Abstract
The investigation of organochlorine pesticides (OCPs) levels in sea turtles is an important issue in conservation research, due to the harmful effects of these chemicals. In the present study, OCPs concentrations were determined in the eggs of two sea turtle species (Eretmochelys imbricata and Chelonia mydas) collected from the Punta Xen and Isla Aguada (Mexican coast) in 2014 and 2015. Concentrations of 20 OCPs were analysed, including isomers of hexachlorocyclohexane, aldrin, chlordanes, endosulfans, methoxychlor, DDTs, and heptachlor. From the group of contaminants considered (analysed as families), the results revealed higher concentrations of ΣHCH and ΣDienes on both selected species. We analysed the relationship between turtle size and the OCPs concentrations; no correlation was found between the size of the female and concentrations in the eggs. In addition, principal component analysis indicated pattern differences between species and years, in good agreement with concentrations differences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Global anthropogenic pollution of the marine environment by organic contaminants, including persistent organic pollutants (POPs), is an issue of great concern. Their presence in aquatic systems around the world is a result of its widespread use and long-distance transport (Hamann et al. 2010). Environmental contaminants of chemical origin can resist chemical, photolytic, and biological degradation (Clark 1992). Due to their lipophilic properties, resistance to breakdown, and biomagnification potential, these chemicals are extremely persistent in the environment and can have many harmful effects on the development and functioning of sea animals (Clark and Krynitsky 1980; Mckenzie et al. 1999; Alava et al. 2006). The bioaccumulation of these toxic substances has become a major cause for concern on several wildlife species (Marcotrigiano and Storelli 2003; Keller et al. 2004b; Ogata et al. 2009) and for the marine turtles communities worldwide (Lake et al. 1994; Storelli and Marcotrigiano 2003; Alava et al. 2006; de Andréa 2008; Alava et al. 2011; Marcovecchio and Freije 2013; da Silva et al. 2014; Guerranti et al. 2014).
Sea turtles have recently been considered as suitable environmental indicators to improve the effectiveness of conservation strategies (Parliament 2008) due to their long life, their trophic position, and their mobility, which allow for the integration of pollutants from extensive areas. Taking into account those characteristics, several studies have reported the worldwide accumulation of pollutant substances in the marine turtles during the past decade (Alam and Brim 2000; Gardner et al. 2003; Lam et al. 2004; Andreani et al. 2008; Monagas et al. 2008; Oros et al. 2009; Jerez et al. 2010; Alava et al. 2011; D’Ilio et al. 2011). Because marine turtles allow the integration of pollutants from extensive areas, they can offer a comprehensive contamination profile within that energy flow ecosystem. The contamination of the marine system is one of the research priorities in the topic of turtle biology and conservation (Hamann et al. 2006).
Some persistent organic pollutants can mimic hormones and may cause adverse health effects in wildlife populations, namely on the fecundity and reproductive competence. According to Camacho et al. (2013a), the bioaccumulation of POPs, such as OCPs, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and polycyclic aromatic hydrocarbon (PAHs), in the tissues and organs of these animals can influence sea turtle natural populations’ growth and development, ultimately causing mortality in the various stages of development. Many sea turtle populations are declining worldwide at alarming rates (Pritchard and Cox 2002) and are considered globally threatened or endangered. (MTSG 1995). Populations of marine turtles suffer greatly by environmental stress and anthropogenic activities being most of the impact on these populations caused by increased commercial and industrial exploitation in the coastal regions. According to the Marine Turtle Specialist Group (MTSG), the main threats to marine turtles currently causing of these species population collapse are coastal development, the incidental capture by fisheries, habitat loss (spawning and feeding) (Derraik 2002), direct use for human consumption and egg poaching (López-Mendilaharsu et al. 2007), climate change, pollution, and pathogens (Shigenaka and Milton 2003).
The Mexican coast represents an area of vital importance for the survival of marine turtles to growth and reproduce. Six of the seven existing species in the world visit the Mexican coasts: Caretta caretta, Chelonia mydas, Dermochelys coriacea, Eretmochelys imbricata, Lepidochelys olivacea, Natator depressus, Lepidochelys kempii, except for Natator depressus, all are listed as vulnerable, endangered, and critically endangered in the IUCN Red List (International Union for Conservation of Nature) (IUCN 2016). The green turtle (Chelonia mydas) can be found in all tropical and subtropical seas, and nesting populations are generally comprised of individuals that have migrated from a wide range of foraging grounds (Godley et al. 2002; Seminoff et al. 2008), and it is the species of sea turtle that presents more coastal habits (de Pádua Almeida et al. 2011). Hawksbill turtles (Eretmochelys imbricata) are circumtropically distributed in coastal waters (Meylan and Donnelly 1999). This species can be found in larger numbers in tropical coastal areas than in subtropical seas (Marcovaldi et al. 2011). Sea turtles are highly migratory, and they undertake complex movements and migrations through geographically disparate habitats. Their movements within the marine environment are less understood, but it is believed that hawksbills turtles in 108 countries and green turtles inhabit coastal waters of more than 140 countries (Groombridge and Luxmoore 1989; IUCN 2016).
