Abstract
Olive mill wastewater (OMW) brings about a major environmental problem in Tunisia as well as in the other Mediterranean countries. Its strong organic load and its toxicity due to the presence of complex phenolic compounds have dire effects when applied to soil. To overcome this difficulty, the OMW pretreatment was investigated in the present work using the Fenton oxidation reaction with zero-valent iron. Then, this pretreated wastewater was valorized in fertigation practice. The effects of the addition of different concentrations of both treated and raw OMW on soil and cropping system were investigated. The treatment by Fenton oxidation with zero-valent iron could reduce 50 % of COD and decrease 53 % of phenolic compounds. OMW application had a temporary effect on the soil pH and EC. The results showed that the evolution of soil pH and EC was related to the organic matter of the soil which depends on the spread concentrations of raw or treated OMW. After 15-day incubation period, the soil pH and EC tended to stabilize and return to the control level. Moreover, this stabilization is faster in treated OMW than that in raw OMW especially for concentrations as high as 3 and 4 %. Plants cultivated with treated OMW showed an increase in their germination. The results pointed an improvement in the stem length of plants which is almost similar to that of the control for both pea and tomato, especially for high concentrations of 3 and 4 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive cultivation practice is widespread throughout the Mediterranean region which produces 97 % of the world’s olive oil (Kapellakis et al. 2006; Tsagaraki et al. 2007). According to recent statistics of the International Olive Oil Council (November 2011), Tunisia is the world’s fourth largest producer with 5.9 % of world production. Despite the economic benefits of the olive oil production, producing countries are facing an acute environmental problem regarding the seasonal generation of huge quantities of effluents: olive mill wastewater (OMW). The annual production of OMW, in the Mediterranean area, reached 30 million m3 and 700,000 m3 in Tunisia alone (Dhouib et al. 2006). This effluent is characterized by high polluting activity related to its low pH, high content of mineral salts, and high organic load (COD between 40 and 210 g/L). Crognale et al. (2006) and Ouzounidou et al. (2010) recorded large amounts of organic molecules constituted by long-chain fatty acids, lipids, sugars, proteins, and pectins increasing their organic load (El Hassani et al. 2009a). Furthermore, OMW contains significant concentrations of polymeric phenolic compounds (between 0.5 and 24 g/L) that display a lignin-like structure constituting the most recalcitrant fraction of this effluent (Tsioulpas et al. 2002). They are gradually oxidized and/or polymerized making OMW highly toxic and phytotoxic (Chatzisymeon et al. 2009; Celano et al. 2008). According to Quaratino et al. (2007) and Hachicha et al. (2009), phenolic compounds generate antimicrobial and phytotoxic effects on seed germination, bacteria, and different species of soil. This is in agreement with studies of Chen et al. (2010) where they show that synergisms between some phenolic compounds could be also responsible for their high toxicity. However, nowadays and especially in the Mediterranean countries, the OMW principal destinies are spreading to agricultural soils and storage in evaporation ponds (El Hassani et al. 2009b) where it was used as a readily available and inexpensive option (Oved et al. 2001). Recent studies have found that the addition of unprocessed OMW causes significant shifts in the structure and function of microbial communities, which in turn influences the soil fertility (Sierra et al. 2001; Mekki et al. 2006, 2007).
