Abstract
The United States town of Aurora, Missouri, USA, stockpiled lead (Pb) and zinc (Zn) mining wastes from the early to mid-1900s in the form of chat piles. Clean-up actions were undertaken at intervals in subsequent years including land leveling and removal of chat. This study assessed the current state of contamination by identifying areas where metals are present at toxic levels. For this purpose, stream sediment samples (N = 100) were collected over a 9 × 12 km area in and around Aurora. Their content of cadmium (Cd), Pb, and Zn were measured, and concentration maps were generated using ArcGIS to categorize affected areas. Metal concentrations varied over a wide range of values with the overall highest values observed in the north-northeast part of Aurora where abundant chat piles had been present. Comparison between observed concentrations and sediment-quality guidelines put the contaminated areas mentioned are above-toxic levels for Cd, Pb and Zn. In contrast, levels in rural areas and the southern part of Aurora were at background levels, thus posing no threat to aquatic habitats. The fact that contamination is constrained to a relatively small area can be advantageously used to implement further remediation and, by doing so, to help protect the underlying karst aquifer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
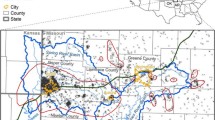
In the early 1900s, the mining town of Aurora was booming as were other mining towns within the Tri-State Mining District of Kansas, Missouri and Oklahoma (TSMD). At that time, the TSMD (Fig. 1) was considered one of the richest lead (Pb) and zinc (Zn) ore deposits in the world (Brockie et al. 1968; Gibson 1972). The December 1905 issue of Lead and Zinc News reported the recent opening of “promising” new mines and total Zn production of 35 million pounds for that year in Aurora. Extensive mining continued in Aurora until the 1950s, when production dwindled rapidly and ended around 1957. Wastes from mining operations were usually disposed of in chat piles, which were stacked in the vicinity of the mine shafts (Gibson 1972). As mining companies moved out, many chat piles remained in place and released toxic elements to the surrounding area.
Aurora is not an isolated case of a mining town dealing with abandoned wastes. In the 1800s, new and improved technology in the mining and refining processes allowed mining companies worldwide to accelerate ore extraction, processing, and transportation. Once ore became depleted, mining companies either closed down or moved out. Much of the mining wastes were abandoned, thus creating environmental nuisances (Hudson-Edwards et al. 2011; Kubit et al. 2015) such as unstable ground, acid drainage, barred land, and mine shafts and buildings, which soon deteriorated. In most places, wastes were exposed for 30–90 years (Romero et al. 2007) with waste piles contaminating nearby soil and groundwater and dust carried by wind causing respiratory health problems (Astete et al. 2009; Wills and Finch 2016).
The problem of abandoned mine wastes is complex because the solutions are governed by legislation, and in most cases it requires a long time for an agreement to be reached and also to attain rehabilitation (Bétourney 2009; Wills and Finch 2016). Studies on Pb–Zn tailings have been focused in regions of (1) impending further contamination through the mobility of metals and acid mine drainage, e.g., McCarthy (2011) in South Africa and Daldoul et al. (2015) in Tunisia; (2) health hazards to nearby residents, e.g., Park et al. (2014) in Korea; and (3) environmental risk assessment, e.g., Antunes et al. (2014) in Portugal and Bétourney (2009) in Canada. Although many studies have addressed the toxic effects of Pb–Zn mine tailings (e.g., Nikolaidis et al. 2010, Schaider et al. 2014) or the response of these wastes to unmanaged treatments, such as wetlands (Jacob and Otte 2004) and bioremediation (Boussen et al. 2013), many other sites lack a characterization study and therefore a viable remediation plan (Hudson-Edwards et al. 2011; Wills and Finch 2016).
The study presented here may apply to towns elsewhere dealing with abandoned mine wastes as well as to those looking for cost-effective ways to assess toxicity to formulate a remediation strategy.
