunisian southern coastal region.
Materials and Methods
Chemicals
Reduced glutathione (GSH), glutathione reductase (EC 1.6.4.2), H2O2, and β-nicotinamide adenine dinucleotide phosphate (NADPH), as well as all other chemicals used in enzymatic assays (namely buffers) were acquired from Sigma Aldrich, Germany. Methanol and acetonitrile were obtained from Fluka (Seelze, Germany). The Bradford test reagent was purchased from Bio-Rad (Hercules, CA).
Sampling Sites
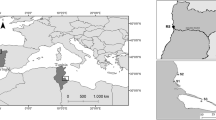
Sediments were collected at 5–10 cm of depth by a 0.1 m2 Van Veen grab sampler in three sites within the Zarzis area (Fig. 1a), located south of the Gulf of Gabes, Medenine governorate, Tunisia. Samples on the coast were raised using the quadrat method. Three samples were collected from each sampling site. An additional sample was collected to determine the composition of particle size of the sediment and to determine heavy metals and PAHs levels. The three sampling sites, which were 13–42 km away from each another, were defined taking into consideration already reported anthropogenic regional pollution profiles (Rabaoui et al. 2014). The first sampling site (S1), designated as “Elmaleha,” is on the coastal area of “Ras Lamsa,” near an untreated domestic wastewater discharge, close to the Zarzis saline, at the end of the tidal channel “Bahara Alouane,” a canal that connects the “Sabkha El Melah” to the Mediterranean (N 33°15′58.172″, E 11°17′28.485″). The second sampling site (S2) was an area in the vicinity of SEPAT Aquaculture (N 33°28′48.09″, E 11°7′47.877″). SEPAT (Société d’Exploitation et de la Promotion de l’Aquaculture en Tunisie) is considered as one of the five main private fish farms in Tunisia (Cherif et al. 2011), covering a 2-hectare area and producing intensively more than 200 tons/year of marine fish species, specifically Dicentrarchus labrax and Sparus aurata in cages (data from the Aquaculture Technical Center; CTA 2017). The third sampling site (S3) is situated next to the Elbibane lagoon entry (N 33°25′18.541″, E 11°5′54.959″), an area characterized by a high conservation value being rich in benthic habitats and dominated by meadows of Cymodocea nodosa, Posidonia oceanica, and Caulerpa prolifera (Pergent and Zaouali 1992). This lagoon hosts the longest algal reef of Neogoniolithon notarisii in the Mediterranean Sea (Jelassi et al. 2015). The sediment samples were manually collected with a grab, over a depth not exceeding 0.5 m (5–10 cm), from an unpolluted reference site, located in the Douro Estuary, Afurada, Gaia, Portugal (Mucha et al. 2010) to allow further comparisons. The here-adopted reference site (RS) is located in the Douro Estuary (Fig. 1b) in the São Paio bay (41°08′29.04″N 8°39′06.77″O), Afurada, Vila Nova de Gaia, Portugal. This area is characterized by a high energetic hydro-dynamism and was considered as a reference area with low levels of both organic matter and heavy metals (Mucha et al. 2010).
Sediment Geochemical Analysis
Sediment Properties
Before chemical characterization, the sediments from all sampling sites were air-dried, crushed, and passed through a 2-mm sieve. The sediment pH was determined by a glass membrane electrode using sediment/water suspensions (1/2.5, w/v) (Mseddi et al. 2016). Sediment organic matter content was obtained according to the Walkley–Black method (Walkley 1947) and was calculated as the weight loss percentage by ignition (4 h at 550 °C). Approximately 100 g of sediment from each sample was dried at 40 °C and sieved using AFNOR mesh-type sieves, varying from 2 mm to 63 microns (Shepard 1954).
Heavy Metal Analysis
The sediments were sieved through 2-mm nylon nets and their metal contents (Cd, Cr, Cu, Hg, Pb, and Zn) were measured following Reis et al. (2009) procedure. 300 mg of each sample were digested in a microwave system (model no. NE-1037, Panasonic) at high pressure using Parr reactor bombs (model no. 4782, Parr). The metals in digested solutions were determined using an atomic absorption spectrometer (AAS; SpectrAA 220 FS, Varian) fitted with a deuterium lamp for background correction, a flame atomization (Marck 7, Varian), an electrothermal atomization (Auto sampler 110, Varian), and cold vapour generation (VGA 77, Varian) depending on the metal levels in the sample and on the element to be analysed. External calibrations were performed with aqueous standards. Analytical accuracy was determined using a marine sediment standard reference material (NIST SRM 2702) with a value of recovery ranging from 100 to 121%. The precision of replicate analyses of individual elements ranged between 2 and 11% of the relative standard deviation (RSD). The limits of detection (LOD) were calculated from the individual calibration curves using the three sigma criteria and were measured in mg/kg (Table 2). According to the guidelines of the classification of environmental quality for coastal sediments, the reference site was considered as class I or insignificantly polluted (all metals are in background levels) (SFT 2007).
Hydrocarbon Analysis
Methanol and acetonitrile were used for the PAHs extraction from sediments following the methodology used by Gonçalves et al. (2016). Sixteen priority PAHs concentrations were quantified in sediment samples to identify the pollution sources. This analytical procedure was optimized and included an ultrasonic extraction with a solvent, clean-up, and preconcentration by solid-phase microextraction (SPME) followed by gas chromatography–mass spectrometry (GC–MS) analysis in selected ion storage (SIS) mode. Chromatographic analyses were performed in a Varian 3900 (Walnut Creek, USA) gas chromatograph. The GC was coupled with a Saturn 2000 ion trap mass spectrometer from Varian Instruments (Sunnyvale, CA). All samples were analysed in triplicate. For quality assurance, along with the samples, blanks were analysed after calibration and control standards to avoid transferring trace amounts of PAHs to samples. A control standard solution at 200 ng/L level (corresponding to a 50 g/k level in sediment matrix) and a spiked sample at the same level were analysed at roughly every ten samples interval. Results were corrected with the recoveries observed in samples spiked at 200 ng/L levels. During the method validation, the reference material IAEA 408 and TAQC proficiency test samples were analysed to check the method accuracy (1.2–9.5% RSD) and to determine the recoveries (86–106%). The limits of detection (LOD) were expressed in ng/g (Table 3). According to the international guidelines for classification of environmental quality of coastal sediments, the reference site was considered as being unpolluted or class I (SFT 2007).
