Abstract
This study investigated the levels and distribution of 17 polychlorinated biphenyls (PCBs) and organochlorine pesticides (HCB, α-HCH, β-HCH, γ-HCH, p,p′-DDE, p,p′-DDD, and p,p′-DDT) in placenta samples from women living in the coastal area of Croatia. During November 2012 to February 2013, 51 placenta samples were collected from healthy mothers. This study presents the first report about Croatian placenta samples. Each of the analysed compounds were found in all of the samples; all of the maximum values were < 1 ng g−1 w.w., and the highest median value found for PCB-28 was 11.2 pg g−1 w.w. PCBs and organochlorine pesticide (OCPs) present in placenta samples were tested for their genotoxic potential using the alkaline comet assay. The alkaline comet assay is one of the most reliable methods in assessing the DNA lesions that occurs in direct interaction of a chemical and the genome. The detected levels of PCBs and OCPs in the placenta did not pose a significant risk to the children’s DNA during embryonic and foetal growth following short-term exposure. PCB and OCP concentrations in the placenta samples did not induce any significant primary damage to DNA in terms of DNA strand breaks and changes in the primary chemical structure, which could be detected by the alkaline comet assay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) are Persistent Organic Pollutants (POPs) because of their physicochemical properties relevant to their environmental fate and commercial purposes. They are released to the environment by intensive human activities in industry and agriculture, and due to their lipophilicity and persistence, they tend to accumulate in the food chain and bioaccumulate in the adipose tissue of humans and animals. PCBs and OCPs act as endocrine disruptors; some epidemiological research suggests possible damage of neural function, reproductive and immune systems, and temporal increases in the incidence of cancers in hormonally sensitive tissues (Longnecker et al. 1997; Ross 2004). Due to worldwide public concern, usage of PCBs and OCPs was banned and/or restricted during the 1970s and 1980s in many industrial countries; however, they are still present in the biotic and abiotic part of the environment. Humans are mainly exposed to PCBs and OCPs via food, although environmental and occupational exposure cannot be excluded. Most PCBs and OCPs are recognized by the IARC and WHO as probable or possible carcinogens for humans. Some PCB congeners (118, 156) are even declared to be known human carcinogens (IARC 2012).
PCBs and OCPs are transferred from the mother to the child primarily via breast milk. In Croatia, the presence of PCBs and OCPs in human milk has been investigated since the early 1970s (Krauthacker et al. 2009). Bergonzi et al. (2009) evaluated levels of PCBs and OCPs in the placenta, maternal and cord blood serum, and maternal adipose tissues. Their results indicated a transplacental transfer of pollutants from maternal to foetal tissues. In another study by Bergonzi et al. (2011), the authors discussed potential health effects on newborns and found a positive association between DDT levels and birth length. In both of these studies, the authors discussed published data of prenatal exposure to PCBs and OCPs and their influence on adverse developmental effects. They indicated that negative results existed to support the thesis that low-level exposure has no impact, and also, opposite results that showed a positive association between exposure to pollutants and adverse developmental effects. Our hypothesis is that such conflicting findings require more extensive investigation and a different approach.
The level of damage to DNA is a commonly accepted biomarker of the risk of cancer development and assessment of the exposure to potentially carcinogenic chemical agents. One of the most reliable methods in assessing the DNA lesions that occur in the direct interaction of a chemical and the genome is the alkaline comet assay (Collins et al. 2014). The method detects a wide range of primary damage to DNA. Basically, these types of damage could be divided into two subgroups: DNA breaks and alkali labile sites. Breaks could be either single or double strand breaks, depending whether one or both DNA strands are affected, respectively. Regarding alkali labile sites, these are all changes in the primary structure of DNA molecule that cause one or both strands to break during the unwinding process under alkaline conditions. Among others, alkali labile sites include alkylation, DNA-adducts, pyrimidine dimers, cross-linking, covalent DNA–protein bonds, apurinic and apyrimidinic sites, oxidatively damaged bases. These primary lesions represent a hazard for DNA stability and preservation of the genetic code. Although they are efficiently repaired, if left unrepaired during DNA replication, they may lead to mutation induction or be further converted in structural genome damage by other complex mechanisms (Azqueta and Collins 2013). As such, comet assay endpoints may be considered relevant biomarkers of exposure to genotoxic chemicals and the assay a surrogate method in the assessment of carcinogenic risk (Singh 2016).
To the best of our knowledge, no data on placental organochlorine contaminant levels in samples from Croatia has been published to date. The present study reports the levels and distribution of PCBs and OCPs in placenta samples from women living in the coastal area of Croatia.