During their reproductive years, C. mydas and E. imbricata show strong fidelity to their foraging and breeding sites, which can be up to thousands of kilometers apart (Carr 1964; Carr and Carr 1972; Limpus et al. 1992; Lohmann et al. 1997). Using satellite telemetry, scientists can track the movements of sea turtles between areas and even across entire oceans (Gaos et al. 2012); however, information on the migrations of sea turtles is currently sparse (Limpus et al. 1992). Marcovaldi and Marcovaldi (1985) describe these species’ general feeding characteristics, indicating that E. imbricata prefer corals and sponges and C. mydas feed on small molluscs and sponges during the first year of life, preferentially feeding on macroalgae and phanerogams after this period; during this foraging time, local environmental nutritional resources are deposited into follicles (which become the yolk of the egg) for the next nesting season.
Most studies focusing on the concentrations of pollutants in sea turtles were based on tissues collected from dead animals. Levels and distribution of various chemical compounds were reported for liver (Malarvannan et al. 2011; Guerranti et al. 2014; Storelli and Zizzo 2014), adipose tissue (Lazar et al. 2011; Yogui 2002), or for more than one organ and tissue (Lake et al. 1994; Corsolini et al. 2000; Miao et al. 2001; Gardner et al. 2003; da Silva 2009; Oros et al. 2009; D’Ilio et al. 2011). Blood samples were successfully used to measure the concentrations of organochlorine pollutants, which is considered to be a non-lethal collection technique (Keller et al. 2004a; Hamann et al. 2006; Swarthout et al. 2010; Camacho et al. 2013b, 2014). Assessments from POP concentrations in eggs and the extent to which contaminants affect these developmental stages of sea turtles has been insufficiently researched, including the embryonic abnormality rates, relationships to hatching success, the timing of reproductive maturation, hatchling growth rates, and hatchling survival rates. Contaminant levels in eggs may offer information for two different life stages—the embryo and the adult females—because contaminants are transferred to the egg from the mother during vitellogenesis (Pagano et al. 1999). Maternal transfer of POPs into eggs has been documented in some turtle species, including sea turtles (Russell et al. 1999; Stewart et al. 2011; Guirlet et al. 2008, 2010).
The OCPs concentrations in sea turtle eggs are of high concern and their potential impact on embryonic and hatchling development is poorly understood. In addition, sea turtle nesting populations are of high interest to determine the range of exposure among different species and locations (Alava et al. 2011). Because nesting females do not feed during migration or nesting periods (Bjorndal et al. 1997), their POPs concentrations are likely to reflect the contamination in their foraging areas and their feeding habits (Bjorndal et al. 1985, 1997; Alava et al. 2006, 2011). Consequently, the POPs concentrations in eggs may represent the contamination levels received on the adult female foraging grounds. Females nesting on the same beach but foraging in different locations would likely produce eggs containing different POPs concentrations. Alternatively, if females from one nesting beach forage in similar locations, then their egg POPs concentrations would be similar and indicative of their foraging regions. Adult females accumulate POPs from their prey as well as from incidentally ingested sediments, which then are deposited, along with lipids, into the follicles. According to Aguirre et al. (2006), the consumption of sea turtle products (tissues, eggs, and blood) poses a number of public health concerns because of the high lipid content and the presence of bacteria, parasites, and environmental contaminants. Thereby, the World Health Organization (WHO) and other regional organizations have provided a guide for consumption of foods containing environmental contaminants and acceptable daily intakes (ADIs) (FAO/WHO 2007). The ADIs are based on human and animal experiments, which investigate the nonobservable adverse effect levels of these chemicals and are generally presented as micrograms per kilogram of body weight per day (Van Oostdam et al. 2005).
Because marine turtles are an endangered species, it is important to understand the responses to long-term impact and conservation measures. Therefore, knowing the species exposure level to these compounds is of paramount importance to make informed management decisions and to perform response measures in order to improve the effectiveness of long-term conservation strategies in developing populations’ recovery (Lam et al. 2004; Casale et al. 2004; Jakimska et al. 2011). The purpose of this study was to determine the POPs and OCPs concentrations in eggs from two species of sea turtles, C. mydas and E. imbricata, nesting on the coasts of Mexico during two consecutive years (2014 and 2015). Understanding chemical contamination, and ultimately the potential risks to the development and reproduction, are crucial elements to the management and conservation of sea turtles.
Materials and methods
Sample collection
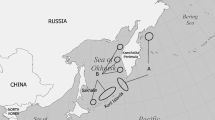
Eggs of two sea turtles species with spawning areas from Campeche were collected to analyse the concentration of organochlorine contaminants. Campeche is located in southeastern Mexico in the Yucatan Peninsula (Fig. 1).