Test Organisms: Collection, Quarantine, Maintenance, and Exposure
Individuals of H. diversicolor were manually collected from estuarine sediments in the Local Natural Reserve of Douro Estuary in the bay of São Paio, Vila Nova de Gaia, Portugal (41°08′29.04″N 8°39′06.77″O), adopted as a reference site in this study. Individuals with a length range of 6.0–8.0 cm and an average wet weight of 0.3 g were selected during the low tide period. Before the start of exposures, worms were subjected to an acclimation and quarantine period of 2 weeks, depurated, kept with filtered natural sea water with salinity adjusted to 25 (Moreira et al. 2005), and placed in natural sediment collected at the reference study site that was previously washed with distilled water and then air dried. A temperature of 20 °C has been chosen as the most adequate for the development of ecotoxicological testing with this species (Moreira et al. 2005). During this quarantine period, the H. diversicolor individuals were daily fed with commercial fish (ActivPet®) ad libitum. The animals were exposed to the different contaminated sediments for 28 days in 1.5-L plastic bottles with continuous aeration. The sediment samples were homogenized by removing large debris with a forceps. Twenty containers were used with three worms randomly positioned in each one respecting five replicates per treatment. The containers were left with approximately 500 g of sediment each, at a temperature of 20 °C, a salinity of 25 and a media change every 2 days. During exposures, no mortality was recorded in any of the exposed groups. By the end of the exposure, the organisms were recovered from the containers, counted, and transferred to Eppendorf microtubes, carefully rinsed with filtered sea water adjusted to the salinity of 25 and stored at − 80° C until further analysis.
Biochemical Analysis
All of the frozen organisms were thawed on ice and tissues were cut in small portions with scissors. A portion of approximately one-third was used for acetylcholinesterase (AChE) activity quantification, and the two remaining thirds were kept for oxidative stress and metabolism parameters determination. The samples were homogenized using ultrasounds (Branson S-250A) at 4 °C. For oxidative stress damage and metabolism parameters determinations (CAT, GSTs, T-GPx, Se-GPX, and TBARS), the samples were homogenized in a volume of 1 mL of 50 mM phosphate buffer pH = 7.0 with Triton X-100 0.1% (homogenization buffer). Then, the suspensions were centrifuged for 10 min at 15,000 g at 4 °C. However, for the determination of AChE activity, the homogenization buffer was 0.1 M phosphate pH = 7.2 and centrifugation was achieved at 3330 g for 3 min at 4 °C. All samples were centrifuged in a refrigerated centrifuge (Eppendorf 5810R) to obtain the supernatant fraction. The spectrophotometric readings were performed in a microplate reader Thermo Scientific; model Multiskan GO, with SkanIt Software 3.2.
CAT Activity Determination
CAT activity was determined by Aebi’s 1984 method by measuring the rate of enzymatic decomposition of H2O2 at a wavelength of 240 nm. The activities were expressed in terms of H2O2 micromoles consumed per min per mg protein (U mg−1 protein).
GPx Determination
Total (T-GPx) and selenium-dependent (Se-GPx) GPx activities were quantified respectively with cumene hydroperoxide and H2O2 as substrates. The activity was monitored by following the decrease in NADPH concentration (at λ = 340 nm) consumed during the generation of GSH from oxidized glutathione. Enzyme activity was calculated as nmol NADPH oxidized/min/mg protein using a molar extinction coefficient of 6.22 × 103 M−1 cm−1 (Livingstone et al. 1993).
GSTs Activity Determination
The GSTs determination was based on the conjugation reaction between 2,4-dinitrochlorobenzene (CDNB) and reduced glutathione (GSH), forming a thioether whose absorbance was monitored by following the increasing absorbance at λ = 340 nm (Habig et al. 1974). The GSTs activity was expressed in terms of total soluble protein present in the samples (activity was expressed in mmol min−1 mg−1 protein).
Levels of Thiobarbituric Acid Reactive Substances
Oxidative damage in lipid membranes was quantified by measuring thiobarbituric acid reactive substances (TBARS) levels by determining their absorbance at a wavelength of 535 nm (Buege and Aust 1978). The molar extinction coefficient (ε) used for the calculation was 1.56 × 106 M−1 cm−1. TBARS concentrations were expressed in terms of malondialdehyde (MDA) amount considering the samples total soluble protein (M mg−1 protein) (Rice-Evans et al. 1991).
AChE Activity Determination
The predominant cholinesterasic form in tissues of H. diversicolor is AChE (Scaps et al. 1996). The quantification method here-used to determine AChE activity was based on the determination of the acetylthiocholine degradation rate, by determining the formation of a yellow coloured compound (5-thio-2-nitrobenzoate) whose presence was quantified at λ = 412 nm (Ellman et al. 1961). AChE activities were expressed as nmole min−1 mg−1 of proteins.
Total Soluble Protein Quantification
The total amount of soluble protein was determined by the Bradford assay (Bradford 1976) by monitoring the samples absorbance at a wavelength of 595 nm. Protein standards were prepared using γ-globulin (1 mg mL−1).
Statistical Analysis
After testing for normality (Shapiro–Wilk test) and homogeneity of variances (Levene test), the biomarker data were compared using a one-way analysis of variance (ANOVA) followed by a Dunnett test if needed (p < 0.05), using the statistical package for social sciences software (SPSS). The principal component analysis (PCA) was applied to establish relationships among geochemical and abiotic parameters. The results were obtained using an R script (FactoMineR library). Data were presented as mean (± standard deviation), and the adopted level of significance (α) was 0.05.
Results
Sediment Properties and Hydrocarbon Contamination
The grain size analysis showed that superficial sediment of all areas consisted mainly of sand (between 63 μm and 2 mm), which widely ranged from 73 to 100%. The mud fraction (< 63 μm) was present mainly in the sediment collected near SEPAT aquaculture (Table 1). The coarser fraction was made up primarily of gravels and fragments of mollusks and gastropods and was found in a considerable proportion at the reference site (25%). The texture sediment characterization classified all the sediments as sand, according to Chassé and Glémarec (1976) (Table 1). The chemical analysis results are given in Table 2. The concentration of each heavy metal varied among the sampling sites. Heavy metals in S1, S2, and S3 sediments ranged from 18.20 to 32.10 mg/kg for Pb; 19.00–36.70 mg/kg for Zn; 5.80–16.40 mg/kg for Cr; 3.90–13.00 mg/kg for Cu; 0.29–0.84 mg/kg for Hg; and 0.83–2.90 mg/kg for Cd. All measured heavy metals were present at the reference site, except for Pb, for which levels were below the limit of detection. The highest Cd, Cu, Cr, Hg, Zn, and Pb concentrations were all found in the sediments collected near SEPAT Aquaculture (S2). Again, the sediments collected at S2 presented the highest PAHs concentrations. Phenanthrene, pyrene, anthracene, fluorine, and fluoanthene were detected in the sediments collected at the coastline of Zarzis. The values ranged from 14.4 to 18.0 ng/g for phenanthrene and reached 12 ng/g for pyrene (Table 3).