Hence, the management of this pollutant wastewater is of major importance nowadays to find an eco-friendly solution. Various treatment methods have been investigated to search the best potential solutions. Successful degradation of organic compounds, reduction of COD, and/or decolorization of OMW have been obtained by physicochemical methods (e.g., ozonation, coagulation, ultrafiltration, electrochemical oxidation, adsorption) and biological treatments (aerobic or anaerobic digestion, fungal/enzymatic treatments) and composting process as reviewed by Mantzavinos and Kalogerakis (2005), Roig et al. (2006), McNamara et al. (2008), Lafi et al. (2009), Abid and Sayadi (2006), Justino et al. (2009), (2010), and Aytar et al. (2013). Advanced oxidation processes (AOPs) should be regarded as a viable alternative with different efficiencies in the phenol degradation (Canizares et al. 2007). These processes employ powerful oxidant agents (e.g., ozone, hydrogen peroxide, and Fenton reagent) as well as the formation of very reactive oxidizing free radicals (OH·), which can remove the OMW partial or total organic load. Among AOPs, Fenton process is the most cost effective and easiest to apply for the high reduction of COD (Lee and Shoda 2008). The reagent components, a mixture of ferrous ion and hydrogen peroxide, are environmentally benign since they generate hydroxyl radicals (OH·) (Kallel et al. 2009). Studies of Aytar et al. (2013) and Justino et al. (2010) demonstrated that photo-Fenton oxidation was an attractive solution with significant reduction of phenolic compounds and OMW decolorization. Furthermore, as reported by Kallel et al. (2009), the application of advanced Fenton processes with iron (0) (zero-valent Fe/H2O2) could be considered as an effective alternative solution for the OMW treatment in optimized experimental conditions. On the other hand, OMW valorization as an amendment in agriculture, either in raw form or after various treatments, the recuperation of their organic components for use in agriculture and industry, are the most commonly proposed practices for their disposal (Capasso et al. 2002; Ouzounidou et al. 2008). Several studies focusing on OMW valorization found that the use of OMW as a fertilizer, with controlled application rates, enhances the herbicidal activity (Kotsou et al. 2004) and has beneficial effects for both crops and hosting soils (Altieri and Esposito 2010). Indeed, the OMWs participate actively in land humification because of their lignocellulosic composition and high levels of polyphenols (Sanchez-Monedero et al. 2008). Nevertheless, OMW direct spreading on agricultural land is limited by constraints imposed by its composition such as the following: oil and grease, high salinity and phenolic compounds with a phytotoxic effect, particularly due to monomeric polyphenols (Greco et al. 2006), and antimicrobial properties that modify the equilibrium of useful soil microorganisms (Barbera et al. 2013).
An integrated approach was proposed in this study, using an OMW treatment by Fenton oxidation with zero-valent iron prior to its valorization in fertigation practice. Thus, the aim of our work was to investigate the effects of the treated OMW application on seed germination, plant growth and soil properties.
Material and methods
OMW characterization
The fresh OMW was collected from a three-phase olive-oil factory located in Sfax (Tunisia) during the olive harvesting season and stored at 4 °C prior to use in the laboratory experiments.
The pH and electrical conductivity (EC) were measured using a pH-meter and conductivity meter, respectively. The dry weight was determined by weighing the sample before and after drying overnight at 105 °C. The organic matter content was determined after combustion of the samples in a furnace at 550 °C for 4 h. Chemical oxygen demand was determined according to the (Knechtel 1978) standard method. Biochemical oxygen demand was measured using the respirometric method. Phenolic compounds were quantified by the Folin–Ciocalteau method (Box 1983) using gallic acid as standard. The absorbance was determined at 760 nm. Identification of these aromatic compounds was carried out by GC/MS after silylation of OMW organic phase. Total nitrogen was determined by the Kjeldahl method. Total phosphorus was measured calorimetrically. Ca, Mg, Na, K were determined by atomic absorption (Fisher Scientific ice 3000). Oily fraction was determined according to the standardized method (JIS K 0102.24.2) after the sample acidification; then, it was extracted by adding hexane.
Experimental procedure
Experiments were carried out in a Floclab jar-test (Prolabo) equipped with six beakers (500-ml capacity). A volume of hydrogen peroxide (H2O2) was introduced in the reactor, followed by 500 ml of OMW sample with natural pH without any dilution. Then, iron metal in a spiral form was introduced. It was obtained from a metal turner and had a relatively wide surface area in order to facilitate the corrosion on the iron metal sheet surface (Kallel et al. 2009). After the introduction of iron spires, a continuous stirring at 200 rpm was applied. This process was performed at room temperature (25 °C).
Soil characterization
Sandy soil was selected in our study because such a type of soil is among the most common in the Mediterranean region, and it provides weak protection against organic molecules (Bustamante et al. 2010). The particle size distribution was determined using the GSA program (Grain Size Analyses: Yaïch 1994). It was composed of sand (65 %), silt (28, 6 %), and clay (6 %). pH and EC were determined, respectively, on a mixture of soil/water (1:2.5 (Pauwels et al. 1992) and 1:5 (Peireira et al. 2009)). The soil organic matter was analyzed by Walkley–Black method (Walkley 1947). The physicochemical characteristics showed an alkaline soil with pH of 8.5, a low electrical conductivity (EC = 124 μS/cm), and an important organic matter content of 1 %. The soil was air-dried and sieved (2 mm in diameter) before use.
