Abstract
Sediment toxicity and metal bioaccumulation were assessed at sites affected by historical copper (Cu) and mercury (Hg) mining activities in the Nalón River basin, Asturias, Spain. Toxicity assessment of stream sediments was based on a 28-day oligochaete Tubifex tubifex sediment bioassay, which allowed the classification of sites into three levels of toxicity: 11 sites were classified as nontoxic (including Cu mine sites), three sites as potentially toxic, and seven sites as toxic (all located in Hg mine districts). The greatest levels of arsenic (As), chromium, Hg, lead (Pb), and zinc (Zn) in T. tubifex were measured at sites affected by Hg mining and the highest Cu levels in tissues at Cu mining sites. Chronic toxicity responses were best explained by As and Hg sediment concentrations and by As, Pb, and Zn tissue residues. Residue levels of As, Hg, Zn, and Pb were successfully used to predict sediment chronic toxicity and estimate effective tissue residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Assessing river sediment quality requires an integrated approach based on several lines of evidence including sediment chemistry, chronic toxicity, and field benthic communities. Several investigators have included additional or alternative measures such as bioaccumulation (Burton et al. 2002; Chapman and McDonald 2005; Grapentine et al. 2002), critical body residues (CBRs; Gust and Fleeger 2005; Rosen and Lotufo 2005), biomarkers (Hollert et al. 2002; Riba et al. 2004), or habitat alterations (Maestre et al. 2009). Incorporating data on tissue residues provides evidence not only on the bioavailability of chemicals but also on their potential for biomagnification (Krantzberg et al. 2000). Most current sediment-quality assessment procedures compare conditions at the study sites with the expected conditions derived from reference sites. This procedure is known as the “reference condition approach” (Reynoldson et al. 1997), which is in agreement with the European Water Framework Directive (WFD; EC 2000) for quality assessment of water bodies. Sediment and biota have been recently recognized as suitable matrices to monitor long-term changes in water quality of European water bodies (EC 2010; Carère et al. 2012); however, in practice environmental quality standards for these compartments have been developed only by some State Members.
Metal-mining activities represent an environmental problem for freshwater ecosystems (Luoma et al. 2010; Solá et al. 2004). Once the mining activity has stopped, the abandoned mine sites usually continue to be sources of pollution to water bodies. Asturias (northern Spain) has historically been a rich metal-mining area. After the Law of Mines from 1825, >800 sites with mining activity were registered in Asturias, and the period between 1950 and 1975 represented the highest level of mining activity (Rodríguez-Terente et al. 2006). In the Nalón River basin (Asturias), two main mining industries were active until the early 1970s: Texeo copper (Cu) mines (Riosa district) and mercury (Hg) mines (Mieres, Pola de Lena,and Somiedo districts). Texeo mines were the most important source of Cu in NW Spain since Roman times, and were exploited from Bronze Age (3810–4090 BP: De Blas Cortina 1996; De Blas Cortina and Suárez Fernández 2009) until the last century. Hg mining also has a long history in Asturias, and extraction of cinnabar dates back to the Roman period (approximately 2000 BP) (Rodríguez-Terente et al. 2006). Since the closure of the mines, spoil heaps, except for the El Terronal site (Mieres) where most of the wastes were isolated in an in situ security landfill in 2002, have not received any type of treatment to avoid mobilization of pollutants. However, no maintenance has been performed since then (Loredo et al. 2010).
Aquatic oligochaetes have been used in metal sediment toxicity and/or bioaccumulation assessment in both laboratory (e.g., Bouché et al. 2000; De Jonge et al. 2012; Maestre et al. 2007; Steen-Redeker et al. 2004) and field exposures (De Jonge et al. 2010; Protano et al. 2014). The study of sediment toxicity and bioaccumulation in environmental risk assessment (ERA) using aquatic oligochaete worms was highlighted by Chapman (2001) and more recently reviewed by Rodriguez and Reynoldson (2011). In the present study, we assessed sediment toxicity and metal bioaccumulation at several sites affected by historical mining activities in the Nalón River basin (Asturias, Spain) using the aquatic oligochaete Tubifex tubifex (Müller). The present study also evaluated the utility of metal tissue residues in T. tubifex to predict chronic toxicity effects due to exposure to metal-polluted sediments.