Biochemical Markers
The CAT activity results revealed significant differences among animals exposed to distinct treatments (one-way Anova: F = 17.028; d.f. = 3, 56; p < 0.05; Fig. 2a), being statistically higher in organisms exposed to the sediment collected near SEPAT Aquaculture (S2) comparatively to the reference site (RS) (Dunnett test, p < 0.05). Differences in the activities of T-GPx (Fig. 2b) and Se-GPx (Fig. 2c) were also noticed among others (one-way Anovas: F = 9.928; d.f. = 3, 56; p < 0.05 and F = 12.465; d.f. = 3, 56; p < 0.05, respectively) having higher activities in organisms exposed to the sediments collected at Elmaleha (S1) and SEPAT Aquaculture (S2) (Dunnett test, p < 0.05).
Biochemical markers (mean ± SD) according to the sampling sites. a Activity of the enzyme catalase (CAT). b Activity of the total GPx (T-GPx). c Activity of the selenium-dependent GPX (Se-GPx). d Activity of the isoenzymes GSTs. e TBARS concentrations. f Activity of the acetilcolinesterase (AChE). *Statistically significant differences compared with the reference sampling site (Dunnett test, p < 0.05)
The response in terms of GSTs activity observed in exposed organisms (Fig. 2d) showed significant differences among individuals exposed to the different sediments (one-way Anova: F = 5.024; d.f. = 3, 56; p < 0.05). Organisms exposed to the sediment collected near SEPAT Aquaculture (S2) exhibited significantly higher GSTs activity values compared with those exposed to the sediment from the reference site (RS) (Dunnett test, p < 0.05). The analysis of the TBARS levels revealed significant differences among the treatments (one-way Anova: F = 6.945; d.f. = 3, 56; p < 0.05; (Fig. 2e). Higher TBARS levels were observed in organisms exposed to the sediment collected at SEPAT Aquaculture (S2) (Dunnett test, p < 0.05). The AChE activity data is depicted in Fig. 2f. The organisms exposed to sediments from all sampling sites (S1, S2, S3) had significantly lower cholinesterasic values compared with those exposed to sediments from the reference site (RS) (one-way Anova: F = 46.978; d.f. = 3, 56; p < 0.05; Dunnett test: p < 0.05).
Relationships Among Biochemical, Geochemical, and Abiotic Parameters
A multivariate analysis was performed to compare the biomarker responses, metal levels, and abiotic parameters. According to PCA, two main components described 92.1% of the variation (Fig. 3). Each component is described according to the dominant group of variables. The first principal component accounting for 86.3% of the variances was associated with metal content as well as an increase in biomarker activities. Only pH was represented by the second component. Correlations were established to assess the relationships between metal content in sediments and biochemical responses in H. diversicolor. The results have shown that Cu, Cr, and Zn were strongly correlated to GSTs, Se-GPx, and T-GPx activities, respectively. The position of stations and variables in a biplot is presented in Fig. 4. The two stations (S1) “Elmaleha” and (S2) “SEPAT Aquaculture” differ from the other stations with their highest levels of heavy metals being associated to increased biological alterations.
Discussion
In the present study, the overall physical–chemical parameters were within the same range of values for all the three impacted sites. These values were similar to those reported in studies done in the Gulf of Gabes (Drira et al. 2014, 2016; Rekik et al. 2014). However, all measured heavy metals (Cd, Cu, Cr, Hg, Pb, and Zn) and some PAHs (phenanthrene, antracene, fluoranthene, and pyrene) were detected at higher levels in S2 compared with the other sites. It is thus possible to hypothesize that the major alterations found in exposed organisms might result from the presence of such amounts of these specific chemicals, whose toxicity is well known. Consequently, the bulk of this discussion will necessarily consider the differences in terms of metals and PAHS that were found in all analyzed sites.
The antioxidant cellular defence system plays a key role in protecting biological systems from ROS by regulating the production of free radicals and their metabolites (Deponte 2013).The primary antioxidant enzymes against superoxide radicals include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). These enzymes act together in the metabolism of ROS and altered activity of one enzyme without compensatory changes in others may lead to lipid peroxidation (Al-Gubory et al. 2012; Deponte 2013). CAT is a fundamental antioxidant enzyme that takes part in the defence against oxidative stress since it controls the amount of hydrogen peroxide (H2O2), resulting from normal and xenobiotic-induced metabolic processes (Ferreira et al. 2005). CAT activity was significantly higher in H. diversicolor individuals exposed to sediments from SEPAT Aquaculture sampling station (S2) being possibly interpreted as an adaptive response to an increase in ROS generated due to metal and PAHs exposure. As suggested by other studies, the trend of higher catalase activity might be related to the oxidant effects elicited by exposure to heavy metals, which can be of great concern in marine and coastal ecosystems (Ferreira et al. 2005; Nunes et al. 2016). An increase in CAT activity in the polychaete Laeonereis acuta exposed to high levels of Cu has already been reported (Geracitano et al. 2004; Hansen et al. 2007). Cd also can interfere with the antioxidant defence system of P. aibuhitensis (Yuan et al. 2010), because this activity decreased at an earlier exposure phase but increased at a later exposure time. Our study detected the presence of some of these metals, namely, Cu, Zn, and Cd, especially at the sampling site near SEPAT aquaculture facility, suggesting that the worms exposed to sediment from this location were under oxidative stress caused by the mentioned metals. However, the contribution of PAHs to the establishment of oxidative conditions cannot be discarded. A significant positive correlation of CAT activity with anthracene concentration in sediment was observed in the liver of the fish species Pomatoschistus microps (Vieira et al. 2008) and Solea senegalensis (Oliva et al. 2010) exposed to 2 mg/L of anthracene. H. diversicolor exposed to increasing sublethal concentrations of B[a]P also had presented higher concentrations of CAT (Catalano et al. 2012). These results are in line with ours; higher levels of fluorene, phenanthrene, and anthracene that were found in sediments from SEPAT Aquaculture (S2) site also may have contributed to higher levels of CAT. This can suggest that PAHs (and more specifically anthracene) may induce oxidative stress requiring the activation of catalase activity (Oliva et al. 2010).