Materials and Methods
Study Design
The study involved healthy mothers who gave birth at the General Hospital in Zadar (Croatia) during the period November 2012 to February 2013; the total number of collected placentas was 51. This study was approved by the Ethics Committee of the General Hospital Zadar (No: 01-1695-1/11, Date: 13 June 2011). Each participant was informed about the study and gave signed consent. The collected placentas and data were coded to protect the participants’ privacy. The recruited participants were interviewed the day after delivery by one of the researchers, and data were collected by a predesigned questionnaire. The collected data included: general personal data (name and date of birth, age, place of birth, educational status, and occupation); maternal body height and weight (before pregnancy/before delivery); data on possible sources of exposure to various contaminants (environmental and/or occupational); potential sources of recent environmental exposure (place of residence, relocation in the past 24 months, vicinity of factory, smelter, highway, large crossroad, or bus terminal with heavy traffic); general health status and data on the last pregnancy and delivery (parity, previous abortions or miscarriages, twins); data on the newborn; data on supplements and/or medicine intake during the pregnancy. Participants were asked to declare their dietary habits as predominant diet type: mixed, vegetarian, vegetarian plus eggs, vegetarian plus eggs and dairy products, as well as providing the data on frequency and approximate quantity of coffee, tea, and alcohol consumption. The exclusion criterion was the presence of a chronic disease.
The study was undertaken on a nonprobabilistic sample of 51 participants from the Zadar region. The study included female subjects who did not have serious medical disorders during pregnancy, childbirth, and breastfeeding in a randomly selected sample after birth in the maternity clinic of the Zadar General Hospital. In the questionnaire, we recorded data about possible health problems during pregnancy, including elevated blood pressure, elevated blood sugar, leg oedema, and other possible side effects, which served as criteria for exclusion.
Placenta Samples
Placenta sampling was performed according to the prepared sampling procedure. Immediately after the delivery, the entire placenta was weighed and placed in a plastic bag, avoiding external contamination. Each plastic bag was marked with the subject’s identification code and frozen at − 20 °C. The frozen samples were carefully transported in a portable refrigerator to the analytical laboratory. Placentas were kept at − 20 °C until analysed. A triangular portion was cut and mechanically homogenised: maternal and foetal sides, and central and peripheral parts.
Chemical Analysis
The placenta samples (approximately 20 g) were ground with 2 g of sodium sulphate in a glass mortar and dissolved in 40 mL of n-hexane. The mixture was passed through a filter paper (Whatman no. 1) to a preweighted test tube. The extracts were concentrated under a gentle stream of nitrogen. Tissue lipid content was determined gravimetrically. The clean-up was first done with 4 mL of 96% sulphuric acid and then repeated two more times. The solvent was evaporated to residues under a gentle stream of nitrogen. Before gas chromatography, the residues were dissolved in 1.0 mL of n-hexane. Analysed compounds: OCPs (HCB (hexachlorobenzene), α-HCH, β-HCH, γ-HCH (α-, β-, γ-hexachlorocyclohexanes), p,p′-DDE, p,p′-DDD, and p,p′-DDT). PCBs are a group of 209 congeners, and methods of analysis usually include six indicator PCBs, whose selection is based on their dominant presence in technical mixtures, the environment, and animal and human tissues. The selection of toxicologically relevant congeners, which mostly include some mono- and di-ortho substituted PCBs, represents the more toxic fraction of PCBs in the sample.
Six indicator PCBs: PCB-28, PCB-52, PCB-101, PCB-138, PCB-153, and PCB-180; toxicologically relevant PCB congeners: PCB-60, PCB-74, PCB-105, PCB-114, PCB-118, PCB-123, PCB-156, PCB-157, PCB-167, PCB-170, PCB-189.
