Abstract
High-throughput acute and chronic toxicity tests using Vibrio fischeri were used to assess the toxicity of a variety of fungicides, herbicides, and neonicotinoids. The use of time points beyond the traditional 30 min of an acute test highlighted the sensitivity and applicability of the chronic toxicity test and indicated that for some compounds toxicity is underestimated using only the acute test. The comparison of EC50 values obtained from acute and chronic tests provided insight regarding the toxicity mode of action, either being direct or indirect. Using a structure–activity relationship approach similar to the one used in hazard assessments, the relationship between toxicity and key physicochemical properties of pesticides was investigated and trends were identified. This study not only provides new information regarding acute toxicity of some pesticides but also is one of the first studies to investigate the chronic toxicity of pesticides using the test organism V. fischeri. The findings demonstrated that the initial bioluminescence has a large effect on the calculated effective concentrations for target compounds in both acute and chronic tests, providing a way to improve and standardize the test protocol. In addition, the findings emphasize the need for additional investigation regarding the relationship between a toxicant’s physicochemical properties and mode of action in nontarget organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Countless xenobiotic compounds, including pesticides, pharmaceuticals, and personal care products, among others, are continuously introduced into the environment and have been detected at concentrations up to μg/L levels. The presence of pesticides in aquatic environments is one of the major challenges for the preservation and sustainability of the environment as a result of large volumes used on an annual basis, their inherent biological activities, and the persistent nature of pesticide residues and their metabolites (Stamatis et al. 2010; Veljanoska-Sarafiloska et al. 2013). Although these compounds were originally designed to act on specific targets, an overwhelming amount of evidence in the literature indicates indirect effects on nontarget organisms (Bonnet et al. 2007; Veljanoska-Sarafiloska et al. 2013).
In an effort to identify and quantify the biological effects on nontarget organisms from pesticides and other xenobiotics, a battery of in vitro and in vivo bioassays are used. Because each bioassay often is designed to measure only one specific endpoint after a specific time of exposure, which leads to a static description of one toxic mode of action as a function of concentration (Froehner et al. 2002; Kokkali and van Delft 2014), it has been strongly encouraged to use a variety of bioassays rather than just one to obtain a more thorough evaluation of potential biological impacts (Blaschke et al. 2010; Froehner et al. 2002; Kokkali and van Delft 2014; Parvez et al. 2006). While it is important to consider the use of a variety of bioassays for the assessment of pesticide bioactivity, it is equally important to consider biological effects at different exposure times both short-term (acute) and long-term (chronic) to understand the toxicants mode of action as well as minimizing the potential to underestimate toxicity by selecting a limited exposure time.
The acute bioluminescence inhibition assay using Vibrio fischeri as a test organism, known as MicroTox®, is a widely used toxicity test commercially distributed by Modern Waters. Although this test has been reported as the most sensitive in comparison to other bacterial based bioassays (Kokkali and van Delft 2014) and is described as being rapid, easy to perform, and cost-efficient (Froehner et al. 2000) there are still notable drawbacks. The main drawback is the limited ecotoxicological relevance of the endpoint selected (Backhaus et al. 1997). It is speculated that evaluating the toxicity at a short incubation time (15 and 30 min) may underestimate the toxicity of a compound, in such cases where toxicities are associated with biosynthetic mechanisms and the toxicity endpoint is determined before the cells enter the respective biological phase (Backhaus et al. 1997; Blaschke et al. 2010; Froehner et al. 2000; 2002). Toxicity development over exposure time remains scarcely investigated, even though studies have highlighted its relevance for toxicity assessment (Backhaus et al. 1997; Blaschke et al. 2010; Deng et al. 2012; Gellert 2000).
The bioactivities of pesticides and other xenobiotics obtained using bioassays often are investigated alongside with physicochemical properties, such as acid dissociation constant (pKa), log octanol/water coefficient (K ow), and molecular weight. The toxicity and quantitative structure–activity relationship is an approach commonly used in hazard assessments that predicts the toxicity of new compounds based on these chemical descriptors (Lee and Chen 2009). Using this approach, toxicity trends have been discovered, including the increase of toxicity with increasing log K ow and pKa values (Diaz et al. 2013; Lee and Chen 2009; Majewsky et al. 2014). Although many studies have used this approach based on results obtain from acute toxicity tests, limited studies have investigated chronic toxicity trends with physicochemical properties using V. fischeri.
The effect of pKa has an established role in ecotoxicity assessments where the ionized and nonionized form of a compound may elicit different effects on multiple targets, including proteins (carriers, enzymes, and receptors), ion channels, and intracellular organelles (Diaz et al. 2013). The influence of a compounds ionization state towards toxicity often is related to the availability of toxicant, where toxic ions may be much more soluble or easily absorbable in different pH environments (Rozman et al. 2001). The ionization state of compounds also may interfere with electron transport mechanisms (Hollingworth 2001), an important component in ATP production, and is required for the production of bioluminescence in V. fischeri (Dunn 2012).