Phytotoxicity test
Phytotoxicity was measured using Zucconi test (Zucconi et al. 1981) by measuring seed germination. Ten seeds from each type of plant, tomato (Lycopersicon esculentum) and pea (Pisum sativum), were placed on filter paper in glass Petri dishes with dimensions of 110 mm × 20 mm. Ten milliliters of OMW samples were then uniformly added to each dish. The dish was capped and kept in a dark incubator at 25 °C temperature for 5 days. Ten milliliters of tap water were used for the controls, instead of OMW. All samples, including the control, were run in triplicate. All seeds were kept in tap water for approximately 12 h prior to the initiation of the experiments to accelerate seed growth.
A germination index (GI) was calculated by counting the grown seeds and determining the average sum of seeds’ root elongation in a sample as related to the control. The results were finally expressed as a percentage.
Fertigation practice
Sixteen seeds of two plant species (pea and tomato) were cultivated in agricultural soil-filled pots (1 kg each). Different concentrations of raw and treated OMW were applied on seed stage. This culture is performed under the same operating conditions with regular watering plants with rainwater. In fact, all the pots were placed in a greenhouse designed as a growth chamber programmed for a 12-h photoperiod with photosynthetic photon flux density of 300 μmol m−2 s−1; temperature, 24 ± 1/18 ± 1 °C day/night; and relative humidity, 60/70 ± 3 %.
The concentrations used are expressed as follows: 0.5 % (50 m3/ha), 1 % (100 m3/ha), 2 % (200 m3/ha), 3 % (300 m3/ha), and 4 % (400 m3/ha). The OMW irrigation effect on these plants was carried out by monitoring their growth during a 20-day period followed by measuring the stem length. The monitoring of the soil properties was also run in parallel during 1 month.
Results and discussion
OMW characteristics
Table 1 summarizes the OMW physicochemical properties. This effluent has an acidic pH 4.33. In this case, the oily fraction, appearing to be highest, reached 1.12 g/L. The OMW polluting character is expressed by its high concentration of organic load characterized with a COD, 64 g/L, an important concentration of phenolic compounds reaching 5 g/L and a high salinity expressed with conductivity equal to 16 mS/cm. The mineral composition shows a high concentration in potassium and sodium with 2.4 and 1.4 g/L, respectively.
Efficiency of Fenton oxidation treatment with zero-valent iron
The study of Kallel et al. (2009) shows that the application of advanced Fenton processes with iron (0) could be considered as an effective alternative solution for the OMW treatment. Based on this previous study, the optimal experimental conditions chosen in our study are as follows: 10 % as a concentration of H2O2 and 16 g /L of metallic iron spiral form with a natural pH and without any dilution.
Experiments show a high performance of Fenton oxidation with zero-valent iron. The results demonstrate that after only 6 h of reaction time, the OMW was well decolorized corresponding to the degradation of phenolic compounds. Moreover, a significant removal of organic load was recorded with the decrease of the rate of COD and phenolic compounds. Figure 1 shows a decrease of COD which reached 50 % after only 6 h of reaction time. In the same level, Fig. 2 indicates an important removal of phenolic compounds of about 53 %. As can be observed in Fig. 3, the obtained chromatograms reveal the high disappearance of phenolic monomer peaks identified in the raw OMW (Fig. 3a).
The performance of Fenton oxidation treatment is summarized in Table 2 with the physicochemical characteristics of treated OMW. The mineral composition of this treated effluent shows important concentrations of HCO3− and K+ which are important fertilizers to the soil.
Phytotoxicity test
The determination of germination index for both raw and treated OMW approves the OMW phytotoxic properties. The results show no germination regarding peas and tomatoes irrigated with raw OMW. As for treated OMW, pea seeds show a weak germination of about 10 %. Nevertheless, tomato seeds do not germinate. This could be due to the acidity of OMW as well as the persistence of some toxic compounds in the treated OMW.