High-resolution gas chromatography with electron capture detector(s) was performed on a CLARUS 500 chromatograph using two capillary columns (Restek, Bellefonte, PA) simultaneously: (1) 60 m × 0.25 mm, Rtx-5 film thickness of 0.25 μm, and (2) 30 m × 0.25 mm, Rtx-1701 film thickness of 0.25 μm. Column temperature was programmed from 100 °C to 110 °C at 4 °C min−1 (isothermally 5 min at 110 °C) and then to 240 °C at 15 °C min−1 (50 min isothermally at 240 °C). The carrier gas was nitrogen. The injector and detector temperatures were 250 and 270 °C, respectively, and the volume of the injected sample was 5 μL. Only the compounds identified on both columns were evaluated. Qualitative and quantitative analyses were done by comparison with an external standard. Method recovery and reproducibility were determined by adding a known amount of all of the analysed compounds (at levels between 5 and 55 pg g−1 of placenta mass for OCPs and between 50 and 75 pg g−1 of placenta mass for PCBs) to five aliquots of homogenised samples before extraction (method of additions). The recoveries of PCBs and OCPs were calculated after subtracting the mean levels of two nonfortified subsamples from the fortified ones. The recoveries for PCBs ranged from 63 to 90%, with a relative standard deviation from 1 to 15%. For OCPs, they ranged from 57 to 87%, with a relative standard deviation from 11 to 16%. The determination limits for PCBs and OCPs were 0.1 and 0.7 pg g−1 of fresh weight, respectively, and were calculated as the average of all determinations based on the signal-to-noise ratio and recovery of compounds. The relative accuracy of the method was determined using certified reference material CRM 430 (Community Bureau of Reference, Commission of the European Communities, Brussels, Belgium), and the obtained results were in agreement with the reference range. Blank reagent was used to show laboratory contamination; the concentrations of analytes in blanks were below the detection limit of the instrument. The results were recalculated by using recovery.
Alkaline Comet Assay
The placenta samples were subjected to extraction in two different types of media. We used principals of extraction as described in a paper by Sandal et al. (2008). The first extraction approach used RPMI 1640 cell culture medium (Sigma-Aldrich, Munich, Germany) in which the placenta samples were kept for 24 h at 37 °C to leach all of the compounds soluble in water medium that may trespass the placental barrier and reach the bloodstream of the foetus. This approach reflects real exposure scenarios more realistically. In the second approach, the placental tissue was leached using dimethyl sulfoxide (DMSO; Sigma-Aldrich, Munich, Germany) for 24 h at 37 °C to extract organic compounds. In both approaches, 0.5 mg of placental tissue was leached by 0.5 mL of the extraction medium of choice.
Detection of primary damage to DNA of human peripheral blood leukocytes was assessed by the alkaline comet assay technique (Collins et al. 2014). All of the chemicals and reagents used in the testing were purchased from Sigma-Aldrich (Munich, Germany).
The blood sample was obtained from a 27-year-old, nonsmoker, male donor without any medical records. Venous blood was collected under sterile conditions in a vacutainer tube (BD Vacutainer® REF 367883, V = 4 mL; Becton-Dickinson, Franklin Lakes, NJ) containing lithium heparin (LH 68 I.U.) as the anticoagulant. Testing was done on a single donor principle to avoid interindividual differences that may result in the dissolution of the results and affect their statistical analysis.
To prepare the agarose microgels, microscopic slides were precoated with 1% (w/w) normal melting point (NMP) agarose. A total of 6 µL of blood was then mixed with 100 µL of 0.5% (w/w) low melting point (LMP) agarose heated to 37 °C. In the next step, it was placed onto microscopic slides, covered by a cover-slip, and placed on ice to polymerise for 7 min.
Following polymerisation, agarose-embedded leukocytes were treated by adopting a slightly modified protocol by Lah et al. (2005). An amount of 60 µL of the extract of placenta was pipetted onto the comet assay slide and covered by the cover-slip. Preparations were incubated in a humid chamber for 30 min at 37 °C in 5% CO2. A total of 19 polar water-medium and 19 nonpolar-medium extracts were tested, each on duplicate slides. Negative (solvent) control slides were treated with the same volume of RPMI 1640 or DMSO (as reference to the extracts obtained by the incubation of the placentas in cell culture medium or DMSO, respectively). Positive control slides were treated with 60 µL of 10 µM hydrogen peroxide for 10 min on ice. After the treatment, the cover-slips were removed and the slides were gently washed with phosphate buffer pH 7.4 and immersed into the lysis solution (1% lauryl sarcosine, 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris–HCl, 1% Triton X-100, 10% dimethyl sulphoxide, pH 10) for 24 h at 4 °C. Slides were denatured (0.3 M NaOH, 1 mM Na2EDTA, pH 13) for 20 min at 4 °C and electrophoresed in the horizontal system (Life Technologies Ltd, Carlsbad, CA) at 0.9 V cm−1, 300 mA for 20 min. Neutralisation of slides was done by redistilled water, and they were stained with ethidium bromide (20 µg mL−1). The analysis of primary DNA damage was performed using Comet Assay IV system (Perceptive Instruments Ltd., Suffolk, Halstead, UK) connected to a epifluorescence microscope Olympus BX 50 (Olympus, Tokyo, Japan). Two comet assay endpoints as markers of DNA damage were recorded: comet tail length (µm) and % of DNA that migrated from the nucleoid to the comet tail (% of DNA). Fifty nucleoids were analysed per duplicate slides, meaning that DNA damage was measured in 100 nucleoids of leukocytes treated with water-based extract and 100 nucleoids of leukocytes treated with nonpolar media extract from a single placenta.