Two species were analysed in the study: green turtles (C. mydas) were sampled in Isla Aguada field (18°47′15.5″N, 91°29′56.5″W), and hawksbill turtles (E. imbricata) were sampled in the Punta Xen field (19°12′39″N, 90°52′09.7″W) (Fig. 1). Sixty eggs for 60 individual sea turtles were collected (1 egg per nest), for each species during the breeding season of 2014 and 2015 (30 eggs per year/species). The curved carapace length (CCL) and curved carapace width (CCW) of the carapace were measured with a flexible tape (Bolten 1999). Sea turtle eggs were collected and wrapped in aluminium foil, stored in Ziploc bags, stored on ice and frozen at − 20 °C. All analyses were performed at the Institute of Ecology, Fishery and Oceanography of the Gulf of Mexico (EPOMEX, Campeche, Mexico).
Pollutants analysed in this study
A total of 20 organochlorine pesticides compounds were investigated in sea turtle eggs, including isomers of hexachlorocyclohexane (alpha, beta, gamma, delta-HCH), aldrin, dieldrin, endrin, endrin aldehyde, ketone endrin, trans chlordane, cis chlordane, endosulfan I, endosulfan II, endosulfan sulfate, methoxychlor, p,p′ DDE, p,p′ DDD, p,p′ DDT, heptachlor, heptachlor epoxide). OCPs were analysed using a mix of standards (SUPELCO 47426-U CLP Organochlorine Pesticide Mix), contaminant concentrations were organized as families: ΣDDT was defined as the sum of p,p′ DDE, p,p′ DDT, and p,p′ DDD; ΣChlordanes as the sum of cis-chlordane and trans-chlordane; ΣHCH as the sum of alpha, beta, gamma, delta; ΣHeptachlor as the sum of heptachlor and heptachlor epoxide; ΣDienes as the sum of aldrin, dieldrin, endrin, endrin aldehyde, and ketone endrin; ΣEndosulfans as the sum of endosulfan I, endosulfan II, and endosulfan sulfate. Limit of detection for each family of compounds in μg g−1 (HCHs—0.007; Aldrin—0.0018; DDTs—0.01; Chlordanes—0.009; Endosulfans—0.007; Heptachlors—0.013; Methoxychlor—0.01).
Contaminant analysis
All the solvents used in the laboratory procedures were of 98% of purity grade (HPLC). Silica gel, alumina, Florisil, and sodium sulfate were purified following the protocol NMX-AA-071-1981 (1981). The glassware was washed with Extran, dried in the oven for 4 h at 200 °C, and washed with acetone and hexane. POP analysis of the eggs followed the method described by Zhang et al. (2007). Fertile eggs were rinsed with distilled water, and the contents were extracted and homogenized thoroughly. The homogenized mix was dried in an oven at 40 °C. Three extractions were performed in an ultrasonic bath. For the first extraction, 50 ml of ethyl acetate-hexane (1:1) was added, and the sample was sonicated for 1 h. The organic layer was transferred to a glass tube, and the extraction was repeated twice with 40 mL of hexane for 1 h. Samples were purified by column chromatography. The column was packed with silica gel (2 g), alumina (2 g), florisil (2 g), and sodium sulfate (2 g). First, 20 ml of methylene chloride was added, followed by 20 ml acetone, and finally 20 ml of hexane. The mobile phase, 35-ml mixture of ethyl acetate: hexane (1: 9) was added. The cleaned extracts were diluted to 5 ml for analysis. The final volume of the solvent used was 0.5 ml.
Instrumental analysis
The contaminants were quantified using a Varian 3800 gas chromatograph equipped with an Ni63 electron capture detector and HT8 capillary column (60 m × 0.25 mm; 25-μm film thickness) (SGE Analytical Science, USA). The temperatures of the injector and detector were 150 and 300 °C, respectively. The oven temperature was maintained at 60 °C min−1 and then increased to 320 °C at a rate of 2 °C min−1 for 5 min. The nitrogen flow into the column was 2 ml/min and a composition of 30 ml/min. Qualitative data were obtained by calculating the area under the curve with the star Chromatography Workstation software version 6 and the calibration patter. The quality of the standard is 99%, and the stock solutions, to make the calibration curve were: 1, 10, 50, 100, and 150 µg/ml.
Quality assurance
Laboratory blanks were analysed for quality assurance. Chicken egg samples were used in triplicate. One milliliter of a 200 ng/ml Decachlorobiphenyl surrogate spike (SK011 Sigma-Aldrich) was added to the samples before the extraction, and they were subsequently refrigerated for 48 h. One of the subsamples was not spiked with the standard as a positive blank. Afterward, the contaminants were extracted and processed in an identical manner to the rest of the samples. Percentages of recovery was > 85%.