GPx is the most important peroxidase enzyme involved in the detoxification of hydroperoxides and of hydrogen peroxide, overlapping catalase activity. This enzyme has been assumed to protect erythrocytes from damage by H2O2 and has been responsible for the reduction of lipid hydroperoxides (Janssens et al. 2000). Therefore, it is hypothesized that this enzyme may protect tissues against oxidative damage, such as lipid peroxidation of membranes (Oliva et al. 2010). It reduces the reactive lipid hydroperoxides to prevent malondialdehyde (MDA) formation (Flohe 1989). Decreased GPx activity can promote susceptibility to oxidative stress by allowing for the accumulation of harmful oxidants, whereas excess GPx activity may promote reductive stress, characterized by a lack of essential ROS needed for cellular signaling processes (Lubos et al. 2011). Both forms of glutathione peroxidase (T-GPx and Se-GPx) quantified in this study showed the same tendency of higher enzymatic levels being found in tissues of organisms exposed to sediments from S2 station. GSTs conjugates electrophilic metabolites with glutathione and so makes them less toxic and more easily excreted (Van der Oost et al. 2003) and are induced by organic contaminants as part of the phase II biotransformation pathway (Banni et al. 2011), whereas GSTs inhibition has been indicated as an unusual response to chemical challenge (Greco et al. 2010). The overexpression of GSTs can be related to an increase of metabolic capacity, namely by conjugation with intracellular cofactors, such as glutathione. This is a common response to exposure to electrophilic pollutants (Nunes et al. 2016). Metals may modulate GSTs activity, causing an increase in the activity of conjugation enzymes, such as GSTs. The increased GSTs activities were observed in rag worms of H. diversicolor collected from contaminated sites with metals, such as the Sado River estuary, Portugal (Moreira et al. 2006), the Seine River estuary, France (Durou et al. 2007), and Spain (Solé et al. 2009). Individuals of H. diversicolor exposed to sediments from SEPAT Aquaculture (S2) site, which are impacted by higher levels of PAHs (phenanthrene, pyrene, and anthracene), presented increases in GSTs activities. Several studies reported GSTs activities to be significantly increased after exposure to PAHs (Beyer et al. 1996; Van der Oost et al. 1996, 1998). Works by Banni et al. (2009a, b) showed as well that after being exposed to the polycyclic aromatic hydrocarbons benzo[a]pyrene, Sparus aurata individuals had higher levels of GSTs. The here-observed increase in GSTs activities may mean that these iso-enzymes were not only involved in the antioxidant response but also were able to act as detoxification isoenzymes.
Lipid peroxidation corresponds to the oxidation of polyunsaturated fatty acids, which occurs in the absence of an effective antioxidant defence system (Ghedira et al. 2011; Buffet et al. 2014). This phenomenon is frequently connected to the toxic effects of environmental pollutants (Hageman et al. 1992; Van Veld et al. 2018) and may be measured as the levels of thiobarbituric acid reactive substances (TBARS), which are frequently used as a biomarker of oxidative damage in response to different environmental pollutants (Romeo et al. 2000; Almroth et al. 2005). Increases in oxidative damage (lipid peroxidation) have been observed for both fish and invertebrates for single and mixed contaminants including heavy metals (Cu, Fe, and Cd) and PAHs (Livingstone 2001). Heavy metals may be involved in lipid peroxidation by various pathways; Cd can indirectly elevate the ROS generation by depleting glutathione and antioxidant enzymes, such as superoxide dismutase and catalase (Van Veld et al. 2018). Increased levels of lipid peroxidation products were observed in the liver of sea bass (Dicentrarchus labrax), and bream (Lepomis macrochirus) exposed to heavy metals (Choi and Oris 2000; Romeo et al. 2000). PAHs have also been reported to induce the development of reactive oxygen and consequently oxidative stress (Livingstone 2001; Sun and Zhou 2008). Higher TBARS levels reported for H. diversicolor exposed to contaminated sediments may indicate that the major antioxidant defences (e.g., the enhanced activities of CAT and GSTs) were not sufficiently effective to prevent the ROS deleterious effect on the lipid membranes as already reported in bivalve species, namely, Scrobicularia plana and Cerastoderma edule from an estuary subjected to large amounts of sewage discharges and industrial effluents, and Donax trunculus exposed to organophosphates and heavy metals (Bergayou et al. 2009; Tlili et al. 2010).
AChE plays an important role in neurotransmission in both invertebrates and vertebrates being responsible for the hydrolysis of acetylcholine into choline and acetic acid at the cholinergic synapses and neuromuscular junctions (Peña-Llopis et al. 2003). AChE is thus involved in nerve impulse termination, and its inhibition is an established biomarker of neurotoxicity caused by exposure to a wide range of contaminants other than pesticides, such as metals, treated effluents, and PAHs (Jebali et al. 2006; Vieira et al. 2008; Ghedira et al. 2009). Several studies showed that metallic contaminants generally inhibit AChE activity, including the fish species Seriola dumerilli exposed to Cd (Jebali et al. 2006), the flathead grey mullet (Mugil cephalus) after sublethal exposure of Cd, Cu, Pb, and Zn (Rajkumar 2011), and zebra fish (Danio rerio) after being exposed to Cu, Fe, Pb, and Cd (Lima et al. 2013). On the other hand, several other recent studies with invertebrates and fish reported an inhibition of this enzyme after exposure to fuel oil or PAHs. In fact, an AChE inhibition was observed in Mytilus galloprovincialis and in Mytilus edulis after an oil spill and in individuals of Nile tilapia (Lima et al. 2013). The here-obtained results in terms of AChE activity are in agreement with previous works and have shown an overall inhibition of this parameter in H. diversicolor individuals exposed to S1, S2, and S3 sediments comparatively to the control site. These sites, whose sediment exposure caused a significant AChE inhibition, are characterized by higher concentrations of both heavy metals and PAHs compared with the reference site. It is thus possible to associate an exposure to the predominant contaminants measured in our samples and the inhibition of the main cholinesterase form in our test species, which can be considered as a clear indication of neurotoxicity.
This methodology was a simplified approach to test the occurrence of biological effects caused by a combination of anthropogenic pollutants on a key species of the benthic communities of the marine environment. By adopting this strategy, it was possible to eliminate a considerable number of confounding factors that could occur if we just collected organisms from the analysed sites and quantified the biomarker response in such organisms. However, issues such as ecological representativity of the here-adopted test species may be considered. Among the few studies that focused on the Zarzis area, one can find the report of Rabaoui et al. (2014), who demonstrated the occurrence of contamination of this area by heavy metals but in mollusks. However, this study from the literature did not assess the profile contamination of our specific sampling sites and was not aimed at using biomarkers assays with polychaetes species. Bouraoui et al. (2010) presented the first report on the application of a classification scale based on biochemical markers on worms of the species H. diversicolor along the Mediterranean coasts, which was until now the only study set with this species in the Tunisian coast. His investigation included the assessment of the marine environment quality along six different sites from the north to the south of Tunisia (i.e., Bizerta Lagoon, Gargour, Nakta, Mahres, Skhira, and a reference site Teboulba) but without including the Zarzis area. The biomarker responses (glutathione S-transferases, acetylcholinesterase, and catalase activities) in this species H. diversicolor were evaluated, and significant differences were detected between contaminated sites compared with the reference samples (Bouraoui et al. 2010) in agreement with our study. All the later studies for biomarkers responses in the polychaeta H. diversicolor evaluated the effect of exposure of these organisms to different concentrations of heavy metals (Bouraoui et al. 2015, 2016) or PAHs levels (Banni et al. 2009a, b). Thus, monitoring pollution in Zarzis area using a scale of classification based on biochemical markers in sentinel organisms worms (e.g., of the species H. diversicolor, or other autochthonous polychaete species) may contribute for a better comprehension of the real toxicological risk of our investigated sites. Until today, the number of studies concerning this area of the Mediterranean Sea is still scarce and demands the adoption of new testing frameworks, encompassing the large biodiversity in the area.