Statistical Analysis
Statistical analysis of the results of comet assay endpoints was done according to most recent recommendation for performing the analysis (Møller and Loft 2014; Lovell 2016; OECD 2016). Testing the normality of distributions was based on Shapiro–Wilk statistics. The distribution comet assay data was normalized by logarithmic transformation in accordance with Anderson and Laubenthal (2013). Comparison was done with Kruskal–Wallis ANOVA rank model (Møller and Loft 2014; Lovell 2016).
For each comet endpoint, tail length and % of DNA in comet tail, parameters of descriptive statistics, were calculated and are presented in Tables 1 and 2. For further analysis, normalisation of the distribution of comet assay data for both parameters was done by base-10 logarithmic transformation (Lovell and Omori 2008). One-way analysis of variance with post hoc Bonferroni test was applied to test the difference in comet assay parameters between negative control leukocytes and leukocytes treated with the placenta extracts.
To address the issue of the ability of PCBs and OCPs present in placenta samples to adversely affect the DNA of foetus, we tested the extracted POPs for their genotoxic potential. The level of primary DNA lesions as the sensitive biomarker of the risk of clastogenicity was determined on human peripheral blood leukocytes treated with POPs leached from the placenta samples. The extent to which the measured primary DNA damage is affected by and linked with the determined mass concentration of specific congeners of PCBs, HCHs, HCB, and DDT/D/E was statistically tested by Spearman’s correlation and multiple regression analysis.
The association between comet assay parameters and the level of α-, β-, γ-HCH, HCB, DDE/D/T, and PCB samples was tested by Spearman’s correlation analysis. Multiple regression analysis was done to evaluate the dependence between tail length and tail intensity values as parameters of primary DNA damage, and organic entities (Table 3). The dependence of comet assay endpoints on α-, β-, γ-HCH, HCBs, toxicologically relevant PCB congeners, an additional group of PCB congeners, and the sum of concentrations of DDT, DDD, and DDE. Age, dietary habits, data on coffee, tea, and alcohol consumption, and medicine intake during pregnancy were considered confounding factors. All of the subjects were nonsmokers. Statistical analysis was done using STATISTICA 12 (StatSoft, Inc., Tulsa, OK).
Results and Discussion
Organochlorine levels: Mass fractions of PCBs and OCPs expressed as range, first, second, and third quartile in picogram per gram of wet weight (pg g−1 w.w.) in the human placenta samples analysed are summarised in Table 1. Although the results are expressed per wet weight, lipid content was determined for each sample, and it proved to be low (median 0.04%).
Each of the analysed compounds was found in all of the samples; all of the maximum values were > 1 ng g−1 w.w. and the highest median value found for PCB-28 was 11.2 pg g−1 w.w. Indicator PCB mass fraction medians had the following pattern: PCB-28 > PCB180 > PCB-52 > PCB-138 > PCB-153 > PCB-101.
Profiles with lower chlorinated biphenyls, such as those of PCB-28 and PCB-52 as major contributors, is rare in species at the top of the food chain, including humans. This pattern indicates possible recent exposure. However, to the best of our knowledge, there was no source of exposure to PCBs in the vicinity of the subjects’ place of residence and no source of exposure to PCBs in all of Croatia. As mentioned in the “Materials and Methods” section, mothers were interviewed the day after delivery by one of the researchers, and the data were collected by the predesigned questionnaire. Data from the questionnaires did not indicate exposure of mothers to pollution, including via dietary habits.
With regard to toxicologically relevant PCB congeners, the highest median levels were found for PCB-170, PCB-74, and PCB-189. The lowest levels were found for PCB congeners 114 and 118. The profile of OCPs found in the placenta samples was as follows: the highest mass fraction was found for γ-HCH, followed by DDE and β-HCH, DDD, DDT, α-HCH, and HCB.
Regarding the investigation of organochlorine levels in humans in Croatia, several studies about organochlorines in human milk samples have been conducted (Herceg Romanić and Krauthacker 2006; Klinčić et al. 2014). In these studies, characteristic profiles of organochlorine compound were found. The typical OCP profile found in human milk samples from Croatia was dominated by p,p′-DDE and β-HCH. As for PCB congeners in human milk, those from the group of indicator congeners (PCB-138, PCB-153 and PCB-180) are the predominant congeners, and mono-ortho congeners PCB-118 and PCB-156 are present. Also observed earlier in the human milk samples from Croatia was the detection of relatively high levels of PCB-170 in human milk.
