Abstract
We assessed human health risk due to mercury (Hg) concentrations in fish from three coastal lagoons (Urías, Huizache, and Teacapán) in the SE Gulf of California. We also determined Hg distribution in muscle and liver of analyzed ichthyofauna and compared the results among studied areas according to tissue, season, and lagoon system by using multivariate analyses. Levels of Hg in most of the analyzed fish followed the sequence liver > muscle. The highest Hg levels in muscle (2.80 µg g−1 dw) and liver (9.51 µg g−1 dw) were measured in Cynoscion reticulatus and Pomadasys macracanthus, respectively, although according to the multivariate analyses, statistical differences of Hg concentrations were not found according to the season and the tissue but were found according to the system. It seems that the higher concentrations were associated with areas where the hydrological regime is lower. With respect to health risk assessment, the highest hazard quotients were estimated for Cynoscion reticulatus (0.45) and Stellifer furthii (0.29) from Urías and Pomadasys macracanthus (0.35) from Huizache. None of the studied fish represent a risk for consumers in terms of Hg levels in the edible portion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) is mobilized by natural and anthropogenic processes in the environment; some of the natural processes include volcanic activity, wind-borne soil particles, sea salt spray, forest wildfires, and biogenic emissions (Nriagu 1989). Anthropogenic activities that release Hg include power plants that use fossil-fuels, manufacturing of ferrous and nonferrous metals, caustic soda production plants, facilities for ore processing, incinerators for urban, medical and industrial wastes, cement plants, and production of chemicals (Pirrone et al. 2010). Hg is known as a global and harmful pollutant to the environment; it may be accumulated by many organisms, transferred, and biomagnified through food webs and eventually found in economically important species for human consumption (Furness and Rainbow 1990). In aquatic biota, fish constitute the main source of Hg to humans (Luoma and Rainbow 2005). In this context, it is necessary to monitor metal levels and assess their potential implications of human health.
Demersal fish species are those that live near to the sea bottom or temporarily in contact with it and reach a depth of about 500 m (Moyle and Cech 2000). Within marine ecosystems, demersal fish transform energy from organic matter, phytoplankton, zooplankton, invertebrates, and other fish and regulate energy transfer between ecosystems (Yáñez-Arancibia and Lara-Domínguez 1988; López Jiménez et al. 2014).
Along the Gulf of California, there are diverse habitats including mangrove forests, marshes, estuarine, and marine systems, which have a high biological diversity and richness and complex pathways of trophic transfer (Flores-Verdugo et al. 1990). These coastal ecosystems provide several ecological services, such as protection and breeding habitats for many species; additionally, they have the ability to accumulate materials that are discharged near to the coast (Montaño-Ley et al. 2015). Discharged materials include Hg and wetlands have been recognized as sinks of Hg, they are also considered as producers of methylmercury (MeHg) (Harbison 1986; Chatterjee et al. 2011). Considering the above scenario, it is necessary to monitor Hg occurrence in coastal ecosystems; for this purpose, fish have been used for many years as indicators of water pollution since they are excellent biomarkers of metals (Marcus et al. 2013). With the aim of comparing Hg concentrations in the three studied areas, we measured elemental concentrations in muscle and liver of several demersal fish. We also assessed human health impact based on the rate of fish consumption and Hg levels in the edible portion of specimens. Additionally, reports on Hg levels in similar fish species were compared with our results.