Materials and Methods
Study Area
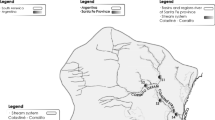
Twenty-five sites were studied in the Nalón River basin during September 2010 and 2011 [three sites were sampled twice (N6, N11, and N15); Table 1]. Four reference sites were located in the study area (N1r, N2r, N18r, and N22r) that belong to the Water Surveillance networks in Spain [(Cantabrian Hydrographical Confederation, CHC)]. Among the study sites, 15 were located in mining districts: 6 in a Cu mine area [Riosa = sites N3 to N8 (Fig. 4a, b in Appendix)] and 9 in Hg mine areas [Pola de Lena = sites N9 to N13; Mieres = N14 to N17 (Fig. 4b, c in Appendix)]. Two of these sites were located upstream any mining or industrial areas (sites N7 and N12) to complete information provided by reference sites on background metal levels in the study area.
Sediment Sampling and Characterization
Sediment sampling was performed under a low-flow regime in September of 2010 to 2011 when most of the fine-grained suspended sediments become deposited on the river bed (Mudroch and Azcue 1995) and when worst conditions for toxicity and bioaccumulation for biota are expected to occur (AQEM Consortium 2002). At each site, a composite sample of sediment was taken with a stainless steel spade from the upper 5 to 10 cm layer of fine sediment settled along an approximately 25-m reach of the river bank. The sediment was sieved in the field through 500-µm mesh to eliminate coarse particles and indigenous fauna (Reynoldson et al. 1995). Samples were taken to the laboratory on ice and stored at 4 °C in the dark during a maximum period of 6 months (as recommended by Reynoldson et al. 1991). Sediment subsamples for metal concentration analyses were air-dried and sieved through a 63-µm mesh. Particle size distribution of unsieved sediment was expressed as dry-weight (dw) percentage according to Udden-Wenworth scale (Teruggi 1982). Sediment total organic carbon (TOC) % was determined through loss-on-ignition method after calcination at 450 °C for 6 h in a muffle furnace (Bryan et al. 1985; USEPA 1990). Several water variables also were measured in situ using a conductivity portable device (Orion 3-Star, Thermo Scientific) and dissolved oxygen, pH, and temperature using a multiparameter portable device (Orion 5-Star, ThermoScientific) (Table 5 in Appendix).
Chronic Toxicity and Metal Bioaccumulation
The 28-day T. tubifex sediment bioassay was developed as a standardized chronic bioassay by Reynoldson et al. (1991) and later published by American Society for Testing and Materials (2005) for sediment toxicity assessment and by the Organisation for Economic Co-operation and Development (2008) for testing of chemicals for bioaccumulation. In the present study, chronic bioassays included survival (%), reproduction [number of total cocoons (TCC), number of empty cocoons (ECC), and number of total young (TYG), and growth end points (total growth rate (TGR); Maestre et al. 2007)]. Twice per week, we measured dissolved oxygen (Orion 5-Star), pH (pH-meter; Crison 2001), and total ammonia (Nessler method; Hach model DR2000 spectrophotometer) in the overlying water, whereas aeration was visually checked daily (Monday through Friday). For a detailed description of T. tubifex culture, see Méndez-Fernández et al. (2013).