The metals mined in Aurora were Zn and Pb with Zn being the main metal. Unlike the rich Pb deposits of southeast Missouri, the Pb–Zn ore within TSMD was formed dispersed over a broad area surrounded by smaller satellite deposits (Brockie et al. 1968; Johnson et al. 2016). Aurora (Fig. 1) is one of these isolated deposits (Gutierrez et al. 2015). Detailed geology of the TSMD ore formation and mineralogy is included in Brockie et al. (1968), Leach (1994), and Johnson et al. (2016).
The large amount of improperly disposed mining wastes within the TSMD prompted various studies to measure the effects of mining wastes on the environment. These studies addressed the most contaminated sites using soils and streambed sediments as sampling medium (e.g., Pope 2005; Schmitt et al. 2005; Andrews et al. 2009; Juracek 2013) as well as surface and subsurface water (Scheider et al. 2014).
Toxicity of Sediments
In this study, contaminants of concern included cadmium (Cd), Pb, and Zn. Cd is a trace metal closely associated with Zn in the TSMD (Brockie et al. 1968; Johnson et al. 2016). All three metals are toxic at high concentrations, whereas Cd is toxic to sediment-dwelling organisms even at small concentrations according to consensus-based sediment toxicity guidelines reported by MacDonald et al. (2000). These guidelines were obtained using the following organisms: amphipod (Hyalella azteca), mayfly (Hexagenia limbata), midge (Chironomus tentans), oligochaete (Lumbriculus variegatus), daphnid (Ceriodaphnia dubia), and bacterial luminescence (Photobacterium phosphoreum). Other studies reporting Cd, Pb, and Zn toxicity to aquatic organisms and various wildlife in the Ozarks region include Besser et al. (2009) who used the amphipod H. Azteca and the daphnid C. dubia; Ingersoll et al. (2009) who reported PEC levels specific for the TSMD based on the amphipod H. azteca; and Beyer et al. (2004) who measured toxicity in birds, including American robins (Turdus migratorius), cardinals (Cardinalis cardinalis), and waterfowl, by comparing metal content in soils and bird tissue.
Sediment-quality guidelines for the TSMD were revised by MacDonald et al. in 2010 to find a relationship between sediment chemistry and sediment toxicity. MacDonald et al. (2010) found that the most reliable method to determine sediment toxicity was to use a combination of the sediment-toxicity threshold values for Cd (11.1 mg/kg), Pb (150 mg/kg), and Zn (2083 mg/kg), which were derived using H. azteca amphiphod’s survival rate. According to their model, sediment samples are considered to pose a low risk if
This approach has been applied successfully to other aquatic organisms, e.g., crayfish (Allert et al. 2012) and freshwater mussels (Besser et al. 2014).
Remediation
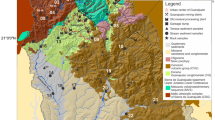
In Aurora, waste piles were more abundant in the northeast part of town as evidenced by air photos (Fig. 2) and reported locations of mine shafts (Missouri Department of Natural Resources [MODNR] 2015). One stream draining this area is Chat Creek, which crosses the town of Aurora from southeast to northwest and changes name to Douger Branch outside the city limits (see Fig. 3). A portion of the study area covered by air photos taken in 1939 and 1975 is shown in Fig. 2. Although the two pictures overlapped over only a small part of the study area, it can be seen that chat piles (white circular mounds) decreased both in size and number between 1939 and 1975. At the present time, no chat piles are left standing.
Remediation in Aurora focused on Pb and targeted the most contaminated soils first. Chat piles were leveled and grass planted on the leveled surface. Remediation took place in intervals depending on the availability of funds. In 2002, Pb-contaminated top soils were removed followed by the addition of high-phosphate poultry litter to the exposed soil. The Pb-contaminated soils were placed at the bottom of a berm constructed in Baldwin Park Dump, a site on the northeast edge of Aurora where mining waste had historically been deposited (MODNR 2013). The City of Aurora received funds from the United States Environmental Protection Agency (USEPA) Brownfields Program in 2007 to further remediate contaminated soil (Carbone et al. 2007; MODNR 2013). As additional funding became available, the next highest contaminated areas of Aurora underwent remediation from 2009 to 2011 (MODNR 2013). The remediated land is now level and is covered with vegetation.