The similarity of the organochlorine profile in human milk and placenta samples lies in the abundance of pesticides γ-HCH and DDE, and concerning PCBs in the relatively high presence of PCB-170. When comparing the placental levels of OCPs found in this study to those reported recently in Italy and Spain, it can be seen that they are generally lower. In placenta samples collected in Italy during 2006, the median (and ranges) placental mass fractions of ∑PCBs (30 congeners), p,p’-DDE, and HCB were 890 (450–4560), 630 (160–5110), and 190 (090–710) pg g−1 of wet weight, respectively (Bergonzi et al. 2009). A similar ∑PCB range (943–4331 pg g−1 of wet weight) but higher median value (2292 pg g−1 of wet weight) was reported for placenta samples from a Spanish population collected during 2003–2004 (Gómara et al. 2012). Unlike in the Italian placenta samples where PCB-28, PCB-52 and PCB-101 were not even detectable, in the Spanish samples lower chlorinated biphenyls PCB-52 and PCB-101 were the major contributors to ∑PCBs, which is in agreement with the results found in our study. In the placenta samples from southern Spain collected 2000–2002, the median mass fraction of p,p′-DDE, p,p′-DDT, and γ-HCH was 1780, 500, and 290 pg g−1 of wet weight, respectively (Lopez-Espinosa et al. 2007). In the Spanish placenta samples collected 2004–2008, Vizcaino et al. (2014) found pesticides in lower mass fractions than Lopez-Espinosa et al. (2007), but they were the same as the reported levels of few PCB congeners and higher than the levels found in our study by one to two orders of magnitude.
Alkaline Comet Assay
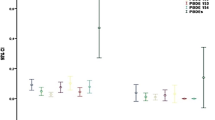
The results of the evaluation of the effect of placental extracts on the induction of primary DNA damage in human leukocytes, using water-based and polar extraction medium, are shown in Table 2. ANOVA was used to test the statistical significance of the alkaline comet assay results obtained for each placenta’s extract with the corresponding vehicle control. Because none of the extracts significantly increased primary damage to DNA, expressed in terms of both comet assay endpoints in Table 2, data for the extracts are presented in a summarised form. For the first sample of the placenta extracted in cell culture medium, an increase in % of DNA compared with the vehicle control was observed at the level of 0.05 (1.03 and 0.45%, respectively), and for the other sample an increase in comet tail length was registered at the significance level of 0.01 (26.5 and 24.0 µm, respectively). Nevertheless, both increases were within the range of regular controls (Collins 2004) for the alkaline comet assay and could not be considered biologically relevant.
With regards to multiple regression analysis, none of the measured organic components significantly affected the primary DNA damage parameters of the alkaline comet assay. The highest contribution to primary DNA damage was obtained for α-, β-, and γ-HCH (β = 0.49; p = 0.21) and the lowest for toxicologically relevant PCBs (β = 0.11; p = 0.75).
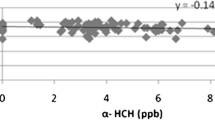
Considering the dependence of comet tail length in the leukocytes treated with placenta extracts, the highest Spearman’s correlation coefficient was observed on a joint level of DDT, DDD, and DDE (R2 = 0.02) and the lowest for indicator PCBs (R2 = 0.00). The highest dependence of % of DNA damage in the comet tail was observed on a joint level of DDT, DDD, and DDE (R2 = 0.20), which also was statistically significant (p = 0.050). The lowest dependence of % of DNA in the comet tail was recorded on HCBs (R2 = 0.01).