Impacts of olive mill wastewater application on soil and plant growth
Evolution of soil pH
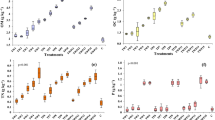
The evolution of soil pH was explored due to the important variations of this parameter on the soil organic matter and the biological activity. The OMW low pH (4.33) tended to reduce soil pH immediately after the applications of both raw and treated OMW (Fig. 4a, b). This reduction was proportional to the increase of OMW concentrations. Moreover, the soil pH continued to decrease for 15 days of incubation (Fig. 4a, b). This decrease in pH may be due to the acidifying effect of biochemical ammonium conversion (Gargouri et al. 2014). In fact, ammonium oxidation produces nitric acid or nitrate (Camberato 2001). Nevertheless, this change in pH was temporary because 30 days after having applied the OMW, the pH tended to return to the same level as that of control, particularly in the case of raw OMW (Fig. 4a) whose pH reached 8.4 after applying the concentration of 0.5 % compared to the same concentration of treated OMW whose pH reached 8.2 (Fig. 4b). These findings are in agreement with those reported by Bustamante et al. (2007) and Haddadin et al. (2009). All these studies concluded that the increase in pH was due to the organic matter, especially mineralization of organic acids and ammonium production.
Evolution of soil EC
The evolution of soil electrical conductivity (EC) reveals the process of mineralization of organic matter. The results show that EC increased proportionally with the OMW doses immediately after their incorporation into the soil (Fig. 5a, b). During the first 15 days, the EC increased from 0.12 dS m−1 to 0.61 dS m−1 and from 0.23 to 0.89 dS m−1 for 0.5 and 4 % raw OMW, respectively (Fig. 5a). The same trend of EC evolution was shown in soils incubated with treated OMW. In fact, during the first 15 days, the EC increased from 0.14 to 0.4 dS m−1 and from 0.26 to 0.63 dS m−1 for 0.5 and 4 % OMW, respectively (Fig. 5b). Throughout this 15-day incubation period, the increase of EC suggests a biological activity inducing mineralization of the organic matter. Also, it can be explained by the OMW high mineral content. An EC increase after the addition of OMW was reported by Piñeiro et al. (2008). However, the results show that after 15 days of incubation, EC decreases and tends to stabilize and returns to the control value. Moreover, this stabilization is faster in treated OMW (Fig. 5b) than that in raw OMW (Fig. 5a) especially for concentrations as high as 3 and 4 % (Fig. 5b). It should be known that the stabilization of EC values after 2 weeks of incubation was also reported by Piotrowska et al. (2006).
Evolution of the soil organic matter
Soils irrigated with raw and treated OMW show an enrichment of organic matter (Fig. 6a, b). Indeed, at the initiation of the experiment, the content of organic matter was greater in these soils than that in the control; it reached around 1.3 % for the greatest dose (4 %) (Fig. 6a, b). Organic matter content generally decreased up to the fourth week of incubation (Gargouri et al. 2014) reaching minimal values as those in control. The decrease in the amount of organic matter observed after a month of incubation could be explained by the digestion of this matter by soil microorganisms activated by OMW addition. In fact, the OMW is composed of sugars and proteins that are easily biodegradable (Kotsou et al. 2004). The results show that the decrease of organic matter reached 20 and 30 % for raw and treated OMW, respectively.
Evolution of the plant growth
Germinability tests on stem length allowed the showing of the evolution of the pea and tomato germination (Fig. 7). The irrigation by raw OMW enhanced the germination of pea with low length of stem which reached a maximum of 1.5 cm for the great dose (4 %) (Fig. 7a). Nevertheless, raw OMW suppressed seed germination of tomato (Fig. 7c) which is more sensitive to OMW than the pea. Thus, this reflects the raw OMW phytotoxicity which is attributed to phenols and fatty acids.
However, irrigation with treated OMW showed the best plant growth when compared to that with raw OMW (Fig. 7b, d). The seed germination proves the reduction of the treated OMW toxicity by the Fenton oxidation, thus the performance of this treatment. The results show that the germination of the stem is almost similar to that of the control for both pea (Fig. 7b) and tomato (Fig. 7d), especially for high concentrations of 3 and 4 %. These findings could be explained by the strongly buffering soil capacity which counterbalances the negative effect of the OMW acidity.
Conclusion
OMW effluent is one of the most polluted industrial wastewater throughout the Mediterranean area. The high organic load and the significant concentrations of phenolic compounds are important parameters regarding the characterization and the toxicity of OMW. In this study, several authors proposed an integrated approach using an OMW treatment by Fenton oxidation with zero-valent iron followed by its valorization in fertigation practice with increasing doses (0.5, 1, 2, 3, and 4 %) of raw and treated OMW. Outstanding results were observed.