Materials and Methods
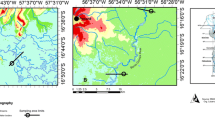
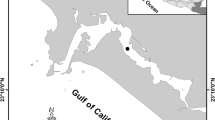
Fish were collected from three coastal lagoons (Urías, Huizache-Caimanero, and Teacapán) located in the state of Sinaloa (SE Gulf of California); these lagoons are surrounded by mangrove forests and have different levels of anthropogenic disturbances (Fig. 1). Urías is located in the city of Mazatlán, Sinaloa; it has an extension of 800 ha and receives domestic untreated sewage, effluents from seafood processing plants, cooling waters from a thermoelectrical plant, and waters discarded from shrimp farming and agricultural fields (Páez-Osuna et al. 1990; Osuna-López et al. 1997). Huizache-Caimanero locates in the southern portion of Sinaloa state; it receives intermittent flows from two rivers connected by narrow channels that transport freshwater to the lagoon during the rainy season. This system has a surface of 17,100 ha. The most relevant impacts derive from agriculture, water course deviations, mining activities, and mangrove deforestation (de la Lanza and García 1991; Zetina-Rejón et al. 2003). Teacapán is located in the boundary between Sinaloa and Nayarit states. Teacapán system accounts for 38,000 ha; the main disturbances are mangrove degradation, agriculture, cattle production, shrimp farming, and shrimp and oyster fisheries (Contreras 1985; Flores-Verdugo et al. 1990). Fishes were collected by local fishermen during the rainy season (August and October 2011, and June and August 2012) and during the dry season (November and December 2011, and from February to May 2012). Fish specimens were transported to the laboratory in ice boxes where taxonomic identification (Fischer et al. 1995), total length, and total weight were determined. Fish were kept frozen (− 19 °C) until dissection and processing. Glassware and plastic utensils were acid washed according to the procedure described by Moody and Lindstrom (1977). Specimens were dissected to extract the liver and a portion of muscle tissue from the median dorsal area.
Liver and muscle samples were freeze-dried in a Labconco Freeze-dry-System-FreeZone 6 (°49 °C and 133 × 10−3 mbar for 72 h); dried samples were ground and homogenized in an agate mortar with pestle. Digestion of duplicate powdered samples was made with concentrated nitric acid (trace metal grade, JT Baker) in capped Teflon vessels (Savillex™) on a hot plate (Barnstead Thermolyne; St. Louis, MO) at 120 °C for 3 h. Analyses of Hg were made by cold vapor atomic absorption spectrophotometry in a Hg analyzer (Buck Scientific 410; Norwalk, CT; UNEP 1993). Quality control of analytical runs was assessed by using certified reference materials of fish muscle (DORM-3, NRC-Canada) and liver (DOLT-4, NRC-Canada). Recovery percentages of Hg in DORM-3 (110.5%) and DOLT-4 (103.2%) reference materials were acceptable. Blanks and reference materials were run with every batch of 20 samples. Results are expressed as µg g−1 on a dry weight basis. Hazard quotient (HQ) was estimated to assess health risk from fish intake by using the equation (Newman and Unger 2002) HQ = E/RfD, where E is the level of exposure or metal intake (Hg) and RfD is the reference dose for mercury (Hg = 0.5 µg kg−1 body weight/day). The level of exposure (E) is calculated as E = C × I/W, where C is the concentration of Hg in fish (µg g−1 wet weight), I is the ingestion rate of fish per capita (25 g day−1), and W is the average weight of an adult (70 kg).
Descriptive statistics (average and standard deviation) of total length and weight of fish and mercury concentrations in studied tissues were calculated. Differences of Hg concentrations between muscle and liver of every fish species were defined by a parametric test (Student’s t test) or a nonparametric test (Mann–Whitney U test). The statistical tests were performed with the software GraphPad Prism 4.0 (Graph Pad Software, San Diego, CA) at a significance level p < 0.05. Besides, mean Hg concentrations in collected fish were compared with an nMDS ordination multivariate analysis considering tissue (liver, muscle), season (rainy, dry), and localities (Urías, Huizache-Caimanero, Teacapán) as factors. Analysis of similarity was applied to define statistical differences of Hg levels among factors. In case of differences, a similarity percentage (SIMPER) analysis was used (Clarke and Warwick 2001). Multivariate statistical analysis was performed with a specialized software (PRIMER-E version 6.0, 2007).