Previous studies performed in the Aurora area consist of mainly two investigations: (1) Trimble (2001) in Chat Creek and (2) Carlson (1999) in Honey Creek. In 2004, there was an attempt to detect element concentrations within the soil and metal content of trees growing in contaminated soil (Carbone et al. 2007) with the investigators reporting that this method had yielded inconclusive results. An assessment of metal contamination that would encompass the city plus the surrounding region was lacking.
This study aimed to assess the state of contamination of Aurora after remediation actions and to identify location and extent of contaminants occurring above toxic levels. To this aim, the content and distribution of Cd, Pb, and Zn in stream sediments were determined and their levels compared against sediment-quality guidelines (SQGs) reported for the area as probable effect concentration (PEC). Furthermore, the distribution of metals were plotted in a map using ArcGIS, and their degree of contamination was compared against the location of former chat piles to monitor the effects of past remediation actions and other disturbances that may have had altered the metal-distribution pattern such as changes in land use.
Study Area
Aurora (population 7500) is located in Lawrence County in southwest Missouri. Aurora is part of the Ozark Plateau, a region of karst topography and gently rolling hills of farmland that produces hay, cattle, and poultry.
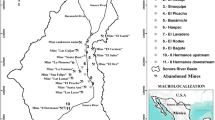
The exposed rock is the Mississippian Burlington limestone, which has well-developed karst features including sinkholes, springs, and losing streams. Several streams cross Aurora and flow east-northeast toward the Spring River. Highway 60 on the southern edge of Aurora approximately parallels the divide that separates the Spring River Basin to the northwest from the James River Basin on the southeast. Chat Creek crosses Aurora and merges with the Spring River. The rural surroundings are crossed by numerous streams, mostly losing streams (Fig. 3). Both Chat Creek and Spring River carry water all year around, whereas the other streams run dry most of the year.
Drinking water for Aurora is supplied by several deep wells that draw water from the Ozarks Aquifer, a confined 366 m-thick limestone aquifer that yields good-quality water (Imes 1989; Richards and Mugel 2008). The Ozark aquifer is overlaid by the Ozark confining unit, a unit comprised by thin layers of shale, among them the Northview Shale, that separate the Ozark Aquifer from the Springfield Plateau Aquifer (75-m thick) above it (Imes 1989). The Ozark confining unit allows leaks from the shallow aquifer to the deeper aquifer. Although no cases of groundwater contamination have been reported for the area, the karst nature of the aquifer makes it prone to contamination due to the large fractures present in the rock, especially if the potentiometric surface of the Ozark aquifer continues to decrease (Richards and Mugel 2008). Toxic metals contained in sediments thus pose a threat to the aquifers below. Because the best protection of karst aquifers is prevention, the removal of contaminated sediments is key to preventing groundwater contamination.
Methods
Surface sediment samples (top 5 cm) were collected in streambeds in and around Aurora. A total of 100 samples were collected, yielding a 1.08 km2/data point coverage. Sample locations are shown in Fig. 3. Samples in Chat Creek were collected at closer intervals to compare the metal content and distribution with values reported for this stream by Trimble in 2001. Trimble (2001) reported high concentrations of Pb and Zn with Zn concentrations ≤24,900 mg/kg.
Stream sediments are a nonhomogeneous medium with respect to their size distribution, mineralogy, and organic matter content (Förster 2004); therefore, they have varying metal concentrations. To estimate and account for this variability in metal content, duplicate samples were collected approximately 1 m apart at 13 random sampling sites.