Adult worms surviving by the end of the 28-day sediment bioassays were used for metal tissue residue analysis. A total of 21 samples were analyzed for metal tissue residues because exposure at four sites (N9, N10, N11b, and N15b) resulted in 100 % mortality. Five laboratory replicates were examined per site, except for N11a and N15a, where a pool of surviving worms was used to obtain enough biomass for metal tissue analyses. Worms were purged in dechlorinated tap water for 5 h. To measure egestion rates in T. tubifex, a gut-clearing period of 4 h is recommended by Martinez-Madrid et al. (1999), whereas based on gut transit of cationic metals in the oligochaete Lumbriculus variegatus, a 6-hour period is proposed by Dawson et al. (2003). Then worms were digested for 1 week in trace element-free nitric acid (70 %; Baker Instra-Analyzed) and afterward for 24 h with H2O2 (30 %; R.P. Normapur Prolabo) in a ratio of 10:1 at room temperature (Clements 1994). Samples were stored at −20 °C until metal analysis was completed.
Metal Analysis
A total of seven metals [cadmium (Cd), Cu, chromium (Cr), Hg, nickel (Ni), lead (Pb), and zinc (Zn)] and one metalloid [arsenic (As)] were measured at SOSPROCAN unit (University of Cantabria, Spain). Acid digestion of sediment samples was performed according to USEPA 3052 and UNE-EN 13656:2003 procedures (9 ml of HNO3 65 % and 4 ml of HF were added to 0.2 g of sediment). For Hg analysis, AuCl3 was added after acid digestion for Hg preservation (USEPA method 6020A). Digested sediment samples were measured by inductively coupled plasma-mass spectrometry (ICP-MS) (7500c; Agilent), and detection limits (DLs) were 0.07 µg l−1 As, 0.01 µg l−1 Cd, 0.10 µg l−1 Cu, 0.02 µg l−1 Cr, 0.03 µg l−1 Hg, 0.06 µg l−1 Ni, 0.01 µg l−1 Pb, and .03 µg l−1 Zn. All batches included Buffalo River sediment as reference material (RM8704; USA) for quality control, and recovery rates (82.5 to 104.4 %) were within certified values.
Metal tissue residues were also measured by ICP-MS, and DLs were 0.002 µg l−1 As, 0.001 µg l−1 Cd, 0.025 µg l−1 Cu, 0.009 µg l−1 Cr, 0.001 µg l−1 Hg, 0.008 µg l−1 nickel (Ni), 0.009 µg l−1 Pb, and 0.002 µg l−1 Zn. Every batch of tissue samples included three blanks and three replicates of a certified reference material (Mussel Tissue ERM-CE278; Belgium). Tissue reference material recovery rates (80.4 to 106.3 %) were within the certified values for Cd, Cr, Cu, Pb, and Zn except for As (140.1 %). No reference values were available for Hg and Ni, but their concentration showed small variations between different batches of reference material (Hg = 0.20 ± 0.04 and Ni = 0.94 ± 0.17 µg/g dw; n = 18). All measurements are expressed in molar mass, related with worm body mass, on a dry-weight basis.
Statistical Analyses
Sites were first classified based on a priori known anthropological pressures on river systems. A total of four pressure groups were indentified: (1) absence of disturbance (CHC reference sites), (2) undetermined/unknown pressures or weak hydromorphological alterations, (3) Cu mining sites, and (4) Hg mining sites.
Metal concentration in sediment and tissue was assessed using nonparametric tests: Kruskal–Wallis followed by multiple comparisons with Dunn´s test (Zar 1996). The validity of pressure groups was assessed by ANOSIM procedure (Clarke 1993). Principal component analysis (PCA) combined with varimax rotation examined dominant patterns of intercorrelation among sediment variables (previously transformed and standardised). Data analyses were performed in IBM SPSS (2011) and PRIMER 6 (Clarke and Gorley 2006) software.
Reference and test sites were included in the same data matrix, and sediment toxicity was evaluated through nMDS using Euclidean distance (PRIMER 6). Reference condition for toxicity assessment was established from a database of 58 reference sites in Northern Spain (Rodriguez et al. 2011; Méndez-Fernández 2013) including four additional sites from the present study area (N1r, N2r, N18r, and N22r). Sediment toxicity assessment in the Nalón River basin was performed site by site in the multivariate space of reference sites using probability ellipses of 80 and 95 % according to the procedure described in detail by Rodriguez et al. (2011). Test sites were assessed as nontoxic (NT) when placed within the 80 % probability ellipse and thus considered similar to the reference condition; sites were assessed as potentially toxic (PT) when placed within 95 and 80 % probability ellipses; and, finally, those sites placed outside the 95 % probability ellipse were assessed as toxic (T) and thus were interpreted as different from the reference condition.