The treatment by Fenton oxidation with zero-valent iron shows great performance by the removal of 50 % of COD after only 6 h of reaction time. In the same level, a significant removal of phenolic compounds was recorded with the decrease of 53 %. The obtained chromatograms reveal the high disappearance of phenolic monomers peaks identified in the raw OMW.
OMW application had a temporary effect on the soil pH and EC. The evolution of these parameters was related to the organic matter of the soil which depends on the OMW concentrations applied on soil. After 15-day incubation period, the soil pH and EC tended to stabilize, especially in treated OMW for concentrations as high as 3 and 4 %. For seed germination, the measure of the germination index showed that raw OMW inhibited totally the seed germination. As for treated OMW, only pea seeds show a weak germination due to the acidity of OMW. Nevertheless, in fertigation practice, under some operating greenhouse conditions, the results showed an improvement in the stem length for both pea and tomato irrigated with treated OMW. Moreover, the germination of plants is comparable to that of the control even in the case of high concentrations of 3 and 4 %. These findings proved the strongly buffering soil capacity which counterbalances the negative effect of the OMW acidity. Then, it allows the opportunity for the best performance for the plant growth.
References
Abid N, Sayadi S (2006) Detrimental effect of olive mill wastewater on the composting process of agricultural wastes. Waste Manag 26:1099–1107. doi:10.1016/j.wasman.2005.06.015
Altieri R, Esposito A (2010) Evaluation of the fertilizing effect of olive mill waste compost in short-term crops. Int Biodeterior Biodegrad 64:124–128. doi:10.1016/j.ibiod.2009.12.002
Aytar P, Gedikli S, Sam M, Farizoğlu B, Çabuk A (2013) Sequential treatment of olive oil mill wastewater with adsorption and biological and photo-Fenton oxidation. Environ Sci Pollut Res 20:3060–3067. doi:10.1007/s11356-012-1212-6
Barbera AC, Maucieri C, Loppolo A, Milani M, Cavallaro V (2013) Effects of olive mill wastewater physico-chemical treatments on polyphenol abatement and Italian ryegrass (Lolium multiflorum Lam.) germinability. Water Res 52:275–281. doi:10.1016/j.watres.2013.11.004
Box JD (1983) Investigation of the Folin–Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res 17:511–522
Bustamante MA, Pérez-Murcia MD, Paredes C, Moral R, Pérez-Espinosa A, Moreno-Caselles J (2007) Short-term carbon and nitrogen mineralization in soil amended with winery and distillery organic wastes. Bioresour Technol 98:3269–3277. doi:10.1016/j.biortech.2006.07.013
Bustamante MA, Said-Pullicino D, Paredes C, Cecilia JA, Moral R (2010) Influences of winery-distillery waste compost stability and soil type on soil carbon dynamics in amended soils. Waste Manag 30:1966–1975. doi:10.1016/j.wasman.2010.03.012
Camberato JJ (2001) Nitrogen in soil and fertilizers. SC Turfgrass Found News 8:6–10
Canizares P, Lobato J, Paz R, Rodrigo MA, Saez C (2007) Advanced oxidation process for the treatment of olive oil mills wastewater. Chemosphere 67:832–838. doi:10.1016/j.chemosphere.2006.10.064
Capasso R, De Martino A, Arienzo M (2002) Recovery and characterization of the metal polymeric organic fraction (polymerin) from olive oil mill wastewaters. J Agric Food Chem 50:2846–2855. doi:10.1021/jf011442c
Celano G, Smejkalová D, Spaccini R, Piccolo A (2008) Reduced toxicity of olive mill waste waters by oxidative coupling with biomimetic catalysis. Environ Sci Technol 42:4896–4901. doi:10.1021/es8000745
Chatzisymeon E, Xekoukoulotakis NP, Mantzavinos D (2009) Determination of key operating conditions for the photocatalytic treatment of olive mill wastewaters. Catal Today 144:143–148. doi:10.1016/j.cattod.2009.01.037