The log octanol/water partition coefficient (K ow) is another physicochemical characteristic that is important for ecotoxicity assessments. This chemical property is commonly used for studying the chemical behavior of organic compounds, mainly reflecting the transfer ability of compounds between water phase and organic phase (Shi et al. 2012). Chemicals with low K ow values (< 10) may be considered relatively hydrophilic, resulting in high water solubilities and biological enrichment factors in aquatic life. Conversely, chemicals that have high K ow values (> 104) are considered to be very hydrophobic (May et al. 2016).
While the pKa and log K ow of compounds are more popular descriptors compared with the molecular weight, this parameter may provide insight to related factors that influence toxicity. These factors include steric, electrostatic, and hydrophobic nature of functional groups present on the compound. A study done by Shi et al. (2012) demonstrated that bulky compounds had an influence on the toxicity of P. phosphoreum, which acts on electron transfer reaction mechanisms, similar to V. fischeri.
Materials and Methods
Compounds Tested
Propiconazole (99% pure), atrazine (99% pure), 2,4-Dichlorophenoxyacetic acid (2,4-D) (97% pure), and zinc sulfate were purchased from Sigma-Aldrich. Tebuconazole (> 98% pure), climbazole (> 98% pure), and myclobutanil (> 97% pure) were purchased from Abcam Biochemicals. Irgarol (> 98% pure), terbutryn (> 98% pure), dicamba (> 98% pure), mecoprop (> 98% pure), diuron (> 98% pure), thiamethoxam (> 98% pure), acetamiprid (> 98% pure), and thiacloprid (> 98% pure) were purchased from Santa-Cruz Biotechnology. Chemicals used in the culture medium were purchased from Fisher Scientific: sodium chloride, trypton, yeast extract; Sigma-Aldrich: potassium chloride and magnesium chloride; and EMD Chemicals: glycerol.
Carrier solvents used to prepare the stock solutions of pesticides were LC–MS grade methanol (MeOH) and dimethyl sulfoxide (DMSO) purchased from Fischer Scientific and Sigma-Aldrich, respectively. Stock solutions of propiconazole, myclobutanil, irgarol, terbutryn, dicamba, 2,4-D, and mecoprop were prepared in MeOH at a concentration of 1000 mg/L. Stock solutions of thiamethoxam, acetamiprid, and thiacloprid were prepared in MeOH at a concentration of 10,000 mg/L. Stock solutions of tebuconazole, climbazole, atrazine, and diuron were prepared in 10% DMSO in MeOH (v/v) at a concentration of 5000 mg/L.
Cultivation of Test Organism
Procedures for cultivation of the test organism have been described in our previous work (Nasuhoglu et al. 2016). Cultivation of the test organism was performed the same way for both acute and chronic toxicity assays. Briefly, freeze-dried luminescent bacteria (Vibrio fischeri, NRRL-B11177, ATCC 49387) were rehydrated in 1 mL of seawater complete medium (SWCM). 0.5 mL of the rehydrated bacteria were then transferred into 100 mL of nutrient supplemented seawater medium (NSSWM) and incubated at 22 °C and shaken at 150 rpm for approximately 24 h (or until exponential growth phase was reached as determined by optical density at 600 nm). Ten-milliliter samples of this incubated liquid broth containing grown V. fischeri were then transferred into 15-mL polyurethane centrifuge tubes and centrifuged at 5000 rpm for 5 min. The supernatant was then decanted, and the remaining bacteria pellets were resuspended with 1 mL of 30% glycerol/NSSWM (v/v) and placed in 1.5-mL cyrovials to be frozen under liquid nitrogen and stored at − 80 °C until the time of experimentation.
A cyrovial containing the preserved V. fischeri culture were thawed on ice and a 250-μL volume of this culture was transferred into 50 mL of fresh NSSWM and incubated at 22 °C at 150 rpm for approximately 10–12 h (or until optical density of 1–1.5 was reached). To maintain a healthy culture exhibiting a stable luminescence response a dilution was performed every 12 h using the following formula:
This approach was used to standardize the initial cell density in NSSWM used for each toxicity assay. Every 12 h of incubation, in addition to OD measurements, luminescence measurements also were performed by transferring 200 μL of samples into black opaque polystyrene Costar 96 microwell plates and measured by a Beckman-Coulter DTX 800 Multimode detector to ensure that bacteria are in their late exponential phase (LEPC).