Results and Discussion
Distribution of Hg in Fish from the Studied Areas
Demersal fish species that were collected in the three areas belong to nine families. In Urías, the collected fish represented seven families; the biometric characteristics and Hg levels are presented in Table 1. In terms of the number of specimens from Urías, Caranx caninus and Mugil cephalus were the best represented species. According to length and size of maturity of fish, most of the individuals (38%) were juveniles. The highest Hg concentrations in muscle (2.80 µg g−1) and liver (3.97 µg g−1) were detected in Cynoscion xanthulus. Overall concentrations of Hg were significantly (p < 0.05) higher in liver than in muscle; considering single species from Urías, Hg levels were significantly (p < 0.05) higher in liver than in muscle of Mugil cephalus. In Huizache, ten fish species of seven families were collected (Table 2). The mugilids M. cephalus and M. curema were the most abundant species. In this area, most of the specimens were juveniles (40%). The highest Hg levels were detected in Pomadasys macracanthus (muscle 1.86 µg g−1; liver 9.51 µg g−1). Considering all the ichthyofauna from Huizache, average Hg levels were liver > muscle. In Caranx caballus and M. cephalus, Hg levels were significantly (p < 0.05) higher in liver than in muscle. Fish from Teacapán belong to 16 species of 9 families (Table 3). In terms of abundance, the milk fish Chanos chanos was the best represented species. Considering biometric information, 49% of the specimens were in a juvenile stage. In muscle, the highest Hg level was 1.36 µg g−1 in Nematistius pectoralis; in liver the most elevated concentration was 6.33 µg g−1 in Stellifer furthii. Considering the averaged Hg levels in all ichthyofauna, Hg levels in liver were significantly higher (p < 0.05) than in muscle. Considering single species, Hg concentrations in liver were significantly higher (p < 0.05) than in muscle in five cases; in N. pectoralis, Hg levels were significantly higher (p < 0.05) in muscle than in liver. In diverse studies with fish, it has been concluded that Hg is preferentially accumulated in liver (Storelli et al. 2005; Mieiro et al. 2012). This behavior has been related to the metabolic function of liver and the presence of metallothioneins. On the other hand, some studies (Kwaśniak and Falkowska 2012) have reported that muscle may contain more elevated levels of Hg than liver because of the affinity of methylmercury to sulfhydryl groups that exist in muscle and also by demethylation of the organic forms of Hg in the liver (Khoshnamvand et al. 2013). With respect to length and weight of specimens, it has been stated that Hg may be accumulated with the age of fish. In our study, fish from the three studied areas had wide weight ranges. Accordingly, Hg levels showed large variations. In this context, essential elements are usually less variable because of homeostatic mechanisms; i.e., they usually do not correlate with length or weight (Jakimska et al. 2011). In the case of Hg, numerous studies have documented the increasing trend of its levels with age or weight of specimens (Kojadinovic et al. 2007). Furthermore, size of fish is considered the most significant factor related to Hg fluctuations (Burger and Gochfeld 2011). Additionally, the process of biomagnification in the marine environment results in elevated Hg levels in top predators. In our study, trophic levels (T.L.) of analyzed ichthyofauna (Amezcua et al. 2015; Fishbase 2017) was variable (range 2.00–4.78). In muscle tissue of fish from Urías and Teacapán, the highest Hg levels were detected in fish of elevated trophic level (T.L. > 4.40). In the case of liver, the most elevated Hg levels corresponded to fish species of T.L. from intermediate (3.18 in Huizache and 3.20 in Teacapán) to elevated (4.78 in Urías).