Linking multivariate biotic with abiotic matrices was performed through BEST procedure and the correlation between the two matrices was evaluated through Spearman’s rank correlation for 999 permutations and 10 restarts. The “best” match between a subset of selected environmental variables and the biotic matrix was examined with RELATE test (PRIMER 6).
Nonlinear dose–response regression models were applied to toxicity and tissue residues, and median lethal and effective residues (LRs and ERs [Meador et al. 2011] ) were estimated using R software and the extension package drc (Ritz and Streibeig 2005). Model selection was performed using custom made R script based on Akaike’s information criterion, and model validation was based on graphical assessment. Potential outliers in the regression models were identified and excluded through the analysis of standardized and Studentised residuals (Zuur et al. 2007). Goodness-of-fit was assessed by R 2 and the Neill’s lack-of-fit test for no-replicates included in the drc package (Ritz and Streibeig 2005).
Results
Sediment Metal Concentration
Metals showed maximum concentration at sites N16 (5,320.9 µg As g−1, 186.9 µg Ni g−1, 265.6 µg Zn g−1), N15 (312.5 µg Hg g−1, 44.9 µg Pb g−1), N8 (1.76 µg Cd g−1), N3 (115.2 µg Cu g−1), and N7 (102.0 µg Cr g−1) (Table 1). In the absence of sediment-quality guidelines (SQGs) in Spain (den Besten et al. 2003), sediment metal concentrations were evaluated using threshold effect concentration (TEC) and probable effect concentration (PEC) values proposed by MacDonald et al. (2000) for North American freshwater sediments. Sediment metal concentration in the mine districts of Asturias showed moderate to high levels. PEC value was exceeded in 28–48 % of study sites for Ni, Hg, and As; TEC value was exceeded for Cd at 84 % of study sites followed by Cr (56 %), Cu (40 %), Ni (36 %), and As (28 %) (Fig. 1). Pb and Zn never exceeded PEC values and only at one or two sites, respectively, had values higher than TEC. Interestingly, some reference sites from the Nalón River basin (N2r, N18r, and N22r), as well as other sites from tributaries not altered by mining works (N7 and N12), showed As and/or Hg sediment concentrations greater than TEC and, occasionally, even PEC values. This fact shows that background geological levels in the study area may naturally contribute to greater metal levels in sediments.
Box-plots comparing sediment metal concentration (μg g−1 dw) in 25 study sites attending to anthropogenic pressure groups: Cu mines n = 6, Hg mines n = 11, reference sites n = 4, and undetermined pressures n = 4. Box is built with 25 and 75 percentiles and shows inside the median marked by a bold line. For each metal, their respective TEC value (dotted line) and, when necessary, the PEC value (dashed line) are indicated. Pie charts show the proportion of test sites in the study area greater than EC (dark grey), greater than TEC (grey), and lower than EC (light grey). Open circles indicate sites with extreme data values (>1.5 times the interquartile range of the data). Significant differences using Dunn’s test are marked as *p < 0.05, **p < 0.01
Significant differences in As, Cu, Cr, Hg, and Ni sediment concentration (Dunn’s test p < 0.05) were obtained by comparing site groups subject to different anthropogenic pressure types (Fig. 2). As, Cr, Hg, and Ni concentration measured in Hg mines were significantly greater than those in other pressure types; Cu concentration in Cu mines was also significantly greater than in undetermined pressure sites. In contrast, no differences were detected for Cd, Pb, and Zn sediment concentration regarding pressure types (Dunn’s test p > 0.05).