Preparation of Dilution and Acute Toxicity Assay
Once the bacteria culture reaches an optical density of 1–1.5, after approximately 12 h of incubation, a series of dilutions were performed to prepare the culture before the exposure to the contaminants. These dilutions are performed to reach an optimal luminescence, 15,000–20,000 relative light units (RLU), which results in obtaining EC50 values of controls that are within the range reported in literature using the commercial MicroTox@. Results obtained for varying initial luminescence are discussed in the results section. Ten milliliters of culture was centrifuged at 5000 rpm for 3 min, and the supernatant was discarded. SWCM was added to the remaining bacteria pellets until an optimal luminescence was reached.
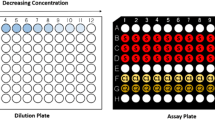
Using a 96-well plate, dilutions of contaminants and controls were performed (Fig. 1). A pesticide stock solution of 200 μL (individual or mixture) was placed in well A1 in the dilution plate. Well B1 contained 140 μL, and wells C1 to H1 contained 180 μl of the respective carrier solvent (MeOH or 10% DMSO/MeOH). A volume of 60 µL from A1 to B1 and a volume of 20 µL from A1 to C1 were transferred. Starting from B1, the even numbered wells were serially diluted by a factor of 10 and similarly the odd numbered wells were serially diluted by a factor of 10 starting from C1. Finally, 10 µL from each well in the dilution plate (for tebuconazole, climbazole, atrazine, and diuron) and 50 µL (for remaining 10 compounds) were transferred into the corresponding well in the black opaque assay plate (columns 2–4, 6–8, 10–12). The same dilution technique was performed for a positive control, ZnSO4 (C1 in Fig. 1), where 10 µL from each well was transferred into the corresponding assay plate in column 1. From the respective carrier solvent, 10 or 50 µL was transferred into all wells of column 9 in the assay plate as the solvent control (C2 in Fig. 1). Solvents were allowed to evaporate prior to the addition of V. fischeri culture. Following the evaporation of the organic solvents, 200 µL of bacteria culture was added to each well in the assay plate. To measure the natural decrease of luminescence over the duration of the test, 200 µL of bacteria culture was added to each well in column 5 (C3 in Fig. 1). Each assay plate consisted of triplicates of three serially diluted samples and three separate controls.

Adapted from Nasuhoglu et al. (2016)
Dilution and assay plate for acute toxicity experimentation.
Preparation of Dilution and Chronic Toxicity Assay
The preparation of dilution and methodology for the chronic toxicity assay is described in Nasuhoglu et al. (2016). Briefly, a similar preparation of dilution was performed for chronic toxicity tests as was described for acute toxicity. Once the bacteria culture reaches an optical density of 1–1.5 after approximately 12 h of incubation a dilution is made using the following formula:
This dilution step is used to standardize initial cell density and obtain a consistent initial luminescence response of approximately 1000 RLU. This lower initial cell density and luminescence response was selected to ensure that the amount of nutrients provided is sufficient to sustain a healthy bacteria culture for approximately 24 h. At a higher initial cell density (corresponding to a higher initial luminescence), there would be a risk that the cultures deteriorate over time due to lack of nutrients, which would be confounded with decreasing luminescence because of the exposure of contaminants. The dilution and assay plates are constructed similarly to acute toxicity tests with minor modifications. The dilutions of samples follow the same procedure; however, the dilutions are made across the plate rather than vertically to maximize the concentration range. Each plate consists of a triplicate serial dilution of a sample and two negative controls (carrier solvent and bacteria culture). Following the evaporation of the organic solvents and the addition of 200 µL of bacteria culture in each well, the plate was covered with permeable film (Breathe-Easy sealing membrane) to allow oxygen diffusion and facilitate aerobic respiration of bacteria. The luminescence in each well was recorded over 20 h every 15 min (shaking for 15 s before each measurement to prevent settling of the bacteria and to homogenize the mixture in each well).
Data Analysis
Acute Toxicity Test
The luminescence of the samples and controls were measured at two endpoints, 15 and 30 min, as recommended in the standard method. The percent inhibition of luminescence was determined by using the following equation:
t is time of measurement (15 or 30 min endpoint), L c,t is the mean of the luminescence values of the bacteria control at t min, L s,t is the observed luminescence of a sample at each dilution at t min, and I luminescence is the inhibition percentage for each dilution at a given endpoint point. Plotting the luminescence inhibition versus the log concentration of a sample generates a dose response curve whereby the effective median concentration (EC50) can be calculated (Fig. 2). For each sample, a dose response curve is generated at the two endpoints and the validity of each plate was established by measuring the EC50 of the positive control, ZnSO4, expected to be within the range of 3–10 mg/L (Ghosh et al. 1996).