Comparison of Hg in Fish from Mexican Waters
With the purpose of contrasting Hg concentrations, reports in similar fish species from Mexican waters were compared (Table 4). Only Hg levels in the edible portion (muscle) were included since this tissue is the most commonly used for monitoring reasons and for human health risk assessments. Our results were presented as ranges of average Hg concentrations for every fish family. The high value of Ariidae (1.16 µg g−1) in our study was an order of magnitude more elevated than in the published studies. On the contrary, the top Hg figure for Carangidae (0.97 µg g−1) and Centropomidae (0.67 µg g−1) were an order of magnitude lower than the corresponding values reported in other studies. For Mugilidae, the Hg ranges (0.08–0.27 µg g−1) of our study were comparable to the other reports (0.03–0.47 µg g−1). In diverse monitoring studies, it has been suggested that closely related species should be compared, because their toxicodynamics and toxicokinetics are similar (Rainbow 1995). Only in the case of Ariidae, our results were more elevated than other reports. For Mugilidae, the results were comparable, and for Carangidae and Centropomidae, Hg levels were lower. There is a need to perform species-specific studies to know more about Hg dynamics in coastal ecosystems from tropical and subtropical latitudes. In temperate latitudes, species diversity is lower compared with areas close to the equator where it is elevated and food webs are more complex (Rohde 1992). This issue makes biomonitoring studies more difficult in tropical regions.
Levels of Hg According to Tissue, Season, and Site
To corroborate statistical differences in the concentrations of Hg according to tissue (muscle and liver), season (rainy and dry), and lagoon system (Urías, Huizache, and Teacapán), multivariate analyses were performed. The nMDS analysis showed clear cut groups between the concentrations in the different lagoons, but a clear separation regarding the other factors was not observed (Fig. 2). This was corroborated by ANOSIM, which indicated that the Hg concentration differed statistically among all systems, but differences were not found in the concentration of Hg according to season or tissue (Table 5). According to SIMPER, the differences between systems were caused due to two factors. First, the species that were not present in all systems were a major source of differences. The other factor that accounted for the differences is related to the species present in all systems but with the average Hg concentration higher depending on the locality (Table 6). In this sense, five species most contributed to the observed differences; the most important was the grunt, Pomadasys macracanthus, which was present in all three systems but with higher concentrations in Huizache, and then decreasing in Urías, and finally the lower values were found in Teacapán. Considering averaged Hg values from all the species in every lagoon (Tables 1, 2, 3), fish from Urías had the highest concentrations in muscle and liver, perhaps as a consequence of the higher anthropogenic activities occurring there. Urías is next to the city of Mazatlán, and therefore, there are more human impacts. Previous studies have highlighted that Urías is a polluted system (Soto-Jiménez and Páez-Osuna 2008). In fact, a significant contribution of atmospheric Hg in this area is represented by a thermoelectrical power plants that has been operating since 1966; in a dated core near the power plant, it was indicated that Hg pollution initiated in 1968 (Ruiz-Fernández et al. 2009). Perhaps this contributed to the highest Hg concentrations found there, as was the case of Cynoscion reticulatus. However, the reason that the Hg concentrations are not very high can be related to the hydrological regime of Urías, in which the water exchange with the sea is much higher than in Huizache and Teacapán (Soto-Jiménez and Páez-Osuna 2001a). As a consequence, the contaminants are removed from this system, which on average makes them less available to the biota. In a similar study, Cd and Pb levels in fish from the same sites were significantly different (Gil-Manrique et al. 2017). It seems that the hydrological regime is a relevant factor related to the presence of trace elements.
Health Risk Assessment
Fish with the highest HQ values were Cynoscion reticulatus (0.45) and Stellifer furthii (0.29) from Urías and Pomadasys macracanthus (0.35) from Huizache (Fig. 3). Estimation of HQ was based on the same rate of fish consumption, so its variation is due to the amount of Hg in the edible portion of the analyzed ichthyofauna. The carnivore fish that had the highest HQ values had relatively higher amounts of Hg and elevated trophic levels (TL > 3.5), it is possible that Hg biomagnification accounts for this behaviour; i.e., elevated HQ values in top predators correspond to elevated TL values. Trophic levels of C. reticulatus (4.78) and S. furthii (4.62) were the highest among the collected ichthyofauna (Amezcua et al. 2015). The other factor that may influence the HQ value is the site where fish were collected; it is not surprising that fish from Urías had higher Hg levels than specimens from the other areas; Urías is more impacted by human activities than the other sites. Although Urías has a lower water residence (5–7 days) time (Soto-Jiménez and Páez-Osuna 2001b) than the other areas (Huizache 67 days; Teacapán 22.8 days), its smaller water surface (21 times smaller than Huizache and 48 times smaller than Teacapán) and circulation pattern favor the accumulation of pollutants (Cardoso-Mohedano et al. 2016).