Statistical analysis
All obtained data were checked for distribution, normality and homogeneity of variances using the Kolmogorov–Smirnov and Levene’s tests, respectively (Zar 1996). A logarithmic transformation (log (x + 1)) was used when data did not fulfil the assumptions of normality or homogeneity of variances. Differences in concentrations among eggs within species and years were determined using a one-way Analysis of Variance (ANOVA) and the interactions using a two-way ANOVA. For these analysis, the IBM SPSS Statistics package, version 22, was used, and the significance level was 0.05. The variation of contaminants was, also, tested by a Permutational multivariate analysis of variance (PERMANOVA) test, including a multifactorial temporal and spatial design (sampling locations, years and interactions). The Principal Coordinates Analysis (PCA) was used visualise the temporal and spatial variation of selected contaminants, with vector overlays (Pearson correlations), indicating correlations between these variables and ordination axes (Anderson et al. 2008). Both PERMANOVA and PCA analysis were based on Euclidian distances between samples, after data transformation Log (x + 1). Multivariate PERMANOVA tests were performed using PRIMER with PERMANOVA software (PRIMER v6 & PERMANOVA v1, PRIMER-E Ltd.).
Results
A total of 120 eggs were sampled for contaminant analysis; data from 6 of these were excluded because of problems during analysis. We identified POPs as ΣChlordane, ΣHCHs, ΣDienes, ΣDDTs, ΣHeptachlor, ΣEndosulfans, and methoxychlor in all 114 of the eggs analysed. Compounds most commonly identified in Punta Xen were ΣDienes, ΣHCHs, and ΣDDT, and in Isla Aguada were ΣDienes, ΣHCHs, ΣChlordane, and ΣHeptachlor (Table 1).
Contaminants concentration in the eggs of hawksbill turtles
In Punta Xen, ΣChlordane, ΣDienes, ΣEndosulfans, and methoxychlor were found to be higher in eggs collected in 2014 than from 2015, whereas ΣDDTs, ΣHCHs, and ΣHeptachlor were found at higher levels in 2015 compared with 2014 (Table 1). For the hawksbill turtles, no significant differences were found for OCPs in eggs between years (p > 0.05). No correlation was found between the CCL and OCP concentrations in eggs (p > 0.05).
Contaminants concentration in the eggs of green turtles
In Isla Aguada, ΣChlordane, ΣHCHs, ΣDienes, ΣEndosulfans, ΣHeptachlor, ΣDDTs, and methoxychlor were the most highly concentrated compounds in 2015 compared with 2014 (Table 1). Significant differences were found between years for OCPs, except DDT, in eggs of green turtles (p = 0.124). No correlation was found between the CCL and OCP concentrations in eggs (p > 0.05).
Interyear and intersite comparisons
The concentrations of OCPs in eggs were significantly differed between green and hawksbill turtles (p < 0.05) in nearly every family of compounds, except for ΣDDT. When analysing the data of two-way ANOVA, according to the year and the sampling location, it was found that the interaction between factors was significant (p < 0.05). Additionally, location and year separately showed differences (p < 0.05; Table 2). The Permutational multivariate analysis of variance (PERMANOVA) test allowed to verify that all the locations were different from each other and showed a significant correlation between location and year (p = 0.015; Table 3). The first Principal Coordinate Analysis (PCA) showed a clear separation between the years and between both sampled locations: Isla Aguada e Punta Xen (Fig. 2). In both PCA analysis, all analysed contaminants were clearly associated with Isla Aguada and the 2015 sampling.
Discussion
Overall, the present research results may provide an important baseline data on contaminant concentrations in sea turtle eggs from south eastern Mexico. OCPs concentrations (ng/g dw) measured in this study and other research (ng/g dw; lw; ww) are reported in Table 4. In the discussion, where the authors measured the concentrations in ng/g wet mass the values were converted to ng/g dry mass.
The green turtle is a typically a nectonic and solitary animal and may occasionally form aggregations in feeding areas (Márquez 1990). The diet of this species varies considerably during its life cycle and during the first year of life, from an omnivorous diet, mainly consuming food of animal origin, to herbivorous when juvenile and adults, being able to feed themselves eventually of living sponges, propagules of mangrove, molluscs, fish, and crustaceans (Bjorndal et al. 1997). According to Meylan (1988), hawksbill turtles are omnivorous with a specialize diet made of sponges. Differences in feeding preferences, foraging strategies and thus trophic levels could explain the differences in OCPs concentrations observed in these two species of sea turtles.
The average hawksbill turtle ΣHCH concentration (0.59 ng/g dw) measured in the current study were lower to that found in hawksbill and green turtle eggs (1.88; 2.64 ng/g dw respectively) from Caribbean region (Dyc et al. 2015), leatherback eggs (1.64 ng/g dw) from Guiana Francesa (Guirlet et al. 2010), and green turtle eggs (2.76 ng/g dw) from Malasia (van de Merwe et al. 2009a), being lower concentrations were found in green turtle eggs (4.24 ng/g dw) found in the present study.
ΣDienes were the second most abundant OCP class measured in Punta Xen and Isla Aguada. The average ΣDienes concentrations measured in hawksbill eggs (0.31 ng/g dw) and green turtle (2.07 ng/g dw) were lower than levels measured in loggerhead eggs samples in Southern Florida (10.12 ng/g dw) (Alava et al. 2006). Alava and collaborators found that the mean 4,4′-DDE concentration in loggerhead eggs (200.8 ng/g dw) was higher than the concentration found in green turtle eggs, concluding that green turtles are herbivores, and as such, they do not accumulate POPs to the same level as omnivorous loggerhead turtles (Alava et al. 2006). Nevertheless, average ΣDDTs concentrations found in the present study were higher in green turtle eggs (1.20 ng/g dw) than in hawksbill turtles (0.25 ng/g dw). Extreme caution must be exercised when comparing values with those of other studies’ different species because of the differences in feeding grounds.