After recording their location with a handheld Global Positioning System, the samples were stored in zip-close bags, labeled, kept in a cool place, and brought within hours to the laboratory where they were air-dried, disaggregated, and sieved to 1-mm particle size. Dry, sieved samples were divided into two subsets. The first subset was sent to ALS, a commercial laboratory, where samples were digested by aqua regia and analyzed using inductively coupled plasma–atomic emission spectroscopy according to USEPA standard method 3050B in compliance with ISO 9001:2008 quality-assurance certification. Aqua regia digestion is a close determination of the total metal content of the sediments (total metal content is extracted by hydrofluoric acid) and the one extraction most investigators use. The second subset was used to determine pH and %fines (<0.14 mm) in the laboratory. A 1:5 wt/wt sediment-water slurry was used to obtain the pH of the samples. The %fines were determined by weight after dry-sieving the <1-mm sample through a 0.14-mm sieve. The percent material that passed the sieve was labeled as %fines.
The correlation among metals was performed using Pearson correlation function of Microsoft Office EXCEL 2007 software among metals (Me). For each correlation coefficient, its significance was calculated as probability level p (e.g., significant at p = 0.05).
Two different methods were used to view the distribution of metal concentrations: box-plot diagrams and ArcGIS-inverse distance weight (IDW) maps. Box-plots provide a quick view of the distribution and are used widely for comparing contaminant content of sediments (United States Environmental Protection Agency 2004; Andrews et al. 2009; Paul et al. 2012). ArcGIS-IDW method enables to construct a map showing areas of increasing metal concentration. The latter method facilitates the visual identification of contaminated areas.
Results and Discussion
The assessment of contamination in this study relied on the chemistry, spatial distribution, and association among metals in sediments collected in 2015. Available data before remediation were nonexistent, except for data collected by Trimble (2001) in Chat Creek. Despite these limitations, the results were able to characterize the state of contamination of Aurora and its surroundings as described later in the text.
Metal concentrations for the Aurora area spread over a wide (up to three orders of magnitude) range of values: 0.5–281 mg/kg for Cd, 6–3450 mg/kg for Pb, and 21–15,500 mg/kg for Zn. To better appraise the differences in the degree of contamination, the data were divided into three subsets: rural Aurora (rural), urban Aurora (urban), and Chat Creek. Chat Creek is an urban stream, and its data are included under “urban,” but they were reported alone for comparison before and after remediation because Chat Creek was the only subset with data available before remediation.
The results (Table 1) list the large range of values obtained for each subset (rural, urban, Chat Creek) with the highest metal content being in Chat Creek. Because large values have the capacity to shift the mean considerably, the median was used instead of the mean as the representative value of each population. Based on the median values and comparing these values with sediment-quality guidelines, it becomes clear that the urban area poses a high risk to benthic organisms (∑PEC-QCd, Pb, Zn > 7.92), whereas the rural area is not toxic (MacDonald et al. 2010). As was expected from the large range of values, extreme values (anomalies) were present.
Correlation analyses were applied to the data to determine which metals showed the closest association and to test the assumption that the source of contamination was the mining waste. In the urban area, strong associations were observed between Cd and Zn with a Pearson correlation coefficient at r = 0.93 (p < 0.01) and between Pb and Zn at r = 0.94 (p < 0.01), whereas Pb and Zn did not correlate to other metals (e.g., iron, chromium, copper) except for Mn (r = 0.87 and 0.83 respectively, p < 0.01). In rural areas, the associations were also strong between Zn, Pb, and Cd despite the much lower concentrations. Strong Cd, Pb, and Zn associations (r > 0.9) were reported for the original ore (Johnson et al. 2016). Although this result is far from construing a validation of the source of metal contamination, it points to mining wastes as a likely source.