None of the HQ results were above the unity; i.e., the total amount of Hg was below the corresponding RfD. If we consider that almost all Hg in muscle tissue of fish is in the organic (mostly methyl Hg) form (Akagi et al. 1994), we may reestimate HQ values using methyl Hg (equivalent to the total of measured Hg) and the RfD of methyl Hg (0.1 µg kg−1 day−1; US EPA 2014) and results are higher by a factor of five (range from 0.05 to 2.25). Under this scenario, in seven cases (from a total of 16, equivalent to 44%), there would be a health risk. The site with more HQ values above one was Urías (3 cases), followed by Teacapán and Huizache (2 cases each). This issue is of concern; however, sound conclusions cannot be drawn on this matter, because no methyl Hg analyses were performed and rates of fish consumption were estimated as apparent consumption (total of the annual fish production in Mexico divided by the population of the country). In a study in the Brazilian Amazon, comparable fish consumption (20 g per day) to ours (25 g per day) was reported but HQ values ranged from 1.5 to 28.5. It was concluded that the elevated risk was associated to artisanal gold mining areas where people catch their fish (Castilhos et al. 2015). On the other hand, the rate of consumption (17.3 g per day) of demersal fish from fishery areas in Greece (Yabanli and Alparslan 2015) resulted in low HQ values in adults (0.07) and children (0.52). Estimation of HQ is highly dependent on the rate of consumption, the origin of fish, and the type of fish. In Italian supermarkets, HQ values were below the unity in all species tested; the rate of consumption was 18 g per day. Nevertheless, top predators, such as swordfish (0.80) and Atlantic bluefin tuna (0.74), had values close to one (Barone et al. 2015).
Although fish is not the only dietary item that incorporates Hg to humans, its relative contribution is relevant. In this sense, studies related to health risk assessments should include more food items and not only fishery products. In a study at an industrial zone in Jiangsu, China (Cao et al. 2010), the HQ from diverse elements in rice and vegetables was assessed. The individual rate of consumption of rice (423.5 g per day) and vegetables (234.6 g per day) was elevated but the HQ for Hg was very low (0.049). In contrast, in an Hg mining area in Wanshan (SE China), although individual rice consumption was similar (401 g per day), vegetable consumption was more elevated (234.6 g per day). The HQ for Hg (0.60) was much higher (Wang et al. 2011).
Conclusions
From length and size of maturity of fish from the studied areas, juvenile stages were common in specimens from Urías (38%), Huizache (40%), and Teacapán (49%). The sequence of Hg levels in analyzed tissues was liver > muscle in most of the ichthyofauna from the three studied lagoons. This is in concordance to other studies of Hg distribution in fish, perhaps as a consequence of the detoxifying role of liver. However, from the multivariate results, it can be concluded that for the compared areas, besides the human activities, the hydrological regime also has an effect on the accumulation of Hg and that the season and type of tissue are not a determining factor. In relation to the health risk assessment, HQ values were below unity for all the fish species. The fish with the highest HQ were predators of elevated trophic position (TL > 3.5). For a more precise estimation of human health risk, it is necessary to perform a detailed survey about fish consumption, use adult fish for Hg measurements, and perform laboratory analysis of methylmercury.