Spatial ordination by nMDS for the 25 study sites based on chronic toxiciy resemblance matrix. Each site is marked by a symbol corresponding to four categories after sediment toxicity risk classification (REF reference) using five endpoints from T. tubifex chronic bioassay (survival, TCC, ECC, TYG, and TGR)
Sediment characteristics [As, Cd, Cu, Cr, Hg, Ni, Pb, Zn, TOC, and silt–clay (SC) fraction] were analyzed through multivariate analysis, and confirmed that site clustering (Euclidean distance) due to metal sediment concentration was at least in part related to mining activity (ANOSIM Global R = 0.411, p = 0.001). ANOSIM analysis indicated that reference sites were significantly different from Cu mines (R = 0.433, p = 0.043) and Hg mines (R = 0.548, p = 0.001) and that undetermined pressure sites showed significant differences with Hg mines (R = 0.700, p = 0.001). No differences were found between other pressure groups.
PCA analysis was run with As, Cu, Cr, Hg, Ni, Zn, TOC %, and SC fraction. PCA after varimax rotation defined two first PCs with eigenvalues >1 (Kaiser criterion) that explained the 80.5 % of the accumulated variance (PC1 = 51.0 % and PC2 = 29.5 %). PC1 was strongly correlated with Cu, Cr, Ni, and Zn concentrations and SC fraction (loadings >0.80), and PC2 was strongly correlated with As and Hg (loadings >0.80) (Table 6 in Appendix). Thus, PC1 defined a gradient from unpolluted reference sites toward polluted mining sites, and PC2 readily distinguished Hg mining sites, with greater As and Hg metal concentration, from other sites (Fig. 5 in Appendix).
Toxicity Assessment
Results from chronic bioassays are listed in Table 2. Site toxicity classification using probability ellipses in the nMDS multivariate space of the reference sites database (n = 58) resulted in 11 sites classified as NT (including Cu mine sites), three sites as PT (N5, N19, and N21), and seven sites as T (N9, N10, N11a, N11b, N15a, N15b, and N16). All T sites were located at Hg mine districts with high levels of As, Hg, and Cd in sediments. Grouping of study sites in the Nalón River basin based on toxicity classification produced, as expected, a high global R value of 0.843 (p = 0.001). However, site grouping based on general anthropogenic pressures did not explain the observed toxicity (ANOSIM Global R = 0.037, p = 0.272) suggesting that toxicity responses were not always attributable to pressures related to mining activities. nMDS analysis based on toxicity data (Fig. 2) showed accurately the dissimilarities between study sites (stress = 0.02) with T sites (all from Hg mines) placed opposite to reference sites. Reference and NT sites showed high values in all bioassay endpoints, whereas T sites showed marked decreases in all studied endpoints (Table 2). Significant differences in sediment toxicity was found between T sites from Hg mining areas and reference sites (ANOSIM R = 0.775, p = 0.003), between T and NT sites (R = 0.923, p = 0.001), as well as between PT and NT sites (R = 0.847, p = 0.003) and PT and T sites (R = 0.659, p = 0.008). Results in the Nalón River basin also indicated nonsignificant differences between reference and NT sites (R = 0.175, p = 0.173) or PT sites (R = 0.278, p = 0.143).
At sites N19 and N21, both assessed as PT, impairment in both ECC and TYG was observed (Table 2), which may be an indication of embryogenesis alterations and/or young mortality after hatching. However, it is noteworthy that the classification of site N5 (from Cu Mines district) as PT is due to high reproduction values (Table 2), i.e., much greater than those found in most reference sites in our database of Northern Spain.
Metal Tissue Residues
The highest Cu (3.75 µmol g−1 dw) tissue residues in bioassay worms were measured at a Cu mining site (N6a), whereas the highest As (28.92 µmol g−1 dw), Cr (0.28 µmol g−1 dw), Hg (104.29 nmol g−1 dw), Pb (46.43 nmol g−1 dw), and Zn (38.21 µmol g−1 dw) tissue residues were measured at an Hg mining site (N11a) (Table 3). Interestingly, two reference sites showed the highest Cd and Ni tissue residues (36.15 nmol Cd g−1 dw at site N18r and 0.26 µmol Ni g−1 dw at site N22r), and none of the reference sites in the Nalón River basin exhibited the lowest metal tissue residues.