Chronic Toxicity Test
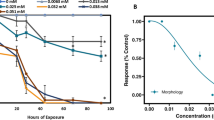
The luminescence of the sample and controls were recorded every 15 min over 20 h (Fig. 3a, b). Plotting the luminescence over time provides evidence of a dynamic relationship between the rate of luminescence and toxicant concentration. Figure 3a shows the change of luminescence with varying concentrations of diuron over time. As depicted in Fig. 3b, the luminescence significantly decreases over the first 5 h representing a period of time for which the bacteria need to reach a critical cell density before exhibiting luminescence (i.e., quorum sensing). As a result of the high variability in cell density and luminescence, data points within this time frame are not included in data analysis. The increase of luminescence between 7 and 15 h follows a linear trend that can be described as the luminescence rate inhibition, which changes for each toxicant concentration. As the concentration of diuron decreases from 75 to 7.5 mg/L the luminescence inhibition compared to the solvent control decreases. The luminescence rate inhibition can be calculated using the following equation:
m c is the initial luminescence rate inhibition calculated from the slope of the mean of luminescence values of the solvent controls, m s is the initial rate of luminescence calculated from the slope of the observed luminescence of a particular sample, and I rate is the inhibition of initial rate of luminescence. Plotting the inhibition of initial rate of luminescence over the concentration of sample will produce a dose response curve (Fig. 3c), where an EC50 can be measured.
For both acute and chronic toxicity tests, the effective concentration showing 50% inhibition (EC50) was calculated using a nonlinear sigmoidal dose response fit using Origin Pro, OriginLab.
Results and Discussion
Acute Toxicity Test
To investigate the toxicity of compounds selected for this study and compare toxicity values reported throughout literature, a series of validation experiments confirming the sensitivity of the adapted high throughput method were completed. The toxicity was determined at two endpoints: 15 and 30 min. As previously discussed, an optimal initial luminescence range of the bacteria culture was established by calculating the EC50 of ZnSO4 at 15 min endpoint with varying initial luminescence (Table 1).
The EC50 of ZnSO4 reported in literature for acute tests is within the range of 3–10 mg/L (Ghosh et al. 1996), which was obtained with the specified initial RLU range of 15,000–20,000 using the high throughput method. The agreement of EC50 values of ZnSO4 at the luminescence range of 15,000–20,000 RLU suggests that the method adapted to 96-well plate works. In addition, considering the lower standard error obtained in that range of initial bioluminescence, all acute toxicity tests were performed within that optimal range.
The acute toxicity method was performed for all fourteen compounds. Each compound was tested in triplicates and percent inhibition was determined by normalizing the data to the bacteria control within each plate. Dose response curves were constructed by plotting the percent inhibition versus the log concentration. The experimental results obtained from the dose response curves shown in Fig. 4 are summarized and compared to the few existing toxicities reported in literature (Table 2). The dose response curves at 15 min for the other compounds and all the curves obtained at an exposure time of 30 min can be found in the supplemental material.
Dose response curves were obtained for all samples except for thiacloprid. As illustrated in Fig. 4, thiacloprid showed no toxic effect towards the bacteria in the concentration range chosen and was not possible to generate a sigmoidal dose response fit. In contrast to acetamiprid, similar in chemical structure and also a pyridine-based neonicotinoid, the EC50 was determined to be 23.0 and 27.6 mg/L at 15 and 30 min, respectively. The dose response curve for atrazine indicated that the EC50 was at a higher concentration than what was investigated within the dilution range. Subsequently, the EC20 provided a more accurate toxicity assessment, and in addition, the EC20 is in agreement with values found in literature (references provided in Table 2).
In general, no significant differences were found between the EC50 values obtained at the two endpoints: 15 and 30 min. Propiconazole, Irgarol, and dicamba had the most noticeable differences between the two time points. Comparing the toxicities of individual compounds within each class, herbicides were found to be less toxic compared with fungicides and neonicotinoids. This trend is most likely due to the modes of action of these compounds. Fungicides have been reported to cause indirect effects on different bacterial species, including respiration, metabolism, and inactivating proteins and enzymes (Yang et al. 2011), which these are the main driving mechanisms for bioluminescence (Dunn 2012). The neonicotinoid compounds showed relatively high toxicity (except for thiacloprid), which is interesting considering the fact that this class of compounds is highly selective in their mode of action at receptor sites in the nervous system of invertebrates (Simon-Delso et al. 2015). A possible explanation for acetamiprid and thiamethoxam toxicity is related to the identified impact of acetamiprid on ATPase activity in which this neonicotinoid caused oxidative stress (Yao et al. 2006).
When investigating the toxicities of the selected compounds throughout literature, there are notable trends that indicate the need for further comprehensive toxicity assessments. The toxicity for most compounds shown in this study do not significantly change between endpoints; however, propiconazole, irgarol, and dicamba had notable decrease in EC50 values at the 30 min timepoint. The difference of EC50 values for the two time points (15 and 30 min) of propiconazole, irgarol, and dicamba were 21.41, 29.86, and 20.37 mg/L, respectively. Without considering and reporting the effective concentrations at 30 min, the toxicity for compounds similar to propiconazole, irgarol, and dicamba may be underestimated.