The results of multiple regression analysis (Table 3) are in alignment with some previous observations (Abhishek et al. 2014; Ennaceur 2017), indicating that hexachlorocyclohexane (HCH) itself does not pose a genotoxic threat to certain cell types. Abhishek et al. (2014) failed to detect primary damage to DNA by alkaline comet assay in human keratinocyte cell line treated by α-HCH. In her work, Ennaceur (2017) also obtained negative genotoxicity results for α-HCH by applying cytokinesis-block micronucleus assay in peripheral blood lymphocyte cultures. For β- and γ-HCH, the author detected an increase in a number of micronuclei, but it was restricted for the highest concentrations tested (50 and 100 mg L−1) at which a significant level of cytotoxicity also was recorded. Thus, the observed genotoxicity could not be attributed to the genotoxic potential of the testing substances but to their cytotoxicity (OECD 2014). Besides, the mass concentrations of HCHs detected in placenta samples in the present study were far below the concentrations used in Ennaceur (2017). Nevertheless, HCHs possess strong synergistic potential, and in the mixture with other POPs, it contributes to an increased formation of intracellular reactive oxygen species (ROS). These are responsible for inducing oxidative damage to nitrogen bases in DNA, and such type of lesions could be sensitively detected by the alkaline comet assay (Abhishek et al. 2014). Based on the obtained results, we can conclude that levels of HCHs measured in the placenta samples analysed in this study are still too low to induce significant deterioration of the primary structure of DNA, but they do contribute to minor changes in it, as detected by the alkaline comet assay.
With regards to the low contribution of toxicologically relevant (TR) PCBs to the overall primary DNA damage, this can be justified by the fact that multiple regression takes into consideration the contribution of a single analysed organic compound to the chosen model with respect to all of the compounds. Following the calculation of Spearman’s correlation coefficient, TR PCBs were the second most relevant chemical group that affected primary DNA damage levels. These are a group of xenobiotics that act by binding to the aryl hydrocarbon receptor (AhR) and through this pathway induce increased transcription of Cyp1 genes, elevated ROS production, and consequently, induction of oxidative DNA damage (Zhang et al. 2015).
Our findings suggest that the allowed frame of treatment period for the comet assay was too short for TR PCBs to entirely exhibit their impact on DNA integrity. A study by Sandal et al. (2008) also assessed the genotoxicity of PCBs dissolved in DMSO by applying the in vitro alkaline comet assay on human peripheral blood leukocytes. But only two congeners were tested: PCB-52 as a nonconplanar and PCB-77 as the planar congener. The DNA damaging potential of both PCBs was observed only at the highest concentrations used, which were far above those that we detected in placenta samples (1 µmol L−1 for PCB-52 and 10 µmol L−1 for PCB-77). The adverse effect on DNA was more prominent for the noncoplanar congener, which in our study is found within toxicologically relevant PCB congeners.
DDT was indicated as a genotoxic agent inducing genome damage by clastogenic pathways of action (Binelli et al. 2008; Canales-Aguirre et al. 2011; Gerić et al. 2012). Some of these studies also assigned its metabolites DDE and DDD as substances able to interact with DNA and affect its primary structure by forming adducts (Binelli et al. 2008; Gerić et al. 2012; Ennaceur et al. 2008). Our results, which confirmed the highest significant correlation of primary DNA damage with DDT and its metabolites, are certainly in alignment with such previously published data (Tables 2, 3). Ennaceur et al. (2008) reported that HCB did not exhibit a genotoxic effect evaluated by micronucleus assay in peripheral blood lymphocytes. Using correlation analysis, we observed the nonsignificant dependence between the comet assay endpoints and levels of HCBs in the placental tissue, although POPs are capable of inducing a genotoxic effect (González-Mille et al. 2010; Juárez-Santacruz et al. 2013). However, the levels of toxicologically relevant PCBs measured in the placenta samples of Croatian women in labor were much lower than those determined in the adipose tissue and blood samples of the general population (100 and 2 pg g−1, respectively) as reported by Kannan et al. (1994) and, thus, not genotoxically active at the measured mass concentrations.
Again, the levels of POPs measured in the fish were much higher compared with those determined in the placenta samples in our study. Thus, it is reasonable that such low concentrations did not induce any significant primary damage to DNA in terms of DNA strand breaks and changes in the primary chemical structure detectable the alkaline comet assay. Thus, considering the results of previous studies on humans and in vitro together with our measurement of mass concentrations of POPs in placenta samples and the assessment of their potential to induce lesions in DNA, it could be concluded that the detected levels of POPs in placenta do not pose a considerable risk to children’s DNA on short term exposure during embryonic and foetal growth.