Box-plot analyses have successfully illustrated distribution variations in geochemical systems that have a nonnormally distributed population and anomalies (USEPA 2004). The box part of the diagram contains 50 % of the concentrations (values between quartiles 1 and 3). The resulting box-plots for each of the three subsets highlighted the large difference between rural and urban areas and allowed visualization of the number and magnitude of the anomalies. Figure 4 shows the box-plot diagrams obtained for Pb and Zn using logarithmic axis, which was necessary to display the large range of values. It is evident from Fig. 4 that among the subsets, there was a large difference between rural and urban areas, and that the subset with the highest metal concentrations was Chat Creek. Data reported by Trimble for Chat Creek in 2001 was included in this figure to compare between 2001 and 2015 conditions. Compared with Trimble’s (2001) values, 2015 levels were approximately the same for Pb and Zn values. These results agree with Schaider et al. (2014), who reported relatively unchanged conditions for Tar Creek (Oklahoma) after decades of chat-pile runoff input.
Searching for evidence that would support a possible explanation for the persistent presence of anomalies in each subset, pH and %fines (wt) were correlated with the metal concentrations. The pH values were in the alkaline range, with values varying between 7.06 and 7.64, and showed no correlation with metal content. Although a strong correlation of metal content was expected with respect to %fines, the %fines did not correlate to any of the Cd, Pb, or Zn. Juracek (2013) also found a lack of a relationship between metal (Pb, Zn) content and grain size in a TSMD contaminated floodplain (Spring River, Kansas, USA). Because neither pH nor %fines were able to explain the presence of anomalous values, one possible explanation is that tiny fragments rich in ore were able to reach the stream, maybe because floodplain soils were disturbed by recent remediation actions, and that these relocated fragments were nor in the stream channel long enough for weathering reactions to reach equilibrium. Once one or more of these unweathered ore fragments were present in the grab sample, and they contained enough metal so as to boost the metal concentration of the sample.
The highest metal concentrations were observed within Chat Creek, a 4-km stream directly adjacent to the former location of chat piles. Using samples collected at close intervals in Chat Creek, the metal concentrations downstream showed peaks of low and high concentrations for all three metals with more than half of the concentrations surpassing PEC levels. Figure 5 shows the results for Cd with a PEC line as reference and a map showing the location where values exceeded the guideline of 11.1 mg/kg (PEC). According to the map, the highest concentrations were located in the northeast corner of Aurora adjacent to the location of former chat piles.
Metal concentrations in this segment of Chat Creek coincide with the pattern and extent of contamination reported by Trimble in 2001. The persisting high metal concentrations in the same 4-km creek segment in both 2001 and 2015, as well as lack thereof downstream, suggest that once contaminants reach the stream, they remain somewhat immobile.
Next, the spatial distribution of metals was displayed in ArcGIS-generated maps using the IDW method. A striking feature is the containment of contamination to a relatively small area. A similar pattern was obtained for each Cd, Pb, and Zn, which was an expected outcome due to their strong association. The spatial distribution of metal contents for Cd, Pb, and Zn are shown in Fig. 6 using color code-delimiting SQG values PEC, 2∙PEC, and >3∙PEC (MacDonald et al. 2010).
The slow dispersion of contaminants may be caused by limestone present in the stream channels, which buffers the acidic pH and favors precipitation of the metals at the greater pH values, which has also been reported by Schaider et al. (2014) for Tar Creek, Oklahoma.
The variation in concentration intrinsic to the heterogeneous nature of sediments was calculated as % variation for each pair. The resulting % variation was consistently approximately 20 % (N = 13 pairs; where one pair corresponds to two grab samples collected within a 1-m distance from each other). Specifically, the % variations and SDs were 17.9 ± 21.8 for Cd, 20.9 ± 16.7 for Pb, and 19.1 ± 16.1 for Zn. The consistency and relatively small values of % variation suggest that the spatial distribution of metals most likely represent the degree of contamination and that their metal content is affected only slightly by the heterogeneity of sediments.