Another notable trend throughout reported studies performing acute toxicity tests with V. fischeri is the wide range of effective concentrations for a given compound. Diuron, for example, has EC50 values at 30 min ranging between 9.2 and 58.07 mg/L (Bonnet et al. 2007; Gatidou et al. 2015; Oturan et al. 2008), and atrazine has reported EC50 values at 15 min ranging from 39 to 150 mg/L (Gaggi et al. 1995; Polo et al. 2011). A possible explanation might be variations of initial bioluminescence used to perform the assay. As demonstrated for ZnSO4 control in Table 1, the initial bioluminescence has a large effect on calculating the effective concentrations.
Chronic Toxicity Test
Of the three data processing methods proposed in Nasuhoglu et al. (2016), the initial luminescence rate inhibition was recommended as the assessment method for measuring the chronic toxicity of the compounds selected for this study. This assessment method was chosen, because it has the benefit of providing time dependent toxicity analysis of samples in an economic and timely fashion, providing a global EC50 value rather than a specific time point. Dose response curves (not shown) were constructed for all compounds studied except for dicamba, 2,4-D, and thiamethoxam for which no effective toxicity was observed within the investigated concentration range (dicamba and 2,4-D: 1.25 × 102 to 3.75 × 10−4 mg/L, thiamethoxam: 1.25 × 103 to 3.75 × 10−3 mg/L). Calculated EC50 values are reported in Table 3.
Although there have been past studies investigating acute toxicity for some of the compounds selected in this study (Table 2), no chronic toxicity tests have been reported for any of the target compounds using V. fischeri. The importance of chronic toxicity testing was highlighted by Backhaus et al. (1997), where it was found that compounds with indirect mode of actions exert their effects after short incubation times and neglecting endpoints after longer exposure time might underestimate toxicity. Indirect modes of action include interfering with cell membranes, causing structural damage and/or disturbance of the energy status, all of which are associated with the production of luminescence.
The comparison of the results obtained from the two high throughput toxicity tests and reported EC50 values highlight the importance of considering time points beyond the conventional 15 and 30 min of acute toxicity. Propiconazole, atrazine, irgarol, mecoprop, and diuron had significantly lower EC50 values based on the chronic toxicity test. Atrazine had the largest difference of EC50’s between the two toxicity tests (EC50,chronic = 9.96 mg/L, EC50,acute at 30 min = 262.64 mg/L), whereas propiconazole, irgarol, mecoprop, and diuron had EC50 values two or more times lower than values obtained using the acute toxicity test. Climbazole, thiacloprid, myclobutanil, and terbutryn had similar EC50 values for both toxicity tests, but climbazole and thiacloprid had lower values in the chronic test while myclobutanil and terbutryn had lower values in the acute test. Tebuconazole was the only compound that had a notably higher EC50 value obtained in the chronic toxicity test. The remaining four compounds (dicamba, 2,4-D, thiacloprid, and thiamethoxam) had either no effective toxicity observed in the acute or chronic test.
As previously described by Nasuhoglu et al. (2016), if acute EC50 values are equivalent or smaller than the values obtained in the chronic test, the compound is considered to exhibit indirect mode of action. Conversely, if chronic EC50 is less than values obtained in the acute test, then the compound exhibits direct mode of action. Based on the experimental results presented in this study, atrazine, propiconazole, climbazole, irgarol, mecoprop, diuron, and thiacloprid can be considered to exhibit direct modes of action inhibiting the biosynthetic pathways of V. fischeri. The remaining compounds, tebuconazole, myclobutanil, terbutryn, dicamba, 2,4-D, acetamiprid, and thiamethoxam, can be considered to have indirect toxicity modes of action towards V. fischeri. The mode of action for acetamiprid varies depending on the EC50 values obtained from the high throughput acute toxicity method and reported in literature. Results obtained for acetamiprid using the high-throughput method were significantly lower than what was reported in the literature (Dell’Arciprete et al. 2009); acetamiprid had a EC50 value at 15 min of 129.0 and 23.0 mg/L obtained by Dell’Arciprete et al. (2009) and this study, respectively. Compared with the EC50 value obtained using the chronic toxicity test, the mode of action of acetamiprid is indirect and direct for experimental and reported values, respectively. A possible explanation for this discrepancy could be due to the type of carrier solvents used to perform the acute toxicity tests. In the present study, MeOH was used as a carrier solvent but was evaporated before exposing the bacteria to the target compound, minimizing the potential impact of organic solvents towards V. fischeri. Although it is not clear what carrier solvent was used in the study conducted by Dell’Arciprete et al. (2009), it has been shown that DMSO (1.45–2.9%) causes an elevated luminescence response up to 40% using Vibrio harveyi (Mariscal et al. 2003). If the carrier solvent was DMSO in the study by Dell’Arciprete et al. (2009), this could result in the damping of the luminescence inhibition caused by acetamiprid leading to a lower toxicity (higher EC50 value).