Green turtle eggs exhibited relatively lower concentrations of ΣChlordane (1.90 ng/g dw) in relation to leatherback eggs (9.12 ng/g dw) from Eastern, Florida (Stewart et al. 2011), and higher concentrations than those found in green turtle eggs (0.24 ng/g dw) from Malaysia (van de Merwe et al. 2009a). Observed results may indicate that different locations in the Gulf of Mexico seem to have a significant influence on OCP concentrations, as well as different years. The average concentration of ΣHeptachlor, ΣEndosulfans, and methoxychlor in Isla Aguada (1.73; 0.82; 0.76 ng/g dw respectively) was greater than that measured in the Punta Xen (0.12; 0.19; 0.07 ng/g dw respectively).
In fact, in the species C. mydas most OCPs concentrations appear to have increased during the two analysed years. The differences in concentrations between species are likely attributable to differing foraging locales, on trophic differences, as well as different metabolic breakdown or elimination of congeners in reptiles inhabiting different climates. For example, leatherback turtles inhabiting waters both much further north and much deeper than the loggerhead.
Future studies should investigate this latter possibility (Alava et al. 2011). In addition, the comparisons between the present and previous studies are limited because of different analytical methodologies, sampling locations, and sample sizes used. Further investigations are necessary to evaluate long-term effects of OCPs and to understand if the concentrations are decreasing or increasing on a temporal scale in green and hawksbill turtles nesting in south eastern Mexico in the Yucatan Peninsula.
Conclusions
The present study provides a foundation for future research and monitoring of sea turtle eggs for contaminant concentrations. Were analysed OCP concentrations in eggs of green and hawksbill turtles, indicated differences between species, which are classified into different trophic levels. The concentration of ΣDDTs was the only OCP group found at similar levels between species. Location and year of sampling were a significant factors influencing OCP concentrations in green turtles. Future studies should evaluate biological effects of contaminants in turtles and relationships with hatchling success, embryonic abnormality rates, hatchling growth rates, and hatchling survival rates.
References
Aguirre AA, Gardner SC, Marsh JC, Delgado SG, Limpus CJ, Nichols WJ (2006) Hazards associated with the consumption of sea turtle meat and eggs: a review for health care workers and the general public. EcoHealth 3:141–153
Alam S, Brim M (2000) Organochlorine, PCB, PAH, and metal concentrations in eggs of loggerhead sea turtles (Caretta caretta) from northwest Florida, USA. J Environ Sci Health Part B 35:705–724
Alava JJ, Keller JM, Kucklick JR, Wyneken J, Crowder L, Scott GI (2006) Loggerhead sea turtle (Caretta caretta) egg yolk concentrations of persistent organic pollutants and lipid increase during the last stage of embryonic development. Sci Total Environ 367:170–181
Alava JJ, Keller JM, Wyneken J, Crowder L, Scott G, Kucklick JR (2011) Geographical variation of persistent organic pollutants in eggs of threatened loggerhead sea turtles (Caretta caretta) from southeastern United States. Environ Toxicol Chem 30:1677–1688
Anderson M, Gorley RN, Clarke RK (2008) Permanova + for Primer: guide to software and statistical methods. Primer-E Limited, Plymouth
Andreani G, Santoro M, Cottignoli S, Fabbri M, Carpenè E, Isani G (2008) Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci Total Environ 390:287–294
Bjorndal KA, Carr A, Meylan AB, Mortimer JA (1985) Reproductive biology of the hawksbill Eretmochelys imbricata at Tortuguero, Costa Rica, with notes on the ecology of the species in the Caribbean. Biol Conserv 34:353–368
Bjorndal KA, Lutz P, Musick J (1997) Foraging ecology and nutrition of sea turtles. Biol Sea Turt 1:199–231
Bolten AB (1999) Techniques for measuring sea turtles. Res Manag Technol Conserv Sea Turt 4:110–114
Camacho M, Luzardo OP, Boada LD, Jurado LFL, Medina M, Zumbado M, Orós J (2013a) Potential adverse health effects of persistent organic pollutants on sea turtles: evidences from a cross-sectional study on Cape Verde loggerhead sea turtles. Sci Total Environ 458:283–289
Camacho M et al (2013b) Potential adverse effects of inorganic pollutants on clinical parameters of loggerhead sea turtles (Caretta caretta): results from a nesting colony from Cape Verde, West Africa. Mar Environ Res 92:15–22