Comparison of T. tubifex metal tissue residues between T, PT, NT, and reference sites showed significant differences only for As when comparing PT with T sites (Dunn’s test p < 0.05). Multivariate analysis of metal bioaccumulation data showed low differences between those four toxicity groups (ANOSIM Global R = 0.335 p = 0.011). Significant differences were only found between NT and T sites (R = 0.662, p = 0.011), whereas differences between reference and T sites were not significant (R = 0.148, p = 0.257) probably due to relatively high metal tissue residues found at two reference sites [N18r and N22r (see Table 3)].
Spearman correlation values showed that As and Hg in sediment were moderately correlated [ρ = 0.57–0.73 (absolute values)] with nMDS site ordination based on toxicity (TOX-SED) and tissue residues (TR-SED) (Table 7 in Appendix). Correlations between metal tissue residues and nMDS site ordination based on toxicity (TOX-TR) showed moderate values for As, Hg, Pb, and Zn [ρ = 0.58–0.74 (absolute values)]. Metals identified by this approach (As, Hg, Pb, and Zn) were tested using RELATE procedure resulting in significant pairwise correlations of metal sediment concentration, chronic toxicity, and tissue residue resemblance matrices (p = 0.001) (Table 7 in Appendix). Toxicity data matrix was best explained by As and Hg sediment concentration (BEST ρ = 0.614), whereas the subset of As, Pb, and Zn tissue residues accounted for toxicity (BEST ρ = 0.739). Tissue residues data matrix was best explained by As, Cu, Hg, and Zn sediment metal concentrations (BEST ρ = 0.588).
Toxicity end points values and As, Hg, Pb, and Zn tissue residues were fitted against several nonlinear dose–response regression models and LR50/20 or ER50/20 estimated for each combination of metal residue and toxicity endpoint (Fig. 3). LR20 and LR50 were estimated from a log-logistic model and were 3.41 and 15.90 µmol g−1 dw for As and 14.79 and 42.10 µmol g−1 dw for Zn. Reproduction ER20 and ER50 values were estimated from Weibull models of TCC for As 2.48 and 10.79 µmol g−1 dw, Zn 9.56 and 32.31 µmol g−1 dw, and Pb 0.031 and 0.032 µmol g−1 dw, respectively, as well as TYG for Hg 0.034 and 0.067 µmol g−1 dw. Other metal tissue residue versus toxicity endpoint relationships were not significantly fitted by either model.
LR and ER values estimated from the best-fitted models, on a tissue-residue basis, after 28-day chronic bioassays for As, Zn, Pb, and Hg (µmol g−1 dw). Dashed line represents LR50 or ER50, and dotted line represents LR20 or ER20. For model descriptions: LL.2 = two-parameter log-logistic models; W1.3, W1.4 = Weibull type 1 model with three and four parameters; W2.3 = Weibull type 2 model with three parameters [dcr package (Ritz and Streibeig 2005)]. Goodness-of-fit was assessed by R 2 and Neill’s lack-of-fit test for no replicates (p value) included in the drc package (Ritz and Streibeig 2005). Outliers are represented by a grey square
Discussion
Forty years after mining activities have ceased, sediment metal concentrations of the Nalón River basin remain high to very high in the Cu and Hg mining districts, respectively. These results are in agreement with those of several studies on soil and surface water contamination reported by Loredo et al. (2003, 2006, 2010). But it is noteworthy that we usually found lower metal levels in the river sediments than those measured several years ago in the same areas (N9, N10, N11, N13, and N16), with the exception of N16, where As was approximately 250 times greater than values reported by Loredo et al. (2005). Studies performed in Hg and Cu mine districts in Asturias suggest that variations in sediment metal concentrations may be severely influenced by climate of the region, e.g., precipitation as a key factor for As leaching (Loredo et al. 2007, 2010).