Toxicity Trends with Respect to Chemical Properties
Correlations between toxicity effect of compounds and their respective physicochemical properties are an important approach in hazard assessments. Evaluating toxicity trends for both acute and chronic experiments is important due to the fact that some chemicals show a different level of toxicity under short- and long-term conditions (May et al. 2016). Investigating potential correlations between acute and chronic toxicity and chemicals properties has been used for other target organisms, such as Daphnia and fish species, and using similar physicochemical properties the relationship between acute and chronic toxicity results were studied. Figure 5 presents the relationship of toxicity (acute and chronic) with respect to three physicochemical properties, acid dissociation constant (pKa), log octanol/water partition coefficient (K ow), and molecular weight.
Trends of acute and chronic toxicities with respect to chemical properties. a Correlation between log K ow and EC50 of compounds. b Correlation between pKa and EC50 of compounds. c Correlation between molecular weight and EC50 of compounds. For all subfigures, acute and chronic EC50 of compounds are noted by outlined and filled shapes, respectively. For all subfigures, the toxicity mode of action for compounds are noted by a circle and triangle for direct and indirect, respectively. For all subfigures, the classes of pesticides are noted by difference colors: fungicides (green), herbicides (blue), and neonicotinoids (orange)
As a result of the similarities between the EC50’s of the two time points obtained using the acute toxicity test, the values obtained at the 15-min exposure time were used for investigating the toxicity trends. In the cases where compounds showed no effect observed or had significant errors (thiacloprid and atrazine), the results reported in literature were used instead. Diuron was removed from trend analysis comparing the pKa to acute and chronic toxicity results due the neutral characteristic.
Of the three chemical properties investigated (pKa, log K ow, and molecular weight), there were larger differences in pKa values among the compounds, thus making it easier to distinguish trends for both acute and chronic toxicity results. The comparison of pKa values to the acute toxicity results (Fig. 5a) showed a notable V-shaped trend for herbicides. Compounds with lower pKa values had lower toxicities until a point (pKa of approximately 1) when compounds with higher pKa values had lower toxicities. Fungicides showed a similar trend of compounds with lower pKa having lower toxicity values, while there was no trend observed for neonicotinoids. The decrease of toxicity with respect to compounds having low pKa’s also is observed for the chronic toxicity test (Fig. 5b), with the exception of atrazine and tebuconazole. The decrease of toxicity with decreasing pKa values is a trend previously reported while investigating the antibacterial activity of sulfamethoxazole transformation products using V. fischeri (Majewsky et al. 2014).
The only trend for the log K ow chemical property in relation to EC50 values was observed for the acute toxicity test where there was an overall V-shaped pattern for fungicides and herbicides, similar to what was described for the comparison of pKa (Fig. 5c). No log K ow trend was observed for neonicotinoid compounds in acute or chronic tests. Although no defined trend was observed for the chronic toxicity test, it is hypothesized that substances with high log K ow values are expected to have higher toxicities upon chronic exposure, because this physicochemical characteristic is associated with the potential of bioaccumulation (Lee and Chen 2009; May et al. 2016).
The comparison of molecular weight of compounds with respect to their EC50 values for the acute and chronic toxicity test showed no notable trends; however, Fig. 5e highlighted the distribution of compounds according to their mode of action. In general, compounds that had lower EC50 values had indirect modes of action.
Results from the three descriptors (log K ow, pKa, and molecular weight) show that none of these properties alone is capable of describing the toxicity of pesticides investigated in the present study, a similar observation for benzoic acids using Pseudokirchneriella subcapitata (Lee and Chen 2009). The trends that are observed regarding the correlation of acute and chronic toxicity with physicochemical properties indicate that both log K ow and pKa are important parameters to consider while the molecular weight is less relevant. The trends highlighted in this paper are in agreement with previous studies that show similar behavior of increasing toxicity with increasing log K ow and pKa, suggesting that this high throughput method for acute and chronic toxicity using V. fischeri is a valuable screening tool for other CEC’s. More investigation is required using larger number of compounds that have wide variations of pKa, log K ow, and molecular weights, thus making it easier to identify prominent correlations between toxicities and physicochemical properties.