Camacho M, Boada LD, Orós J, López P, Zumbado M, Almeida-González M, Luzardo OP (2014) Monitoring organic and inorganic pollutants in juvenile live sea turtles: results from a study of Chelonia mydas and Eretmochelys imbricata in Cape Verde. Sci Total Environ 481:303–310
Carr A (1964) Transoceanic migrations of the green turtle. Bioscience 14:49–52
Carr A, Carr MH (1972) Site fixity in the Caribbean green turtle. Ecology 53:425–429
Casale P, Laurent L, De Metrio G (2004) Incidental capture of marine turtles by the Italian trawl fishery in the north. Adriat Sea Biol Conserv 119:287–295
Clark R (1992) Marine pollution. Clarendon Press, Oxford
Clark D Jr, Krynitsky A (1980) Organochlorine residues in eggs of loggerhead and green sea turtles nesting at Merritt Island, Florida–July and August 1976. Pestic Monit J 14:7–10
Clark DR, Krynitsky AJ (1985) DDE residues and artificial incubation of loggerhead sea turtle eggs. Bull Environ Contam Toxicol 34:121–125
Corsolini S, Aurigi S, Focardi S (2000) Presence of polychlorobiphenyls (PCBs) and coplanar congeners in the tissues of the Mediterranean loggerhead turtle Caretta caretta. Mar Poll Bull 40:952–960
D’Ilio S, Mattei D, Blasi MF, Alimonti A, Bogialli S (2011) The occurrence of chemical elements and POPs in loggerhead turtles (Caretta caretta): an overview. Mar Poll Bull 62:1606–1615
da Silva J (2009) Ocorrência de pesticidas organoclorados e bifenilos policlorados em tartarugas marinhas Chelonia mydas. Universidade de São Paulo, São Paulo
da Silva CC, Varela AS, Barcarolli IF, Bianchini A (2014) Concentrations and distributions of metals in tissues of stranded green sea turtles (Chelonia mydas) from the southern Atlantic coast of Brazil. Sci Total Environ 466:109–118
de Andréa MM (2008) Bioindicadores ecotoxicológicos de agrotóxicos
De Andrés E, Gómara B, González-Paredes D, Ruiz-Martín J, Marco A (2016) Persistent organic pollutant levels in eggs of leatherback turtles (Dermochelys coriacea) point to a decrease in hatching success. Chemosphere 146:354–361
de Pádua Almeida A, Santos AJ, Thomé JC, Belini C, Baptistotte C, Marcovaldi MÂ, dos Santos AS, Lopez M (2011) Avaliação do estado de conservação da tartaruga marinha Chelonia mydas (Linnaeus, 1758) no Brasil Biodiversidade Brasileira
Derraik JG (2002) The pollution of the marine environment by plastic debris: a review. Mar Poll Bull 44:842–852
Dyc C, Covaci A, Debier C, Leroy C, Delcroix E, Thomé J-P, Das K (2015) Pollutant exposure in green and hawksbill marine turtles from the Caribbean region. Reg Stud Mar Sci 2:158–170
Gaos AR, Lewison RL, Wallace BP, Yañez IL, Liles MJ, Nichols WJ, Seminoff JA et al (2012) Spatial ecology of critically endangered hawksbill turtles Eretmochelys imbricata: implications for management and conservation. Mar Ecol Prog Ser 450:181–194
García-Besné G, Valdespino C, Rendón-von Osten J (2015) Comparison of organochlorine pesticides and PCB residues among hawksbill (Eretmochelys imbricata) and green (Chelonia mydas) turtles in the Yucatan Peninsula and their maternal transfer. Mar Pollut Bull 91:139–148
Gardner SC, Pier MD, Wesselman R, Juárez JA (2003) Organochlorine contaminants in sea turtles from the Eastern Pacific. Mar Pollut Bull 46:1082–1089
Godley B, Richardson S, Broderick A, Coyne M, Glen F, Hays G (2002) Long-term satellite telemetry of the movements and habitat utilisation by green turtles in the Mediterranean. Ecography 25:352–362
Groombridge B, Luxmoore R (1989) The green turtle and hawksbill (Reptilia: Cheloniidae): world status, exploitation and trade. CITES, Washington, DC
Guerranti C, Baini M, Casini S, Focardi SE, Giannetti M, Mancusi C, Marsili L, Perra G, Fossi MC (2014) Pilot study on levels of chemical contaminants and porphyrins in Caretta caretta from the Mediterranean Sea. Mar Environ Res 100:33–37
Guirlet E, Das K, Girondot M (2008) Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat Toxicol 88:267–276
Guirlet E, Das K, Thomé J-P, Girondot M (2010) Maternal transfer of chlorinated contaminants in the leatherback turtles, Dermochelys coriacea, nesting in French Guiana. Chemosphere 79:720–726
Hamann M, Schäuble CS, Simon T, Evans S (2006) Demographic and health parameters of green sea turtles Chelonia mydas foraging in the Gulf of Carpentaria, Australia. Endanger Species Res 2:81–88
Hamann M, Godfrey M, Seminoff J, Arthur K, Barata P, Bjorndal K, Bolten A, Broderick A, Campbell L, Carreras C (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endanger Species Res 11:245–269
IUCN (2016) The IUCN red list of threatened species, version 2016.3. www.iucnredlist.org. Downloaded on 10 June 2016