References
Akagi H, Branches F, Kinjo Y (1994) Methylmercury pollution in Tapajos river basin, Amazon. Environ Sci 3(1):25–32
Amezcua F, Muro-Torres V, Soto-Jiménez MF (2015) Stable isotope analysis versus TROPH: a comparison of methods for estimating fish trophic positions in a subtropical estuarine system. Aquat Ecol 49:235–250. https://doi.org/10.1007/s10452-015-9517-4
Barone G, Storelli A, Garofalo R, Busco VP, Quaglia NC, Centrone G, Storelli MM (2015) Assessment of mercury and cadmium via seafood consumption in Italy: estimated dietary intake (EWI) and target hazard quotient (THQ). Food Addit Contam Part A 32(8):1277–1286. https://doi.org/10.1080/19440049.2015.1055594
Burger J, Gochfeld M (2011) Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci Total Environ 409(8):1418–1429. https://doi.org/10.1016/j.scitotenv.2010.12.034
Cao H, Chen J, Zhang J, Zhang H, Qiao L, Men Y (2010) Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. J Environ Sci 22(11):1792–1799. https://doi.org/10.1016/S1001-0742(09)60321-1
Cardoso-Mohedano G, Páez-Osuna F, Amezcua-Martínez F, Ruiz-Fernández AC, Ramírez-Reséndiz G, Sánchez-Cabeza J (2016) Combined environmental stress from shrimp farm and dredging releases in a subtropical coastal lagoon (SE Gulf of California). Mar Pollut Bull 104:83–91. https://doi.org/10.1016/j.marpolbul.2016.02.008
Castilhos Z, Rodrigues-Filho S, Cesar R, Rodrigues AP, Villas-Bôas R, de Jesus I, Lima M, Faial K, Miranda A, Brabo E, Beinhoff C, Santos E (2015) Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ Sci Pollut Res 22:11255–11264. https://doi.org/10.1007/s11356-015-4340-y
Chatterjee M, Canario J, Sarkar SK, Branco V, Godhantaraman N, Bhattacharya BD, Bhattacharya A (2011) Biogeochemistry of mercury and methylmercury in sediment cores from Sundarban mangrove wetland, India—a UNESCO World Heritage Site. Environ Monit Assess 184:5239–5254. https://doi.org/10.1007/s10661-011-2336-8
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd, Plymouth
Contreras F (1985) Las Lagunas Costeras Mexicanas. Centro de Ecodesarrollo, Secretaría de Pesca, México
de la Lanza EG, García JL (1991) Sistema lagunar Huizache-Caimanero, Sin. Un estudio socio ambiental, pesquero y acuícola. Hidrobiológica 1:1–27
Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE (1995) Guía FAO para la identificación de especies para los fines de la pesca: Pacífico centro-oriental. FAO, Rome
Fishbase (2017) www.fishbase.org. Accessed 1 Dec 2017
Flores-Verdugo F, González-Farías F, Ramírez-Flores O, Amezcua-Linares F, Yáñez-Arancibia A, Álvarez-Rubio M, Day JW (1990) Mangrove ecology, aquatic primary productivity and fish community dynamics in the Teacapán-Agua Brava lagoon-estuarine system (Mexican Pacific). Estuaries 13:219–230. https://doi.org/10.2307/1351591
Furness RW, Rainbow PS (1990) Heavy metals in the marine environmental. CRC Press, Boca Raton
Gil-Manrique B, Nateras-Ramírez O, Martínez-Salcido AI, Ruelas-Inzunza J, Páez-Osuna F, Amezcua F (2017) Cadmium and lead concentrations in hepatic and muscle tissue of demersal fish from three lagoon systems (SE Gulf of California). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-8901-0
Harbison P (1986) Mangrove muds sink and a source for trace metals. Mar Pollut Bull 17:246–250. https://doi.org/10.1016/0025-326X(86)90057-3
Jakimska A, Konieczka P, Skóra K, Namiesnik J (2011) Bioaccumulation of metals in tissues of marine animals, part I: the role and impact of heavy metals on organisms. Pol J Environ Stud 20(5):1117–1125