The broad range of concentrations typical of sediments and soils contaminated with mine wastes makes a comparison between this and other studies difficult. However, if we use the median as an approximation of the contamination level, Chat Creek in Aurora has Cd, Pb, and Zn values comparable with other contaminated sites within the TSMD but affecting a smaller area (Pope 2005; Andrews et al. 2009; Juracek 2013; Johnson et al. 2016). Sites contaminated with abandoned Pb–Zn mines elsewhere report similar patterns of contamination including the large range of values, a Zn content much greater than Pb, a significant amount of Cd in sediments, and limited mobility of metals in neutral to alkaline conditions (Romero et al. 2007; Nikolaidis et al. 2010; Daldoul et al. 2015). Extreme values have been reported next to an active Pb–Zn mine (Yanqun et al. 2005) where the median values of Cd, Pb, and Zn are, respectively, 925, 4969, and 11,630 mg/kg corresponding to 28×, 25×, and 2× the Chat Creek values reported here.
The low dispersion and high constrain of metals found in Aurora can be advantageously used to remediate these contaminated stream segments before metals will develop conditions (e.g., by sediment buildup), at which point the pH and redox would change to acidic pH and/or reducing conditions, which would in turn facilitate the mobilization of metals either downstream and/or to seep into the underlying aquifer.
Suggested remediation treatments lead to the further removal of metals from sediments. Remediation treatments have been discussed (Johnson et al. 2016) for areas within the TSDM. General recommendations include active treatments such as (1) the addition of a buffering agent (e.g., lime) followed by removal (dredging) of the contaminated sediment and/or (2) passive treatments, including planting of known hyperaccumulators, which are plants able to transport the metals from the soil and into the plant tissue. For the latter method, removal of metals will be achieved after the plants are harvested. Both of these methods require proper disposal of the metal-rich material.
Conclusions
Correlation among metals and spatial distribution maps point to mining wastes as the source of metal contamination despite the fact that the chat piles have been remediated and are not visible anymore. The spatial distribution maps of metal content showed a small area of high contamination (surpassing the PEC used as a guideline to separate toxic to nontoxic levels) contrasting with a larger extension of land with no contamination.
The area most contaminated was a 4-km (2.5-mile) segment of Chat Creek where metals levels are toxic to wildlife, particularly aquatic organisms. The maps constructed for each of Cd, Pb, and Zn show that the area with toxic levels of metal is constrained to a relative small area at or near the former location of chat piles.
Limestone’s buffering capacity seems to be keeping the metals in place as insoluble precipitates, which has resulted in little dispersion, a characteristic favorable to conduct remediation efforts.
Continued removal of metal contaminated sediment is advisable because metals could be remobilized under acidic conditions, and, once in a mobile form, they could compromise drinking-water sources, especially considering the fractured nature of the underlying aquifer.
References
Allert AL, DiStefano RJ, Schmitt CJ, Fairchild JF, Brumbaugh WG (2012) Effects of mining-derived metals on riffle-dwelling crayfish in southwestern Missouri and southeastern Kansas, USA. Arch Environ Contam Toxicol 63:563–573
Andrews WJ, Becker MF, Mashburn SL, Smith J (2009) Selected metals in sediments and streams in the Oklahoma part of the Tri-State Mining District, 2000–2006. USGS Survey Scientific Investigations Report 2009–5032