Jakimska A, Konieczka P, Skóra K, Namiesnik J (2011) Bioaccumulation of metals in tissues of marine animals, part II: metal concentrations in animal tissues. Pol J Environ Stud 20:1127–1146
Jerez S, Motas M, Cánovas RÁ, Talavera J, Almela RM, del Río AB (2010) Accumulation and tissue distribution of heavy metals and essential elements in loggerhead turtles (Caretta caretta) from Spanish Mediterranean coastline of Murcia. Chemosphere 78:256–264
Keller JM (2013) Forty-seven days of decay does not change persistent organic pollutant levels in loggerhead sea turtle eggs. Environ Toxicol Chem 32:747–756
Keller J, Kucklick J, McClellan-Green P (2004a) Organochlorine contaminants in loggerhead sea turtle blood: extraction techniques and distribution among plasma and red blood cells. Arch Environ Contam Toxicol 46:254–264
Keller JM, Kucklick JR, Stamper MA, Harms CA, McClellan-Green PD (2004b) Associations between organochlorine contaminant concentrations and clinical health parameters in loggerhead sea turtles from North Carolina, USA. Environ Health Perspect 112:1074–1079
Lake JL, Haebler R, McKinney R, Lake CA, Sadove SS (1994) PCBs and other chlorinated organic contaminants in tissues of juvenile Kemp’s Ridley Turtles (Lepidochelys kempi). Mar Environ Res 38:313–327
Lam JC, Tanabe S, Chan SK, Yuen EK, Lam MH, Lam PK (2004) Trace element residues in tissues of green turtles (Chelonia mydas) from South China waters. Mar Pollut Bull 48:174–182
Lazar B, Maslov L, Romanić SH, Gračan R, Krauthacker B, Holcer D, Tvrtković N (2011) Accumulation of organochlorine contaminants in loggerhead sea turtles, Caretta caretta, from the eastern Adriatic Sea. Chemosphere 82:121–129
Limpus C, Miller J, Paramenter C, Reimer D, McLachlan N, Webb R (1992) Migration of green (Chelonia mydas) and loggerhead (Caretta caretta) turtles to and from eastern Australian rookeries. Wildl Res 19:347–357
Lohmann K, Witherington BE, Lohmann CM, Salmon M (1997) Orientation, navigation, and natal beach homing in sea turtles. Biol Sea Turt 1:107–136
López-Mendilaharsu M, Sales G, Giffoni B, Miller P, Fiedler FN, Domingo A (2007) Distribución y composición de tallas de las tortugas marinas (Caretta caretta y Dermochelys coriacea) que interactúan con el palangre pelágico en el. Atlántico Sur Collect Vol Sci Pap ICCAT 60:2094–2109
Malarvannan G, Takahashi S, Isobe T, Kunisue T, Sudaryanto A, Miyagi T, Nakamura M, Yasumura S, Tanabe S (2011) Levels and distribution of polybrominated diphenyl ethers and organochlorine compounds in sea turtles from Japan. Mar Pollut Bull 63:172–178
Marcotrigiano G, Storelli M (2003) Heavy metal, polychlorinated biphenyl and organochlorine pesticide residues in marine organisms: risk evaluation for consumers. Vet Res Commun 27:183–195
Marcovaldi M, Marcovaldi G (1985) Projeto Tamar: área de desova, ocorrência e distribuição das espécies, época de reprodução, comportamento de postura e técnicas de conservação das tartarugas marinhas no Brasil Brasília, MA-IBDF, p 46
Marcovaldi MA, Lopez GG, Soares LS, Santos AJB, Bellini C, Santos AS, Lopez M (2011) Avaliação do estado de conservação da tartaruga marinha eretmochelys imbricata (linnaeus, 1766) no Brasil Biodiversidade Brasileira 1
Marcovecchio J, Freije R (2013) Procesos químicos en Estuarios. Universidad Tecnológica Nacional, Aires
Márquez M (1990) FAO species catalogue, vol 11: sea turtles of the world. FAO, Rome
McKenzie C, Godley B, Furness R, Wells D (1999) Concentrations and patterns of organochlorine contaminants in marine turtles from Mediterranean and Atlantic waters. Mar Environ Res 47:117–135
Meeting JFWECoFA, World Health Organization (2007) Evaluation of certain food additives and contaminants: sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives, vol 68. World Health Organization
Meylan AB, Donnelly M (1999) Status justification for listing the hawksbill turtle (Eretmochelys imbricata) as critically endangered on the 1996 IUCN Red List of Threatened Animals Chelonian. Conserv Biol 3:200–224
Miao X-S, Balazs GH, Murakawa SK, Li QX (2001) Congener-specific profile and toxicity assessment of PCBs in green turtles (Chelonia mydas) from the Hawaiian Islands. Sci Total Environ 281:247–253
Monagas P, Oros J, Araña J, Gonzalez-Diaz O (2008) Organochlorine pesticide levels in loggerhead turtles (Caretta caretta) stranded in the Canary Islands, Spain. Mar Pollut Bull 56:1949–1952
MTSG (Marine Turtle Specialist Group) (1995) A global strategy for the conservation of marine turtles. IUCN, Gland
Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A (2009) International Pellet Watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar Pollut Bull 58:1437–1446
Oros J, Gonzalez-Diaz O, Monagas P (2009) High levels of polychlorinated biphenyls in tissues of Atlantic turtles stranded in the Canary Islands, Spain. Chemosphere 74:473–478
Pagano JJ, Rosenbaum PA, Roberts RN, Sumner GM, Williamson LV (1999) Assessment of maternal contaminant burden by analysis of snapping turtle eggs. J Great Lakes Res 25:950–961
Parliament U (2008) Climate change act 2008. London
Podreka S, Georges A, Maher B, Limpus CJ (1998) The environmental contaminant DDE fails to influence the outcome of sexual differentiation in the marine turtle Chelonia mydas. Environ Health Perspect 106:185
Pritchard JK, Cox NJ (2002) The allelic architecture of human disease genes: common disease–common variant… or not? Hum Mol Genet 11:2417–2423
Russell RW, Gobas FA, Haffner GD (1999) Maternal transfer and in ovo exposure of organochlorines in oviparous organisms: a model and field verification. Environ Sci Technol 33:416–420
Seminoff JA, Zárate P, Coyne M, Foley DG, Parker D, Lyon BN, Dutton PH (2008) Post-nesting migrations of Galápagos green turtles Chelonia mydas in relation to oceanographic conditions: integrating satellite telemetry with remotely sensed ocean data. Endanger Species Res 4:57–72
Shigenaka G, Milton S (2003) Oil and sea turtles: biology, planning, and response. National Oceanic and Atmospheric Administration, NOAA’s National Ocean Service, Office of Response and Restoration
Stewart KR, Keller JM, Templeton R, Kucklick JR, Johnson C (2011) Monitoring persistent organic pollutants in leatherback turtles (Dermochelys coriacea) confirms maternal transfer. Mar Poll Bull 62:1396–1409
Storelli M, Marcotrigiano G (2003) Heavy metal residues in tissues of marine turtles. Mar Pollut Bull 46:397–400
Storelli MM, Zizzo N (2014) Occurrence of organochlorine contaminants (PCBs, PCDDs and PCDFs) and pathologic findings in loggerhead sea turtles, Caretta caretta, from the Adriatic Sea (Mediterranean Sea). Sci Total Environ 472:855–861
Swarthout RF, Keller JM, Peden-Adams M, Landry AM, Fair PA, Kucklick JR (2010) Organohalogen contaminants in blood of Kemp’s ridley (Lepidochelys kempii) and green sea turtles (Chelonia mydas) from the Gulf of Mexico. Chemosphere 78:731–741
van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Ibrahim K, Lee SY (2009a) Chemical contamination of green turtle (Chelonia mydas) eggs in peninsular Malaysia: implications for conservation and public health. Environ Health Perspect 117:1397
van de Merwe JP, Hodge M, Whittier JM, Lee SY (2009b) Analysing persistent organic pollutants in eggs, blood and tissue of the green sea turtle (Chelonia mydas) using gas chromatography with tandem mass spectrometry (GC-MS/MS). Anal Bioanal Chem 393:1719–1731
van de Merwe JP, Hodge M, Whittier JM, Ibrahim K, Lee SY (2010) Persistent organic pollutants in the green sea turtle Chelonia mydas: nesting population variation, maternal transfer, and effects on development. Mar Ecol Prog Ser 403:269–278
Van Oostdam J, Donaldson S, Feeley M, Arnold D, Ayotte P, Bondy G, Chan L, Dewaily E, Furgal C, Kuhnlein H (2005) Human health implications of environmental contaminants in Arctic Canada: a review. Sci Total Environ 351:165–246
Yogui GT (2002) Ocorrência de compostos organoclorados (pesticidas e PCBs) em mamíferos marinhos da costa de São Paulo (Brasil) e da Ilha Rei George (Antártica). Universidade de São Paulo
Zar J (1996) Biological analysis. Prentice Hall, Englewood Cliffs
Zhang H, Wang Z, Lu B, Zhu C, Wu G, Walter V (2007) Occurrence of organochlorine pollutants in the eggs and dropping-amended soil of Antarctic large animals and its ecological significance. Sci China D Earth Sci 50:1086–1096
Acknowledgements
The license (SGPA/DGVS/03974/14) to collect the eggs samples of 120 turtles was provided by the Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT). The authors want to thank the turtle camp Grupo Ecologista Quelonios A.C., Punta Xen and Campamento Tortuguero de Isla Aguada, who aided in the fieldwork. This work was supported by Coordination for the Improvement of Higher Education Personnel (CAPES Brazil), (1201/2013-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Salvarani, P.I., Morgado, F., Vieira, L.R. et al. Organochlorines Contaminants in Eggs of Hawksbill (Eretmochelys imbricata) and Green Sea Turtles (Chelonia mydas) from Mexico coast. Arch Environ Contam Toxicol 76, 425–434 (2019). https://doi.org/10.1007/s00244-018-00589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-00589-3