Conclusions
Given the increasing number and diversity of pollutants dumped into the marine environment, especially in vulnerable areas, such as the Mediterranean Sea, the present study showed that the biomarker assessment in organisms exposed to sediments from putatively contaminated sites is an effective and complimentary method to predict detrimental effects and toxicity by presenting a potential alternative to the constraints of highly costly chemical measurements of water and sediment. Therefore, it is important to note that the responses of biochemical biomarkers depend on the analysed tissue and sampling sites, and on the need of a judicious selection of an adequate species to serve as a test organism. This work contributed to the first characterization of the environmental status of Zarzis area by evidencing the biological responses caused by potentially anthropogenic pollution near aquaculture centres and diffusely impacted sites in a highly representative marine polychaete species. The establishment of potential causal relationships between biomarkers and metals/PAHs studied has clearly been observed. These relationships were clearly more pronounced in the vicinity of an aquaculture facility. Hence, additional work is still needed to develop and validate new biomarkers in order to ensure a better understanding of the real toxicological risk of the investigated sites and for a long-term comprehension of putative effects at varied physiological levels that may signal population and ecological deleterious consequences.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ait Alla A, Mouneyrac C, Durou C, Moukrim A, Pellerin J (2006) Tolerance and biomarkers as useful tools for assessing environmental quality in the Oued Souss estuary (Bay of Agadir, Morocco). Comp Biochem Physiol Part C: Toxicol Pharmacol 143(1):23–29. https://doi.org/10.1016/j.cbpc.2005.11.015
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36. https://doi.org/10.1016/j.jclepro.2014.08.009
Al-Gubory KH, Garrel C, Faure P, Sugino N (2012) Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod Biomed Online 25(6):551–560. https://doi.org/10.1016/j.rbmo.2012.08.004
Almroth BC, Sturve J, Berglund Å, Förlin L (2005) Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol 73(2):171–180. https://doi.org/10.1016/j.aquatox.2005.03.007
Amado LL, Da Rosa CE, Leite AM, Moraes L, Vaz W, Lea GL, Martinez C, Geracitano LA (2006) Biomarkers in croakers Micropogonias furnieri (Teleostei: Sciaenidae) from polluted and non-polluted areas from the Patos Lagoon estuary (Southern Brazil): evidences of genotoxic and immunological effects. Mar Pollut Bull 52(2):199–206. https://doi.org/10.1016/j.marpolbul.2005.11.006
Banni M, Bouraoui Z, Ghedira J, Clerandeau C, Guerbej H, Narbonne JF, Boussetta H (2009a) Acute effects of benzo[a]pyrene on liver phase I and II enzymes, and DNA damage on sea bream Sparus aurata. Fish Physiol Biochem 35(2):293–299. https://doi.org/10.1007/s10695-008-9210-9
Banni M, Bouraoui Z, Clerandeau C, Narbonne JF, Boussetta H (2009b) Mixture toxicity assessment of cadmium and benzo [a] pyrene in the sea worm Hediste diversicolor. Chemosphere 77(7):902–906. https://doi.org/10.1016/j.chemosphere.2009.08.041
Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I (2011) Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals 24(6):981–992. https://doi.org/10.1007/s10534-011-9456-z
Barhoumi B, Clérandeau C, Gourves PY, Le Menach K, Megdiche Y, Peluhet L, Budzinski H, Baudrimont M, Driss MR, Cachot J (2014) Pollution biomonitoring in the Bizerte lagoon (Tunisia), using combined chemical and biomarker analyses in grass goby, Zosterisessor ophiocephalus (Teleostei, Gobiidae). Mar Environ Res 101(1):184–195. https://doi.org/10.1016/j.marenvres.2014.07.002
Bergayou H, Mouneyrac C, Pellerin J, Moukrim A (2009) Oxidative stress responses in bivalves (Scrobicularia plana, Cerastoderma edule) from the Oued Souss estuary (Morocco). Ecotoxicol Environ Saf 72(3):765–769. https://doi.org/10.1016/j.ecoenv.2008.09.012
Beyer J, Sandvik M, Hylland K, Fjeld E, Egaas E, Aas E, Skåre JU, Goksøyr A (1996) Contaminant accumulation and biomarker responses in flounder (Platichthys flesus L.) and Atlantic cod (Gadus morhua L.) exposed by caging to polluted sediments in Sørfjorden, Norway. Aquat Toxicol 36(1–2):75–98. https://doi.org/10.1016/S0166-445X(96)00798-9
Bouraoui Z, Banni M, Ghedira J, Clerandeau C, Narbonne JF, Boussetta H (2009) Evaluation of enzymatic biomarkers and lipoperoxidation level in Hediste diversicolor exposed to copper and benzo[a]pyrene. Ecotoxicol Environ Saf 72(7):1893–1898. https://doi.org/10.1016/j.ecoenv.2009.05.011
Bouraoui Z, Banni M, Chouba L, Ghedira J, Clerandeau C, Jebali J, Narbonne JF, Boussetta H (2010) Monitoring pollution in Tunisian coasts using a scale of classification based on biochemical markers in worms Nereis (Hediste) diversicolor. Environ Monit Assess 164(1–4):691–700. https://doi.org/10.1007/s10661-009-0921-x
Bouraoui Z, Ghedira J, Boussetta H (2015) Biomarkers responses in different body regions of the polychaeta Hediste diversicolor (Nereidae, Polychaete) exposed to copper. Rev Gestão Costeira Integr J Integr Coast Zone Manage 15(3). https://doi.org/10.5894/rgci594
Bouraoui Z, Ghedira J, Banni M, Boussetta H (2016) Acute effects of cadmium and copper on cytochemical responses in the polychaete Hediste diversicolor. Int J Environ Res 10(1):131–138. https://doi.org/10.22059/IJER.2016.56895
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Buffet PE, Poirier L, Zalouk-Vergnoux A, Lopes C, Amiard JC, Gaudin P, de Faverneye CR, Guibbolini M, Gilliland D, Ettajani HP, Valsami-Jones E (2014) Biochemical and behavioural responses of the marine polychaete Hediste diversicolor to cadmium sulfide quantum dots (CdS QDs): waterborne and dietary exposure. Chemosphere 100:63–70. https://doi.org/10.1016/j.chemosphere.2013.12.069
Catalano B, Moltedo G, Martuccio G, Gastaldi L, Virno-Lamberti C, Lauria A, Ausili A (2012) Can Hediste diversicolor (Nereidae, Polychaete) be considered a good candidate in evaluating PAH contamination? A multimarker approach. Chemosphere 86(9):875–882. https://doi.org/10.1016/j.chemosphere.2011.10.040