Conclusions
In addition to identifying toxicities of relatively new pesticides, this study highlighted important data gaps preventing an accurate assessment of risks toward nontarget organisms from pesticides. The results obtained using the chronic toxicity method revealed that conventional acute toxicity endpoints for some pesticides might not provide an underestimation of the toxicity. In addition, the chronic test allowed for a more in-depth investigation of toxicity risks toward V. fischeri by taking into account the dynamic relationship of luminescence over time. The comparison of results obtained from the two toxicity tests identified modes of action of pesticides (direct or indirect), and by plotting the toxicity data alongside physicochemical properties, it was shown that most often pesticides with indirect modes of action had higher toxicities to V. fischeri. In addition, a correlation of toxicity with physicochemical properties was identified such that the toxicity increases with increasing pKa and log K ow. Based on the results presented in this study the addition of chronic toxicity to routine acute toxicity tests provides a more comprehensive assessment of environmental risks of pesticides and other CECs, which will minimize the potential for underestimating toxicity.
References
Backhaus T, Froehner K, Altenburger R, Grimme LH (1997) Toxicity testing with Vibrio fischeri: a comparison between the long-term (24 h) and the short-term (30 min) bioassay. Chemosphere 35:2925–2938. https://doi.org/10.1016/S0045-6535(97)00340-8
Blaschke U, Paschke A, Rensch I, Schüürmann G (2010) Acute and chronic toxicity toward the bacteria Vibrio fischeri of organic narcotics and epoxides: structural alerts for epoxide excess toxicity. Chem Res Toxicol 23:1936–1946. https://doi.org/10.1021/tx100298w
Bonnet JL, Bonnemoy F, Dusser M, Bohatier J (2007) Assessment of the potential toxicity of herbicides and their degradation products to nontarget cells using two microorganisms, the bacteria Vibrio fischeri and the ciliate Tetrahymena pyriformis. Environ Toxicol 22:78–91
Dell’Arciprete ML et al (2009) Reactivity of hydroxyl radicals with neonicotinoid insecticides: mechanism and changes in toxicity This article was published as part of the themed issue in honour of Esther oliveros. Photochem Photobiol Sci 8:1016–1023
Deng Z, Lin Z, Zou X, Yao Z, Tian D, Wang D, Yin D (2012) Model of hormesis and its toxicity mechanism based on quorum sensing: a case study on the toxicity of sulfonamides to Photobacterium phosphoreum. Environ Sci Technol 46:7746–7754. https://doi.org/10.1021/es203490f
Diaz D et al (2013) Pharmacokinetic drivers of toxicity for basic molecules: strategy to lower pKa results in decreased tissue exposure and toxicity for a small molecule Met inhibitor. Toxicol Appl Pharmacol 266:86–94. https://doi.org/10.1016/j.taap.2012.10.026
Dunn AK (2012) Vibrio fischeri metabolism: symbiosis and beyond. Adv Microb Physiol 61:37–68. https://doi.org/10.1016/b978-0-12-394423-8.00002-0
Fernández-Alba AR, Hernando MD, Piedra L, Chisti Y (2002) Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassays. Anal Chim Acta 456:303–312. https://doi.org/10.1016/S0003-2670(02)00037-5
Froehner K, Backhaus T, Grimme LH (2000) Bioassays with Vibrio fischeri for the assessment of delayed toxicity. Chemosphere 40:821–828
Froehner K, Meyer W, Grimme LH (2002) Time-dependent toxicity in the long-term inhibition assay with Vibrio fischeri. Chemosphere 46:987–997
Gaggi C, Duccini M, Bacci E, Sbrilli G, Bucci M, Naby AMHE (1995) Toxicity and hazard ranking of s-triazine herbicides using microtox two green algal species and a marine crustacean. Environ Toxicol Chem 14:1065–1069
Gatidou G, Stasinakis AS, Iatrou EI (2015) Assessing single and joint toxicity of three phenylurea herbicides using Lemna minor and Vibrio fischeri bioassays. Chemosphere 119:S69–S74
Gellert G (2000) Sensitivity and significance of luminescent bacteria in chronic toxicity testing based on growth and bioluminescence. Ecotoxicol Environ Saf 45:87–91. https://doi.org/10.1006/eesa.1999.1849
Ghosh SK, Doctor PB, Kulkami PK (1996) Toxicity of zinc in three microbial test systems. Environ Toxicol Water Qual 11:13–19. https://doi.org/10.1002/(SICI)1098-2256(1996)11:1<13:AID-TOX3>3.0.CO;2-C
Hernando MD, De Vettori S, Martínez Bueno MJ, Fernández-Alba AR (2007) Toxicity evaluation with Vibrio fischeri test of organic chemicals used in aquaculture. Chemosphere 68:724–730. https://doi.org/10.1016/j.chemosphere.2006.12.097