Antunes IMHR, Albuquerque MTD, Sanches FAN (2014) Spatial risk assessment related to abandoned mining activities, an environmental management tool. Environ Earth Sci 72:2631–2641
Astete J, Caceres W, Gastanaga MC, Lucero M, Sabastizagal I, Oblitas T et al (2009) Lead intoxication and other health problems in children population who live near mine tailing. Rev Peru Med Exp Salud Publica 26:15–19
Besser JM, Brumbaugh WG, Allert AL, Poulton BC, Schmitt CJ, Ingersoll CG (2009) Ecological impacts of lead mining on Ozark streams: toxicity of sediment. Ecotoxicol Environ Saf 72:516–526
Besser JM, Ingersoll CG, Brumbaugh WG, Kremble NE, May TW, Wang N et al (2014) Toxicity of sediments from lead-zinc mining areas to juvenile freshwater mussels (Lampsilis siliquoidea) compared to standard test organisms. Environ Toxicol Chem 34:626–639
Beyer WN, Dalgarn J, Dudding S, French JB, Mateo R, Miesner J et al (2004) Zinc and lead poisoning in wild birds in the Tri-State Mining District. Environ Contam Toxicol 48:108–117
Bétourney MC (2009) Abandoned metal mine stability risk evaluation. Risk Anal 29:1355–1370
Boussen S, Soubrand M, Bril H, Ouerfelli K, Abdeljaouad S (2013) Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 192:227–236
Brockie DC, Hare EH, Dingess PR (1968) The geology and ore deposits of the Tri-State District of Missouri, Kansas and Oklahoma. In: Ridge JD (ed) Ore deposits of the United States, 1933–1967. The American Institute of Mining, Metallurgical, and Petroleum Engineers, New York, pp 400–443
Carbone N, Blodgett C, Muenks S, Lea R, Franson R (2007) Evaluation of hyperspectral imagery for Missouri Mine Waste Inventory Sept. 1, 2006—June 30, 2007. Missouri Department of Natural Resources and U.S. Environmental Protection Agency Brownfields Program
Carlson JL (1999) Zinc mining contamination and sedimentation rates of historical overbank deposits, Honey Creek watershed, southwest Missouri. Master thesis, Missouri State University
Daldoul G, Souissi R, Souissi F, Jemmali N, Chakroun HK (2015) Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. J African Earth Sci 110:150–159
Förstner U (2004) Traceability of sediment analysis. Trends Anal Chem 23:217–236
Gibson AM (1972) Wilderness bonanza—the Tri-State District of Missouri, Kansas, and Oklahoma. University of Oklahoma Press, Norman
Gutierrez M, Wu SS, Peebles JL (2015) Geochemical mapping of Pb- and Zn-contaminated streambed sediments in southwest Missouri, USA. J Soil Sediment 15:189–197
Hudson-Edwards KA, Jamieson HE, Lottermoser BG (2011) Mine wastes: past, present, future. Elements 7:375–380
Imes JL (1989) Analysis of the effect of pumping on ground-water flow in the Springfield Plateau and Ozark aquifers near Springfield, Missouri. USGS-WRI, Open File Report 89–4079, Rolla, MO
Ingersoll CG, Ivey CD, Brumbaugh WG, Besser JM, Kremble NE (2009) Toxicity assessment of sediments from the Grand Lake O’ the Cherokees with the amphipod Hyalella azteca. US Geological Survey Administrative Report CERC-8335-FY09-20-01
Jacob DL, Otte ML (2004) Long-term effects of submergence and wetland vegetation on metals in a 90-year old abandoned Pb-Zn mine tailings pond. Environ Pollut 130:337–345
Johnson AW, Gutierrez M, Gouzie D, McAlily LR (2016) State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 144:1132–1141
Juracek KE (2013) Occurrence and variability of mining-related lead and zinc in the spring river flood plain and tributary flood plains, Cherokee County, Kansas, 2009–11. USGS Scientific Investigations Report 2013–5028
Kubit OE, Pluhar CJ, De Graff JV (2015) A model for prioritizing sites and reclamation methods at abandoned mines. Environ Earth Sci 73:7915–7931
Leach DL (1994) Genesis of the Ozark Mississippi valley-type metallogenic province, Missouri, Arkansas, Kansas, and Oklahoma, USA. In: Fontbote L, Boni M (eds) Sediment-hosted Zn-Pb ores. Special publication of the society for geology applied to mineral deposits, vol. 10. Springer, Berlin, pp 104–38