CTA, Centre Technique d’Aquaculture (2017) Guide des fermes aquacoles en Tunisie, Tunisie, 50
Chassé C, Glémarec M (1976) Principes généraux de la classification des fonds pour la cartographie biosédimentaire. J Rech Océanogr 1(3):1–18
Cherif N, Attia El Hili H, Mzoughi N, Chouba L, El Bourl M, El-Amri D, Hamza A, Maatoug K, Zaafrane S, Hammami S (2011) Tunisian aquaculture: present situation and potentialities. Fish Farms Manage Dis Control Environ 1(4):1–19
Choi J, Oris JT (2000) Evidence of oxidative stress in bluegill sunfish (Lepomis macrochirus) liver microsomes simultaneously exposed to solar ultraviolet radiation and anthracene. Environ Toxicol Chem 19(7):1795–1799. https://doi.org/10.1002/etc.5620190713
Cognetti G, Maltagliati F (2000) Biodiversity and adaptive mechanisms in brackish water fauna. Mar Pollut Bull 40(1):7–14. https://doi.org/10.1016/S0025-326X(99)00173-3
Coll M, Piroddi C, Steenbeek J, Kaschner K, Lasram FBR, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, Estrada M, Froglia C, Galil BS, Gasol JM, Gertwagen R, Gil J, Guilhaumon F, Kesner-Reyes k, Kitsos MS, Koukouras A, Lampadariou N, Laxamana E, López-Fé de la Cuadra CF, Lotze hk, Martin D, Mouillot D, Oro D, Raicevich S, Rius-Barile J, Saiz-Salinas JI, San Vicente C, Somot, S, Templado J, Turon X, Vafidis D, Villanueva R, Voultsiadou E (2010) The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5(8). https://doi.org/10.1371/journal.pone.0011842
de Lima D, Roque GM, de Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51. https://doi.org/10.1016/j.marenvres.2012.11.005
Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta (BBA)-Gen Subj 1830(5):3217–3266. https://doi.org/10.1016/j.bbagen.2012.09.018
Drira Z, Elloumi J, Guermazi W, Hassen MB, Hamza A, Ayadi H (2014) Seasonal changes on planktonic diatom communities along an inshore-offshore gradient in the Gulf of Gabes (Tunisia). Acta Ecol Sin 34(1):34–43. https://doi.org/10.1016/j.chnaes.2013.11.005
Drira Z, Kmiha-Megdiche S, Sahnoun H, Hammami A, Allouche N, Tedetti M, Ayadi H (2016) Assessment of anthropogenic inputs in the surface waters of the southern coastal area of Sfax during spring (Tunisia, Southern Mediterranean Sea). Mar Pollut Bull 104(1–2):355–363. https://doi.org/10.1016/j.marpolbul.2016.01.035
Durou C, Mouneyrac C, Amiard-Triquet C (2005) Tolerance to metals and assessment of energy reserves in the polychaete Nereis diversicolor in clean and contaminated estuaries. Environ Toxicol 20(1):23–31. https://doi.org/10.1002/tox.20074
Durou C, Poirier L, Amiard J-C, Budzinski H, Gnassia-Barelli M, Lemenach K, Peluhet L, Mouneyrac C, Roméo M, Amiard-Triquet C (2007) Biomonitoring in a clean and a multi-contaminated estuary based on biomarkers and chemical analyses in the endobenthic worm Nereis diversicolor. Environ Pollut 148(2):445–458. https://doi.org/10.1016/j.envpol.2006.12.022
El Zrelli R, Courjault-Radé P, Rabaoui L, Castet S, Michel S, Bejaoui N (2015) Heavy metal contamination and ecological risk assessment in the surface sediments of the coastal area surrounding the industrial complex of Gabes city, Gulf of Gabes, SE Tunisia. Mar Pollut Bull 101(2):922–929. https://doi.org/10.1016/j.marpolbul.2015.10.047
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2005) Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 71(1):39–48. https://doi.org/10.1016/j.aquatox.2004.10.009
Flohé L (1989) Structure and catalytic mechanism of glutathione peroxidase. In: Taniguchi N, Higashi T, Sakamoto Y, Meister A (eds) Glutathione centennial: molecular perspectives and clinical implications. Academic Press, London, p 103–114
Geracitano LA, Bocchetti R, Monserrat JM, Regoli F, Bianchini A (2004) Oxidative stress responses in two populations of Laeonereis acuta (Polychaeta, Nereididae) after acute and chronic exposure to copper. Mar Environ Res 58(1):1–17. https://doi.org/10.1016/j.marenvres.2003.09.001
Ghedira J, Jebali J, Bouraoui Z, Banni M, Chouba L, Boussetta H (2009) Acute effects of chlorpyryphos-ethyl and secondary treated effluents on acetylcholinesterase and butyrylcholinesterase activities in Carcinus maenas. J Environ Sci 21(10):1467–1472. https://doi.org/10.1016/S1001-0742(08)62441-9
Ghedira J, Jebali J, Banni M, Chouba L, Boussetta H, López-Barea J, Alhama J (2011) Use of oxidative stress biomarkers in Carcinus maenas to assess littoral zone contamination in Tunisia. Aquat Biol 14(1):87–98. https://doi.org/10.3354/ab00377
Gonçalves C, Teixeira C, Basto MCP, Almeida CMR (2016) PAHs levels in Portuguese estuaries and lagoons: salt marsh plants as potential agents for the containment of PAHs contamination in sediments. Reg Stud Mar Sci 7:211–221. https://doi.org/10.1016/j.rsma.2016.05.004
Greco L, Serrano R, Blanes MA, Serrano E, Capri E (2010) Bioaccumulation markers and biochemical responses in European sea bass (Dicentrarchus labrax) raised under different environmental conditions. Ecotoxicol Environ Saf 73(1):38–45. https://doi.org/10.1016/j.ecoenv.2009.09.011
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hageman JJ, Bast A, Vermeulen NPE (1992) Monitoring of oxidative free radical damage in vivo: analytical aspects. Chemico-Biol Interact 82(3):243–293. https://doi.org/10.1016/0009-2797(92)90001-2
Hansen BH, Rømma S, Garmo ØA, Pedersen SA, Olsvik PA, Andersen RA (2007) Induction and activity of oxidative stress-related proteins during waterborne Cd/Zn-exposure in brown trout (Salmo trutta). Chemosphere 67(11):2241–2249. https://doi.org/10.1016/j.chemosphere.2006.12.048
Janssens BJ, Childress JJ, Baguet F, Rees J-F (2000) Reduced enzymatic antioxidative defense in deep-sea fish. J Exp Biol 203(24):3717–3725
Jebali J, Banni M, Guerbej H, Almeida EA, Bannaoui A, Boussetta H (2006) Effects of malathion and cadmium on acetylcholinesterase activity and metallothionein levels in the fish Seriola dumerilli. Fish Physiol Biochem 32(1):93–98. https://doi.org/10.1007/s10695-006-0041-2
Jelassi R, Khemaissia H, Zimmer M, Garbe-Schönberg D, Nasri-Ammar K (2015) Biodiversity of talitridae family (Crustacea, Amphipoda) in some Tunisian coastal lagoons. Zool Stud 54(1):17. https://doi.org/10.1186/s40555-014-0096-1