Hollingworth RM (2001) Inhibitors and uncouplers of mitochondrial oxidative phosphorylation A2. In: Krieger WC (ed) Handbook of pesticide toxicology, 2nd edn. Academic Press, San Diego, pp 1169–1261. https://doi.org/10.1016/B978-012426260-7.50060-4
Kokkali V, van Delft W (2014) Overview of commercially available bioassays for assessing chemical toxicity in aqueous samples. Trends Anal Chem 61:133–155
Kross BC, Vergara A, Raue LE (1992) Toxicity assessment of atrazine, alachlor, and carbofuran and their respective environmental metabolites using Microtox. J Toxicol Environ Health 37:149–159
Lee PY, Chen CY (2009) Toxicity and quantitative structureactivity relationships of benzoic acids to Pseudokirchneriella subcapitata. J Hazard Mater 165:156–161
Majewsky M, Wagner D, Delay M, Bräse S, Yargeau V, Horn H (2014) Antibacterial activity of sulfamethoxazole transformation products (TPs): general relevance for sulfonamide TPs modified at the para position. Chem Res Toxicol 27:1821–1828. https://doi.org/10.1021/tx500267x
Mariscal A, Peinado MT, Carnero-Varo M, Fernández-Crehuet J (2003) Influence of organic solvents on the sensitivity of a bioluminescence toxicity test with Vibrio harveyi. Chemosphere 50:349–354
May M, Drost W, Germer S, Juffernholz T, Hahn S (2016) Evaluation of acute-to-chronic ratios of fish and Daphnia to predict acceptable no-effect levels. Environ Sci Eur 28:1–9. https://doi.org/10.1186/s12302-016-0084-7
Nasuhoglu D, Westlund P, Isazadeh S, Neamatallah S, Yargeau V (2016) Development of a facile and high-throughput bioluminescence assay using Vibrio fischeri to determine the chronic toxicity of contaminated samples. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-016-2008-z
Oturan N, Trajkovska S, Oturan MA, Couderchet M, Aaron J-J (2008) Study of the toxicity of diuron and its metabolites formed in aqueous medium during application of the electrochemical advanced oxidation process “electro-Fenton”. Chemosphere 73:1550–1556. https://doi.org/10.1016/j.chemosphere.2008.07.082
Parvez S, Venkataraman C, Mukherji S (2006) A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ Int 32:265–268. https://doi.org/10.1016/j.envint.2005.08.022
Polo AM, Tobajas M, Sanchis S, Mohedano AF, Rodriguez JJ (2011) Comparison of experimental methods for determination of toxicity and biodegradability of xenobiotic compounds. Biodegradation 22:751–761. https://doi.org/10.1007/s10532-010-9448-7
Rozman KK, Doull J, Hayes WJ Jr (2001) Dose, time, and other factors influencing toxicity A2. In: Krieger WC (ed) Handbook of pesticide toxicology, 2nd edn. Academic Press, San Diego, pp 1–93. https://doi.org/10.1016/B978-012426260-7.50004-5
Shi JQ, Cheng J, Wang FY, Flamm A, Wang ZY, Yang X, Gao SX (2012) Acute toxicity and n-octanol/water partition coefficients of substituted thiophenols: determination and QSAR analysis. Ecotoxicol Environ Saf 78:134–141. https://doi.org/10.1016/j.ecoenv.2011.11.024
Simon-Delso N et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34
Stamatis N, Hela D, Konstantinou I (2010) Occurrence and removal of fungicides in municipal sewage treatment plant. J Hazard Mater 175:829–835. https://doi.org/10.1016/j.jhazmat.2009.10.084
Tchounwou PB, Wilson B, Ishaque A, Ransome R, Huang M-J, Leszczynski J (2000) Toxicity assessment of atrazine and related triazine compounds in the microtox assay, and computational modeling for their structure-activity relationship. Int J Mol Sci 1:63–74
Veljanoska-Sarafiloska EM, Jordanoski M, Stafilov T (2013) Presence of DDT metabolites in water, sediment and fish muscle tissue from Lake Prespa, Republic of Macedonia. J Environ Sci Health B 48:548–558
Yang C, Hamel C, Vujanovic V, Gan Y (2011) Fungicide: modes of action and possible impact on nontarget microorganisms. Ecology 2011:1–8
Yao XH, Min H, Lv ZM (2006) Response of superoxide dismutase, catalase, and ATPase activity in bacteria exposed to acetamiprid. Biomed Environ Sci 19:309–314
Acknowledgements
Funding for this study was provided by a research grant to Viviane Yargeau (PI) from the Natural Sciences and Engineering Research Council (NSERC) of Canada through the Discovery Grant Program (RGPIN/04635-2015) and by the McGill Engineering Doctoral Award (MEDA).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Westlund, P., Nasuhoglu, D., Isazadeh, S. et al. Investigation of Acute and Chronic Toxicity Trends of Pesticides Using High-Throughput Bioluminescence Assay Based on the Test Organism Vibrio fischeri . Arch Environ Contam Toxicol 74, 557–567 (2018). https://doi.org/10.1007/s00244-017-0483-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-017-0483-9