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
MacDonald DD, Ingersoll CG, Crawford M, Prencipe H, Besser JM, Brumbaugh WG et al. (2010) Advanced screening-level ecological risk assessment (SLERA) for aquatic habitats within the Tri-State Mining District, Oklahoma, Kansas and Missouri. Draft Final Technical Report submitted to the United States Environmental Protection Agency, Region 6, Dallas Texas, Region 7, Kansas City Kansas and the United States Fish and Wildlife Service, Columbia
McCarthy TS (2011) The impact of acid mine drainage in South Africa. S African J Sci 107:1–7
Missouri Department of Natural Resources (2013) Baldwin Park. In Registry of confirmed abandoned or uncontrolled hazardous waste disposal sites in Missouri. Missouri Registry annual report, pp 199–202. http://www.dnr.mo.gov/pubs/pub337.pdf. Accessed:
Missouri Department of Natural Resources (2015) GeoSTRAT—Geosciences Technical Resource Assessment Tool. http://dnr.mo.gov/geology/geostrat.htm. Accessed 9 Nov 2015
Nikolaidis C, Zafiriadis I, Mathioudakis V, Const T (2010) Heavy metal pollution associated with an abandoned lead–zinc mine in the Kirki region, NE Greece. Bull Environ Contam Toxicol 85:307–312
Park DUK, Kim DS, Yu SD et al (2014) Blood levels of cadmium and lead in residents near abandoned metal mine areas in Korea. Environ Monit Assess 186:5209–5220
Paul AP, NV Paretti, DE MacCoy, AMD Brasher (2012) The occurrence of trace elements in bed sediment collected from areas of varying land use and potential effects on stream macroinvertebrates in the conterminous western United States, Alaska, and Hawaii, 1992–2000. USGS Scientific Investigations Report 2012–5272
Pope LM (2005) Assessment of contaminated streambed sediment in the Kansas part of the historic Tri-State Lead and Zinc Mining District, Cherokee County, 2004. USGS Survey Scientific Investigations Report 2005–5251. http://pubs.usgs.gov/sir/2005/5251/pdf/SIR20055251.pdf
Richards JM, Mugel DN (2008) Potentiometric surface of the Ozark Aquifer near Springfield, Missouri, 2006–07. USGS Scientific Investigations Map 3003
Romero FM, Armienta MA, González-Hernández G (2007) Solid-phase control on the mobility of potentially toxic elements in an abandoned lead/zinc mine tailings impoundment, Taxco, Mexico. Appl Geochem 22:109–127
Schaider LA, Senn DB, Estes ER, Brabander DJ, Shine JP (2014) Sources and fates of heavy metals in a mining-impacted stream: temporal variability and the role of iron oxides. Sci Total Environ 490:456–466
Schmitt CJ, Whyle JJ, Brumbaugh WG, Tillitt DE (2005) Biochemical effects of lead, zinc, and cadmium from mining on fish in the tri-states district of northeastern Oklahoma, USA. Environ Toxicol Chem 24:1483–1495
Trimble JC (2001) Spatial patterns and floodplain contributions of mining-related contaminants in Chat Creek watershed, southwest Missouri, MS Thesis, Missouri State University
United States Environmental Protection Agency (2004) The incidence and severity of sediment, contamination in surface waters of the United States. National Sediment Quality Survey (2nd ed). Report EPA-823-R-04-007
Wills BA, Finch JA (2016) Tailings disposal. In: Wills’ mineral processing technology: An introduction to the practical aspects of ore treatment and mineral recovery (8th ed). pp 439–448
Yanqun Z, Yuan L, Jianjun C, Hauyan C, Li Q, Schvartz C (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environ Int 31:755–762
Acknowledgments
This study was funded by a Missouri State University faculty research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez, M., Wu, SS., Rodriguez, J.R. et al. Assessing the State of Contamination in a Historic Mining Town Using Sediment Chemistry. Arch Environ Contam Toxicol 70, 747–756 (2016). https://doi.org/10.1007/s00244-016-0265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0265-9