Chen H, Yao J, Wang F, Zhou Y, Chen K et al (2010) Toxicity of three phenolic compounds and their mixtures on the Gram positive bacteria Bacillus subtillis in the aquatic environment. Sci Total Environ 408:1043–1049. doi:10.1016/j.scitotenv.2009.11.051
Crognale S, D’Annibale A, Federici F, Fenice M, Quaratino D, Petruccioli M (2006) Olive oil mill wastewater valorisation by fungi. J Chem Technol Biotechnol 81:1547–1555. doi:10.1002/jctb.1564
Dhouib A, Ellouz M, Aloui F, Sayadi S (2006) Effect of bioaugmentation of activated sludge with white-rot fungi on olive mill wastewater detoxification. J Appl Microbiol 42:405–411. doi:10.1111/j.1472-765X.2006.01858.x
El Hassani FZ, Zinedine A, Bendriss Amraoui M, Errachidi F, Mdaghri Alaoui S et al (2009a) Characterization of the harmful effect of olive mill wastewater on spearmint. J Hazard Mater 170:779–785
El Hassani FZ, Bendriss Amraoui M, Zinedine A, Aissam H, Mdaghri Alaoui S et al (2009b) Changes in leaf phenols and other physiological parameters of peppermint in response to olive mill wastewater application. Int J Agric Biol 11:413–418
Gargouri K, Masmoudi M, Rhouma A (2014) Influence of Olive Mill Wastewater (OMW) spread on carbon and nitrogen dynamics and biology of an arid sandy soil. Commun Soil Sci Plant Anal 45:1–14. doi:10.1080/00103624.2013.849727
Greco G, Colarieti ML, Toscano G, Iamarino G, Rao MA, Gianfreda L (2006) Mitigation of olive mill wastewater toxicity. J Agric Food Chem 54:6776–6782. doi:10.1021/jf061084j
Hachicha S, Cegarra J, Sellami F, Hachicha R, Drira N et al (2009) Elimination of polyphenols toxicity from olive mill wastewater sludge by its co-composting with sesame bark. J Hazard Mater 161:1131–1139. doi:10.1016/j.jhazmat.2008.04.066
Haddadin MSY, Haddadin J, Arabiyat OI, Hattar B (2009) Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Bioresour Technol 100:4773–4782. doi:10.1016/j.biortech.2009.04.047
International Olive Council report (2011). http://www.internationaloliveoil.org. Accessed 5 Nov 2012
Justino CI, Duarte K, Loureiro F, Pereira R, Antunes SC et al (2009) Toxicity and organic content characterization of olive oil mill wastewater undergoing a sequential treatment with fungi and photo-Fenton oxidation. J Hazard Mat 172:1560–1572. doi:10.1016/j.jhazmat.2009.08.028
Justino C, Marques AG, Duarte KR, Duarte AC, Pereira R et al (2010) Degradation of phenols in olive oil mill wastewater by biological, enzymatic, and photo-Fenton oxidation. Environ Sci Pollut Res 17:650–656. doi:10.1007/s11356-009-0256-8
Kallel M, Belaid C, Mechichi T, Ksibi M, Elleuch B (2009) Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem Eng J 150:391–395. doi:10.1016/j.cej.2009.01.017
Kapellakis IE, Tsagarakis KP, Avramaki C, Angelakis AN (2006) Olive mill wastewater management in river basins: a case study in Greece. Agric Water Manag 82:354–370. doi:10.1016/j.agwat.2005.08.004
Knechtel RJ (1978) A more economical method for the determination of chemical oxygen demand. Water Pollut Control 1978:25–29
Kotsou M, Mari I, Lasaridi K, Chatzipavlidis I, Balis C, Kyriacou A (2004) The effect of live oil mill wastewater (OMW) on soil microbial communities and suppressiveness against Rhizoctonia solani. Appl Soil Ecol 26:113–121
Lafi WK, Shannak B, Al-Shannag M, Al-Anber Z, Al-Hasan M (2009) Treatment of olive mill wastewater by combined advanced oxidation and biodegradation. Sep Purif Technol 70:141–146. doi:10.1016/j.seppur.2009.09.008
Lee H, Shoda M (2008) Removal of COD and color from livestock wastewater by the Fenton method. J Hazard Mater 153:1314–1319. doi:10.1016/j.jhazmat.2007.09.097
Mantzavinos D, Kalogerakis N (2005) Treatment of olive mill effluents:Part I. Organic matter degradation by chemical and biological processes—an overview. Environ Int 31:289–295. doi:10.1016/j.envint.2004.10.005