Khoshnamvand M, Kaboodvandpour S, Ghiasi F (2013) A comparative study of accumulated total mercury among white muscle, red muscle and liver tissues of common carp and silver carp from the Sanandaj Gheshlagh Reservoir in Iran. Chemosphere 90:1236–1241. https://doi.org/10.1016/j.chemosphere.2012.09.061
Kojadinovic J, Potier M, Corre ML, Cosson RP, Bustamante P (2007) Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ Pollut 146(2):548–566. https://doi.org/10.1016/j.envpol.2006.07.015
Kwaśniak J, Falkowska L (2012) Mercury distribution in muscles and internal organs of the juvenile and adult Baltic cod (Gadus morrhua callarias Linnaeus, 1758). Oceanol Hydrobiol Stud 41(2):65–71. https://doi.org/10.2478/s13545-012-0018-y
López Jiménez LN, González Solis A, Torruco D (2014) Peces bentónicos y demersales de la Sonda de Campeche: sur del Golfo de México. CONABIO. Biodiversitas 113:12–16
Luoma SN, Rainbow P (2005) Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol 39(7):1921–1931. https://doi.org/10.1021/es048947e
Marcus AC, Okoye CO, Ibeto CN (2013) Bioaccumulation of trace metals in shellfish and fish of Bonny River and creeks around Okrika in Rivers State, Nigeria, Nigeria. Bull Environ Contam Toxicol 90(6):708–713. https://doi.org/10.1007/s00128-013-0992-9
Mieiro CL, Coelho JP, Pacheco M, Duarte AC, Pereira ME (2012) Evaluation of species-specific dissimilarities in two marine fish species: mercury accumulation as a function of metal levels in consumed prey. Arch Environ Contam Toxicol 63(1):125–136. https://doi.org/10.1007/s00244-011-9740-5
Montaño-Ley Y, Carbajal N, Páez-Osuna F (2015) Sediment dynamics in a complex coastal lagoon system of the Gulf of California. J Coast Conserv 19(3):295–306. https://doi.org/10.1007/s11852-015-0391-y
Moody JR, Lindstrom RN (1977) Selection and cleaning of plastic containers for age of trace element samples. Anal Chem 49:2264–2267
Moyle PB, Cech JJ (2000) Fishes: an introduction to ichthyology. Prentice-Hall, Saddle River
Newman MC, Unger MA (2002) Fundamentals of Ecotoxicology. Lewis Publishers, Boca Raton
Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338(6210):47–49. https://doi.org/10.1038/338047a0
Osuna-López JI, Zazueta-Padilla H, Frías-Espericueta M, Izaguirre-Fierro G, López-López G (1997) Metales pesados en sedimentos superficiales del sistema Arroyo Jabalines-Estero del Infiernillo, Mazatlán, Sinaloa, México. Revista de Ciencias del Mar UAS 15:43–49
Páez-Osuna F, Montaño-Ley Y, Bojórquez-Leyva H (1990) Intercambio de agua, fósforo y material suspendido entre el sistema lagunar del puerto de Mazatlán y las lagunas costeras adyacentes. Rev Int Contam Ambient 6:19–32
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10(13):5951–5964. https://doi.org/10.5194/acp-10-5951-2010
Rainbow PS (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31(4–12):183–192. https://doi.org/10.1016/0025-326X(95)00116-5
Reimer AA, Reimer RD (1975) Total mercury in some fish and shellfish along the Mexican coast. Bull Environ Contam Toxicol 14(1):105–111
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65(3):514–527
Ruelas-Inzunza J, Meza-Lópeza G, Páez-Osuna F (2008) Mercury in fish that are of dietary importance from the coasts of Sinaloa (SE Gulf of California). J Food Compos Anal 21:211–218. https://doi.org/10.1016/j.jfca.2007.11.004
Ruelas-Inzunza J, Páez-Osuna F (2005) Mercury in fish and shark tissues from two coastal lagoons in the Gulf of California, Mexico. Bull Environ Contam Toxicol 74:294–300. https://doi.org/10.1007/s00128-004-0583-x
Ruelas-Inzunza J, Páez-Osuna F, Zamora-Arellano N, Amezcua-Martínez F, Bojórquez-Leyva H (2009) Mercury in biota and surficial sediments from Coatzacoalcos estuary, Gulf of Mexico: distribution and seasonal variation. Water Air Soil Pollut 197:165–174. https://doi.org/10.1007/s11270-008-9799-4