References
Abhishek A, Ansari NG, Shankhwar SN, Jain A, Singh V (2014) In vitro toxicity evaluation of low doses of pesticides in individual and mixed condition on human keratinocyte cell line. Bioinformation 10:716–720. https://doi.org/10.6026/97320630010716
Anderson D, Laubenthal J (2013) Analysis of DNA damage via single-cell electrophoresis. In: Makovets S (ed) DNA electrophoresis. Springer, Edinburgh, pp 209–218
Azqueta A, Collins AR (2013) The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Arch Toxicol 87:949–968. https://doi.org/10.1007/s00204-013-1070-0
Bergonzi R, Specchia C, Dinolfo M, Tomasi C, De Palma G, Frusca T, Apostoli P (2009) Distribution of persistent organochlorine pollutants in maternal and foetal tissues: data from an Italian polluted urban area. Chemosphere 76:747–754. https://doi.org/10.1016/j.chemosphere.2009.05.026
Bergonzi R, De Palma G, Specchia C, Dinolfo M, Tomasi C, Frusca T, Apostoli P (2011) Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators of fetal growth and health. Sci Total Environ 409:2888–2893. https://doi.org/10.1016/j.scitotenv.2011.04.031
Binelli A, Riva C, Cogni D, Provini A (2008) Genotoxic effects of p,p′-DDT (1,1,1-trichloro-2,2-bis-(chlorophenyl)ethane) and its metabolites in Zebra mussel (D. polymorpha) by SCGE assay and micronucleus test. Environ Mol Mutagen 49:406–415. https://doi.org/10.1002/em.20400
Canales-Aguirre A, Padilla-Camberos E, Gómez-Pinedo U, Salado-Ponce H, Feria-Velasco A, De Celis R (2011) Genotoxic effect of chronic exposure to DDT on lymphocytes, oral mucosa and breast cells of female rats. Int J Environ Res Public Health 8:540–553. https://doi.org/10.3390/ijerph8020540
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26:249–261. https://doi.org/10.1385/MB:26:3:249
Collins AR, Koppen G, Valdiglesias V, Dusinska M, Kruszewski M, Møller P, Rojas E, Dhawan A, Benzie I, Coskun E, Moretti M, Speit G, Bonassi S, For the ComNet Project (2014) The comet assay as a tool for human biomonitoring studies: the ComNet Project. Mutat Res Rev Mut Res 759:27–39. https://doi.org/10.1016/j.mrrev.2013
Ennaceur S (2017) Study of the genotoxic and cytotoxic effects of the α-, β-, and γ-hexachlorocyclohexane isomers in human lymphocyte cells using the cytokinesis-block micronucleus assay. Drug Chem Toxicol 40(1):85–89. https://doi.org/10.1080/01480545.2016.1175008
Ennaceur S, Ridha D, Marcos R (2008) Genotoxicity of the organochlorine pesticides 1,1-dichloro-2,2- bis(p-chlorophenyl)ethylene (DDE) and hexachlorobenzene (HCB) in cultured human lymphocytes. Chemosphere 71:1335–1339. https://doi.org/10.1016/j.chemosphere.2007.11.040
Gerić M, Ceraj-Cerić N, Gajski G, Vasilić Ž, Capuder Ž, Garaj-Vrhovac V (2012) Cytogenetic status of human lymphocytes after exposure to low concentrations of p,p′-DDT, and its metabolites (p,p′-DDE, and p,p′-DDD) in vitro. Chemosphere 87:1288–1294. https://doi.org/10.1016/j.chemosphere.2012.01.037
Gómara B, Athanasiadou M, Quintanilla-López JE, González MJ, Bergman Å (2012) Polychlorinated biphenyls and their hydroxylated metabolites in placenta from Madrid mothers. Environ Sci Pollut Res 19:139–147. https://doi.org/10.1007/s11356-011-0545-x
González-Mille DJ, Ilizaliturri-Hernández CA, Espinosa-Reyes G, Costilla-Salazar R, Díaz-Barriga F, Ize-Lema I, Mejía-Saavedra J (2010) Exposure to persistent organic pollutants (POPs) and DNA damage as an indicator of environmental stress in fish of different feeding habits of Coatzacoalcos, Veracruz, Mexico. Ecotoxicology 19:1238–1248. https://doi.org/10.1007/s10646-010-0508-x
Herceg Romanić S, Krauthacker B (2006) Organochlorine pesticides and PCB congeners in human milk from two population groups in Croatia. Bull Environ Contam Toxicol 76:705–711. https://doi.org/10.1007/s00128-006-0977-z
IARC—International Agency on Research on Cancer (2012) Estimated cancer incidence, mortality and prevalence worldwide. http://globocan.iarc.fr/Default.aspx. Accessed 21 July 2017
Juárez-Santacruz L, García-Nieto E, Costilla-Salazar R, García-Gallegos E, Coronel-Olivares C, Gómez-Camarillo M, Gaytán-Oyarzún J (2013) Assessment of the genotoxic potential of sediments contaminated with POPs and agricultural soils using vicia faba micronucleus assay. Soil Sediment Contam 22:288–300. https://doi.org/10.1080/15320383.2013.726293