McNamara CJ, Anastasiou CC, O’Flaherty V, Mitchell R (2008) Bioremediation of olive mill wastewater. Int Biodeterior Biodegrad 61:127–134. doi:10.1016/j.ibiod.2007.11.003
Mekki A, Dhouib A, Aloui F, Sayadi S (2006) Olive wastewater as an ecological fertilizer. Agron Sust Dev 26:61–67. doi:10.1051/agro:2005061
Mekki A, Dhouib A, Sayadi S (2007) Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J Environ Manag 84:134–140. doi:10.1016/j.jenvman.2006.05.015
Ouzounidou G, Asfi M, Sortirakis N, Papadopoulou P, Gaitis F (2008) Olive millwastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate. J Hazard Mater 158:523–530
Ouzounidou G, Zervakis GI, Gaitis F (2010) Raw and microbiologically detoxified olive mill waste and their impact on plant growth. Terr Aquat Environ Toxicol 4:21–38
Oved T, Shaviv A, Goldrath T, Raphi T, Mandelbaun MD (2001) Influence of effluent irrigation on community composition and function of ammoniaoxidizing bacteria in soil. Appl Environ Microbiol 67:3426–3433. doi:10.1128/AEM. 67.8.3426-3433.2001
Pauwels JM, Van Rust E, Verloo M, Mvondo ZEA (1992) Manual of soil laboratory analytical methods of soil and plants. Agricultural publications, 28th edn. AGCD, Belgium
Peireira R, Ksibi M, Sousa J.P, Jörg R, Haddioui A et al (2009). Workshop on risk assessment of contaminated sites. National Engineering school of Sfax, Laboratory water energy and environment
Piñeiro AL, Albarrán A, Rato Nunes JM, Barreto C (2008) Short- and medium-term effects of two-phase olive mill waste application on olive grove production and soil properties under semiarid Mediterranean conditions. Bioresour Technol 99:7982–7987. doi:10.1016/j.biortech.2008.03.051
Piotrowska A, Iamarino G, Rao MA, Gianfreda L (2006) Short-term effects of olive mill waste water (OMW) on chemical and biochemical properties of a semiarid Mediterranean soil. Soil Biol Biochem 38:600–610. doi:10.1016/j.soilbio.2005.06.012
Quaratino D, D'Annibale A, Federici F, Cereti CF, Rossini F, Fenice M (2007) Enzyme and fungal treatments and a combination thereof reduce olive mill wastewater phytotoxicity on Zea mays L. seeds. Chemosphere 66:1627–1633. doi:10.1016/j.chemosphere.2006.07.092
Roig A, Cayuela ML, Sánchez-Monedero MA (2006) An overview on olive mill wastes and their valorisation methods. Waste Manag 26:960–969. doi:10.1016/j.wasman.2005.07.024
Sanchez-Monedero MA, Cayuela ML, Mondini C, Serramia N, Roig A (2008) Potential of olive mill wastes for soil C sequestration. Waste Manag 28:767–773. doi:10.1016/j.wasman.2007.09.029
Sierra J, Marti E, Montserrat G, Cruanas R, Garau MA (2001) Characterization and evolution of a soil affected by olive oil mill wastewater disposal. Sci Total Environ 279:207–214
Tsagaraki E, Larazides HN, Petrotos KB (2007) Olive mill wastewater treatment. In: Oreopoulou V, Russ W (eds) Utilization of by-products and treatment of waste in the food industry. Springer, New York, pp 133–157
Tsioulpas A, Dimou D, Iconomou D, Aggelis G (2002) Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour Technol 84:251–257. doi:10.1016/S0960-8524(02)00043-3
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–264
Zucconi F, Pera A, Forte M, Bertoldi M (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Acknowledgments
The authors would like to thank the Ministry of Higher Education, Scientific Research and Technology for the financial support to the current work. We wish to thank Mr. Jamil JAOUA, founder and former Head of the English Teaching Unit at the Sfax (Tunisia) Faculty of science, for having patiently edited this Paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mseddi, S., Chaari, L., Belaid, C. et al. Valorization of treated olive mill wastewater in fertigation practice. Environ Sci Pollut Res 23, 15792–15800 (2016). https://doi.org/10.1007/s11356-015-4353-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4353-6