Ruiz-Fernández AC, Frignani M, Hillaire-Marcel C, Ghaleb B, Arvizu MD, Raygoza-Viera JR, Páez-Osuna F (2009) Trace metals (Cd, Cu, Hg, and Pb) accumulation recorded in the intertidal mudflat sediments of three coastal lagoons in the Gulf of California, Mexico. Estuaries Coasts 32:551–564. https://doi.org/10.1007/s12237-009-9150-3
Soto-Jiménez MF, Páez-Osuna F (2001a) Distribution and normalization of heavy metal concentrations in mangrove and lagoonal sediments from Mazatlan Harbor (SE Gulf of California). Estuar Coast Shelf Sci 53(3):259–274. https://doi.org/10.1006/ecss.2000.0814
Soto-Jiménez M, Páez-Osuna F (2001b) Cd, Cu, Pb, and Zn in lagoonal sediments from Mazatlán harbor (SE Gulf of California): bioavailability and geochemical fractioning. Bull Environ Contam Toxicol 66(3):350–356. https://doi.org/10.1007/s00128-001-0012-3
Soto-Jiménez MF, Páez-Osuna F (2008) Diagenetic processes on metals in hypersaline mudflat sediments from a subtropical saltmarsh (SE Gulf of California): postdepositional mobility and geochemical fractions. Appl Geochem 23(5):1202–1217. https://doi.org/10.1016/j.apgeochem.2007.11.011
Storelli MM, Giacominelli-Stuffler R, Storelli A, Marcotrigiano GO (2005) Accumulation of mercury, cadmium, lead and arsenic in swordfish and bluefin tuna from the Mediterranean Sea: a comparative study. Mar Pollut Bull 50(9):1004–1007. https://doi.org/10.1016/j.marpolbul.2005.06.041
UNEP/FAO/IOC/IAEA (1993) Guidelines for monitoring chemical contaminants in the sea using marine organisms. Reference methods for marine pollution studies No. 6. UNEP, Monaco
US EPA—United States Environmental Protection Agency (2014) IRIS—integrated risk information system. http://www.usepa.gov/iris. Accessed 10 May 2016
Vàzquez F, Florville-Alejandre TR, Herrera M, Díaz de León LM (2008) Heavy metals in muscular tissue of the catfish, ariopsis felis, in the southern Gulfof México (2001–2004). Lat Am J Aquat Res 36:223–233. https://doi.org/10.4067/S0718-560X2008000200005
Wang X, Lia Y-F, Lia B, Dongc Z, Quc L, Gaoa X, Chaia Z, Chen C (2011) Multielemental contents of foodstuffs from the Wanshan (China) mercury mining area and the potential health risks. Appl Geochem 26:182–187. https://doi.org/10.1016/j.apgeochem.2010.11.017
Yabanli M, Alparslan Y (2015) Potential health hazard assessment in terms of some heavy metals determined in demersal fishes caught in eastern Aegean Sea. Bull Environ Contam Toxicol 95:494–498. https://doi.org/10.1007/s00128-015-1584-7
Yáñez-Arancibia A, Lara-Domínguez AL (1988) Ecology of three sea catfishes (Ariidae) in a tropical coastal ecosystem, southern Gulf of Mexico. Mar Ecol Progr Ser 49(3):215–230
Zetina-Rejón RM, Arreguín SF, Chávez E (2003) Trophic structure and flows of energy in the Huizache-Caimanero lagoon complex on the Pacific coast of Mexico. Estuar Coast Shelf Sci 57:803–815. https://doi.org/10.1016/S0272-7714(02)00410-9
Funding
This study was funded by Programa de Mejoramiento del Profesorado para el Tipo Superior (PRODEP) and Universidad Nacional Autónoma de México (PAPIIT IN208911-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Martínez-Salcido, A.I., Ruelas-Inzunza, J., Gil-Manrique, B. et al. Mercury Levels in Fish for Human Consumption from the Southeast Gulf of California: Tissue Distribution and Health Risk Assessment. Arch Environ Contam Toxicol 74, 273–283 (2018). https://doi.org/10.1007/s00244-017-0495-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-017-0495-5