Kalman J, Palais F, Amiard JC, Mouneyrac C, Muntz A, Blasco J, Riba I, Amiard-Triquet C (2009) Assessment of the health status of populations of the ragworm Nereis diversicolor using biomarkers at different levels of biological organisation. Mar Ecol Prog Ser 393:55–67. https://doi.org/10.3354/meps08239
Kalman J, Buffet PE, Amiard JC, Denis F, Mouneyrac C, Amiard-Triquet C (2010) Assessment of the influence of confounding factors (weight, salinity) on the response of biomarkers in the estuarine polychaete Nereis diversicolor. Biomarkers 15(5):461–469. https://doi.org/10.3109/1354750X.2010.491162
Kharroubi A, Gzam M, Jedoui Y (2012) Anthropogenic and natural effects on the water and sediments qualities of costal lagoons: case of the Boughrara Lagoon (Southeast Tunisia). Environ Earth Sci 67(4):1061–1067. https://doi.org/10.1007/s12665-012-1551-0
Lionetto F, Del Sole R, Cannoletta D, Vasapollo G, Maffezzoli A (2012) Monitoring wood degradation during weathering by cellulose crystallinity. Materials 5(10):1910–1922. https://doi.org/10.3390/ma5101910
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666. https://doi.org/10.1016/S0025-326X(01)00060-1
Livingstone DR, Lemaire P, Matthews A, Peters L, Bucke D, Law RJ (1993) Pro-oxidant, antioxidant and 7-ethoxyresorufin O-deethylase (EROD) activity responses in liver of dab (Limanda limanda) exposed to sediment contaminated with hydrocarbons and other chemicals. Mar Pollut Bull 26(11):602–606. https://doi.org/10.1016/0025-326X(93)90498-9
Louati A, Elleuch B, Kallel M, Saliot A, Dagaut J, Oudot J (2001) Hydrocarbon contamination of coastal sediments from the Sfax area (Tunisia), Mediterranean Sea. Mar Pollut Bull 42(6):444–451. https://doi.org/10.1016/S0025-326X(00)00179-X
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15(7):1957–1997. https://doi.org/10.1089/ars.2010.3586
Lucan-Bouché ML, Biagianti-Risbourg S, Arsac F, Vernet G (1999) An original decontamination process developed by the aquatic oligochaete Tubifex exposed to copper and lead. Aquat Toxicol 45(1):9–17. https://doi.org/10.1016/S0166-445X(98)00091-5
Maranho LA, Baena-Nogueras RM, Lara-Martín PA, Del Valls TA, Martín-Díaz ML (2014) Bioavailability, oxidative stress, neurotoxicity and genotoxicity of pharmaceuticals bound to marine sediments. The use of the polychaete Hediste diversicolor as bioindicator species. Environ Res 134:353–365. https://doi.org/10.1016/j.envres.2014.08.014
Matthiessen P, Law RJ (2002) Contaminants and their effects on estuarine and coastal organisms in the United Kingdom in the late twentieth century. Environ Pollut 120(3):739–757. https://doi.org/10.1016/S0269-7491(02)00175-6
Moreira SM, Moreira-Santos M, Guilhermino L, Ribeiro R (2005) A short-term sub-lethal in situ toxicity assay with Hediste diversicolor (polychaeta) for estuarine sediments based on post exposure feeding. Environ Toxicol Chem 24(8):2010–2018. https://doi.org/10.1897/04-473R1.1
Moreira SM, Lima I, Ribeiro R, Guilhermino L (2006) Effects of estuarine sediment contamination on feeding and on key physiological functions of the polychaete Hediste diversicolor: laboratory and in situ assays. Aquatic Toxicol 78(2):186–201. https://doi.org/10.1016/j.aquatox.2006.03.001
Mseddi S, Chaari L, Belaid C, Chakchouk I, Kallel M (2016) Valorization of treated olive mill wastewater in fertigation practice. Environ Sci Pollut Res 23(16):15792–15800. https://doi.org/10.1007/s11356-015-4353-6
Mucha AP, Bordalo AA, Vasconcelos MT (2010) Anthropogenic impact on the benthic dynamics in the Douro estuary—an integrated assessment. Situation and Perspectives for the European Union, 6–10 May 2010 Porto
Nunes B, Vidal D, Barbosa I, Soares AMVM, Freitas R (2016) Pollution effects on biochemical pathways determined in the polychaete Hediste diversicolor collected in three Portuguese estuaries. Environ Sci Processes Impacts 18(9):1208–1219. https://doi.org/10.1039/C6EM00297H
Oliva M, González de Canales ML, Gravato C, Guilhermino L, Perales JA (2010) Biochemical effects and polycyclic aromatic hydrocarbons (PAHs) in Senegal sole (Solea senegalensis) from a Huelva estuary (SW Spain). Ecotoxicol Environ Saf 73(8):1842–1851. https://doi.org/10.1016/j.ecoenv.2010.08.035
Oliva M, José Vicente J, Gravato C, Guilhermino L, Dolores Galindo-Riaño M (2012) Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): seasonal and spatial variation. Ecotoxicol Environ Saf 75(1):151–162. https://doi.org/10.1016/j.ecoenv.2011.08.017
Peña-Llopis S, Ferrando MD, Peña JB (2003) Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol 65(4):337–360. https://doi.org/10.1016/S0166-445X(03)00148-6
Pergent G, Zaouali J (1992) Analyse phénologique et lépidochronologique de Posidonia oceanica dans une lagune hyperhaline du Sud Tunisien. In: Rapport de la Commission Internationale pour l’Exploration Scientifique de la Mer Mediterranee, vol 33, pp 48–49
Rabaoui L, Balti R, Zrelli R, Tlig-Zouari S (2014) Assessment of heavy metals pollution in the gulf of Gabes (Tunisia) using four mollusk species. Mediterr Mar Sci 15(1):45–58. https://doi.org/10.12681/mms.504
Rajkumar J (2011) Biochemical markers of oxidative stress in Mugil cephalus exposed to cadmium, copper, lead and zinc. Int J Pharma Bio Sci 2(3):41–50
Reis PA, Antunes JC, Almeida CMR (2009) Metal levels in sediments from the Minho estuary salt marsh: a metal clean area? Environ Monitor Assess 159(1–4):191. https://doi.org/10.1007/s10661-008-0622-x
Rekik A, Denis M, Dugenne M, Barani A, Maalej S, Ayadi H (2014) Seasonal distribution of ultra phytoplankton and heterotrophic prokaryotes in relation to abiotic variables on the north coast of Sfax after restoration. Mar Pollut Bull 84(1–2):280–305. https://doi.org/10.1016/j.marpolbul.2014.05.003
Rice-Evans C, Diplock AT, Symons MC (1991) Techniques in free radical research. Sole distributors for the USA and Canada. Elsevier, Amsterdam. https://doi.org/10.1016/0014-5793(92)81064-S
Romeo M, Bennani N, Gnassia-Barelli M, Lafaurie M, Girard JP (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquat Toxicol 48(2–3):185–194. https://doi.org/10.1016/S0166-445X(99)00039-9