Kannan N, Schulz-Bull DE, Petrick G, Duinker JC, Macht-Hausmann M, Wasserman O (1994) Toxic chlorobiphenyls in adipose tissue and whole blood of an occupationally/accidentally exposed man and the general population. Arch Environ Health 49:375–383. https://doi.org/10.1080/00039896.1994.9954990
Klinčić D, Herceg Romanić S, Matek Sarić M, Grzunov J, Dukić B (2014) Polychlorinated biphenyls and organochlorine pesticides in human milk samples from two regions in Croatia. Environ Toxicol Pharmacol 37:543–552. https://doi.org/10.1016/j.etap.2014.01.009
Krauthacker B, Votava-Raić A, Herceg Romanić S, Tješić-Drinković D, Do Tješić-Drinković, Reiner E (2009) Persistent organochlorine compounds in human milk collected in croatia over two decades. Arch Environ Contam Toxicol 57:616–622. https://doi.org/10.1007/s00244-009-9301-3
Lah B, Zinko B, Narat M, Marinsek-Logar R (2005) Monitoring of genotoxicity in drinking water using in vitro comet assay and ames test. Food Technol Biotechnol 43:139–146
Longnecker MP, Rogan WJ, Lucier G (1997) The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBs (polychlorinated biphenyls) and an overview of organochlorines in public health. Ann Rev Public Health 18:211–244. https://doi.org/10.1146/annurev.publhealth.18.1.211
Lopez-Espinosa M-J, Granada A, Carreno J, Salvatierra M, Olea-Serrano F, Olea N (2007) Organochlorine pesticides in placentas from Southern Spain and some related factors. Placenta 28:631–638. https://doi.org/10.1016/j.placenta.2006.09.009
Lovell DP (2016) Statistical analysis of comet assay data. In: Dhawan A, Anderson D (eds) The comet assay in toxicology, 2nd edn. Royal Society of Chemistry, Cambridge, pp 551–580
Lovell DP, Omori T (2008) Statistical issues in the use of the comet assay. Mutagenesis 23:171–182. https://doi.org/10.1093/mutage/gen015
Møller P, Loft S (2014) Statistical analysis of comet assay results. In: Azqueta A, Langie S, Collins A (eds) 30 years of the comet assay: an overview with some new insights. Frontiers Media SA, Lausanne Switzerland, pp 171–174. https://doi.org/10.3389/fgene.2014.00292
OECD (2014) OECD guideline for the testing of chemicals 487: in vitro mammalian cell micronucleus test. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg487-2014-508.pdf
OECD—Organization for Economic Cooperation and Development (2016) OECD guideline for the testing of chemicals: in vivo mammalian alkaline comet assay. http://www.oecd-ilibrary.org/docserver/download/9716431e.pdf?expires=1515408406&id=id&accname=guest&checksum=95E473F30C0584318C453EA16FE7A374
Ross G (2004) The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Saf 59:275–291. https://doi.org/10.1016/j.ecoenv.2004.06.003
Sandal S, Yilmaz B, Carpenter DO (2008) Genotoxic effects of PCB 52 and PCB 77 on cultured human peripheral lymphocytes. Mutat Res Genet Toxicol Environ Mutagen 654(1):88–92. https://doi.org/10.1016/j.mrgentox.2008.05.005
Singh NP (2016) The comet assay: reflections on its development, evolution and applications. Mutat Res Rev Mut Res 767:23–30. https://doi.org/10.1016/j.mrrev.2015.05.004
Vizcaino E, Grimalt JO, Fernández-Somoano A, Tardon A (2014) Transport of persistent organic pollutants across the human placenta. Environ Int 65:107–115. https://doi.org/10.1016/j.envint.2014.01.004
Zhang J, Zhang X, Xia P, Zhang R, Wu Y, Xia J, Su G, Zhang J, Giesy JP, Wang Z, Villeneuve DL, Yu H (2015) Activation of AhR-mediated toxicity pathway by emerging pollutants polychlorinated diphenyl sulfides. Chemosphere 144:1754–1762. https://doi.org/10.1016/j.chemosphere.2015.09.107
Acknowledgements
This study was supported by the Croatian Science Foundation (Project No. 8366).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Želježić, D., Herceg Romanić, S., Klinčić, D. et al. Persistent Organochlorine Pollutants in Placentas Sampled from Women in Croatia and an Evaluation of Their DNA Damaging Potential In Vitro. Arch Environ Contam Toxicol 74, 284–291 (2018). https://doi.org/10.1007/s00244-017-0503-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-017-0503-9