Ruilian YU, Xing Y, Ruiliananhui Z, Gongren HU, Xianglin TU (2008) Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J Environ Sci 20(6):664–669. https://doi.org/10.1016/S1001-0742(08)62110-5
Saliot A (2004) Évolution de la pollution de l’océan mondial et de la Méditerranée par les hydrocarbures sur les quarante dernières années (1960–2000). Oceanis 30(4):449–459
Scaps P (2002) A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (OF Müller) (Annelida: Polychaeta). Hydrobiologia 470(1–3):203–218. https://doi.org/10.1023/A:1015681605656
Scaps P, Demuynck S, Descamps M, Dhainaut A (1996) Biochemical and enzymatic characterization of an acetylcholinesterase from Nereis diversicolor (Annelida, Polychaeta): comparison with the cholinesterases of Eisenia fetida (Annelida, Oligochaeta). Biol Bull 190(3):396–402. https://doi.org/10.2307/1543032
SFT (2007) Guidelines for classification of environmental quality in fjords and coastal areas. Revision of classification of metals and organic contaminants in water and sediment. Norwegian Statens ForurensningsTilsyn (SFT), Norwegian Pollution Control Authority SFT TA-2229/2007
Shepard FP (1954) Nomenclature based on sand-silt-clay ratios SEPM. J Sediment Res, vol 24. http://dx.doi.org/10.1306/D4269774-2B26-11D7-8648000102C1865D
Solé M, Kopecka-Pilarczyk J, Blasco J (2009) Pollution biomarkers in two estuarine invertebrates, Nereis diversicolor and Scrobicularia plana, from a Marsh ecosystem in SW Spain. Environ Int 35(3):523–531. https://doi.org/10.1016/j.envint.2008.09.013
Sun FH, Zhou QX (2008) Oxidative stress biomarkers of the polychaete Nereis diversicolor exposed to cadmium and petroleum hydrocarbons. Ecotoxicol Environ Saf 70(1):106–114. https://doi.org/10.1016/j.ecoenv.2007.04.014
Tlili S, Métais I, Boussetta H, Mouneyrac C (2010) Linking changes at sub-individual and population levels in Donax trunculus: assessment of marine stress. Chemosphere 81(6):692–700. https://doi.org/10.1016/j.chemosphere.2010.07.064
Van der Oost R, Goksøyr A, Celander M, Heida H, Vermeulen NPE (1996) Biomonitoring of aquatic pollution with feral eel (Anguilla anguilla) II biomarkers: pollution-induced biochemical responses. Aquat Toxicol 36(3–4):189–222. https://doi.org/10.1016/S0166-445X(96)00802-8
Van der Oost R, Lopes SCC, Komen H, Satumalay K, den Bos R, Heida H, Vermeulen NPE (1998) Assessment of environmental quality and inland water pollution using biomarker responses in caged carp (Cyprinus carpio): use of a bioactivation: detoxication ratio as a biotransformation index (BTI). Mar Environ Res 46(1–5):315–319. https://doi.org/10.1016/S0141-1136(97)00096-2
Van der Oost D, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Van Veld PA, Sanders BM, Fowler BA, Lars F, Di Giulio RT, Marius B, Stegeman JJ (2018) Molecular responses to environmental contamination: enzyme and protein systems as indicators of chemical exposure and effect. Biomarkers. CRC Press, Boca Raton, pp 235–336
Vieira LR, Sousa A, Frasco MF, Lima I, Morgado F, Guilhermino L (2008) Acute effects of benzo[a]pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Sci Total Environ 395(2–3):87–100. https://doi.org/10.1016/j.scitotenv.2008.01.052
Vieira C, Morais S, Ramos S, Delerue-Matos C, Oliveira MBPP (2011) Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra- and inter-specific variability and human health risks for consumption. Food Chem Toxicol 49(4):923–932. https://doi.org/10.1016/j.fct.2010.12.016
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils-Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63(4):251–264
Wang Y, Yang L, Kong L, Liu E, Wang L, Zhu J (2015) Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake, Shandong, East China. Catena 125:200–205. https://doi.org/10.1016/j.catena.2014.10.023
Yuan X, Chen A, Zhou Y, Liu H, Yang D (2010) The influence of cadmium on the antioxidant enzyme activities in polychaete Perinereis aibuhitensis Grube (Annelida: Polychaeta). Chin J Oceanol Limnol 28(4):849–855. https://doi.org/10.1007/s00343-010-9127-x
Zaghden H, Kallel M, Louati A, Elleuch B, Oudot J, Saliot A (2005) Hydrocarbons in surface sediments from the Sfax coastal zone, (Tunisia). Mediterr Sea Mar Pollut Bull 50(11):1287–1294. https://doi.org/10.1016/j.marpolbul.2005.04.045
Zaghden H, Kallel M, Elleuch B, Oudot J, Saliot A, Sayadi S (2014) Evaluation of hydrocarbon pollution in marine sediments of Sfax coastal areas from the Gabes Gulf of Tunisia, Mediterranean Sea. Environ Earth Sci 72(4):1073–1082. https://doi.org/10.1007/s12665-013-3023-6
Zaghden H, Serbaji MM, Saliot A, Sayadi S (2016) The Tunisian Mediterranean coastline: potential threats from urban discharges Sfax-Tunisian Mediterranean coasts. Desalin Water Treat 57(52):24765–24777. https://doi.org/10.1080/19443994.2016.1149107
Acknowledgements
The authors are grateful to the “Erasmus Mundus Programme” for providing scholarship to R. Ghribi through the “Battuta” program (Building Academic Ties towards Universities through Training Activities) dedicated to the development of relations between Europe and North Africa through grants of excellence between the two regions. The authors are grateful to the Tunisian Ministry of Higher Education and Scientific Research also supported this thesis. This work was conducted in the framework of Researcher FCT Program (Operational Program for Human Potential, QREN, EU) who hired Bruno Nunes. The authors are particularly thankful to Prof. Abdelmajid Dammak for his contribution to correct grammatical errors and to improve the English writing skills of this paper. This research was also supported by the European Regional Development Fund (ERDF) through the COMPETE—Operational Competitiveness Program and by national funds through FCT—Foundation for Science and Technology, under the projects “PEst-C/MAR/LA0015/2013” and “UID/Multi/04423/2013” in the framework of the programme PT2020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghribi, R., Correia, A.T., Elleuch, B. et al. Toxicity Assessment of Impacted Sediments from Southeast Coast of Tunisia Using a Biomarker Approach with the Polychaete Hediste diversicolor. Arch Environ Contam Toxicol 76, 678–691 (2019). https://doi.org/10.1007/s00244-019-00611-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00611-2