The EQS directive (EC 2008) was an important improvement of long-term water-quality monitoring at the European level, pointing toward the use of sediments and biota as matrices for assessment of priority substances under the WFD (EC 2000), with emphasis on Cd and Hg. Some European countries have developed independent SQGs such as in Flemish basins (De Cooman et al. 1999) or in the Netherlands (Crommentuijn et al. 1997), but the absence of SQGs in Spain limits the development of a sound ERA and water-quality protection plans. Unfortunately, the only mandatory requirement by the European directives (EC 2000, 2008) for sediment and biota quality is that contamination levels should not increase. This is clearly insufficient for the objective of attaining good ecological status in rivers subject to historical high contamination.
Maximum As, Cu, Hg, and Zn tissue residues in our laboratory study were greater than those previously reported for sediment-dwelling annelids in the field (Table 4), although most field data have not been obtained from mining sites. When comparing laboratory tissue residues with values from field-collected aquatic annelids in the literature (Table 4), As and Hg bioaccumulated at T sites showed greater values, whereas Cd had always lower values. For essential metals, such as Cu and Ni, mean concentrations were relatively constant in the present study, independent of sediment toxicity classification, and were within the range of concentrations reported in the literature. In the case of Zn, mean concentration was not only greater at T sites but also greater than most values reported for field aquatic annelids. Cr showed less variation from field to laboratory studies, and in all cases Cr tissue residues in aquatic annelids were <1 μmol g−1 dw.
Adverse effects of As on some aquatic organisms are expected to occur at tissue concentrations between 0.17 and 0.67 μmol g−1 dw (Eisler 2000). Threshold values based on field ecological effects were greater: For instance, Rainbow et al. (2012) reported 1.13 µmol As g−1 dw in Hydropsyche siltalai related to mayfly population impairment, a value close to the ER20 for T. tubifex reproduction (2.48 μmol g−1 dw) in the present study. For Hg, the proposed criteria for the protection of freshwater species is approximately 0.150 µmol g−1dw (Eisler 2000). However, tissue residues as low as 0.067 μmol Hg g−1 dw were related to 50 % reduction in total young production (TYG) in the present study. Nevertheless, at T sites from Hg mines, high As and Hg tissue residues were measured suggesting that the combination of both As and Hg, as well as that of other metals, are likely responsible for the observed toxicity impairments.
Pb is a known accumulative metabolic poison, and existing data suggest that it may have adverse effects on organisms (Eisler 2000). However, no protection criteria based on tissue residues for freshwater invertebrates are known by the authors. Rainbow et al. (2012) reported benthic community alterations in metal-rich streams when the Pb tissue concentration in H. siltalai exceeded 1.45 µmol g−1 dw, but laboratory effective tissue residues (ER20/50) in the present study were lower than that value (0.03 µmol g−1 Pb dw). Regarding Zn, ER50 for reproduction (TCC) and LR50 in the present study were 33.31 and 42.10 µmol Zn g−1 dw, respectively. Similar threshold values were reported for Zn tissue concentration in Simulidae (14.8–30.3 µmol g−1 dw) and Leuctra sp (27.5–58.6 µmol g−1 dw) (De Jonge et al. 2013) or Hydropsyche spp. (18.6–49.1 µmol g−1 dw) (Solá et al. 2004) related to field ecological effects on macroinvertebrate fauna. In the present study, laboratory Pb and Zn tissue residues appear to be related to sediment toxicity. However, Zn is an essential metal for all living organisms, which complicates the toxicity assessment of this element with respect to bioaccumulation (Eisler 2000), and estimated Zn-ER values should be taken with caution.
Data reported in the literature suggest that Cr, Cd, Ni, and Cu tissue residues in the present study are not likely responsible of causing the observed toxicity effects. Méndez-Fernández et al. (2013) calculated a Cr-CBR50 for reproduction in T. tubifex of 0.65 µmol Cr g−1 dw, a value 2.3 times greater than the maximum tissue residues measured in the present study. Regarding Cd, metal tissue residues in T. tubifex exposed to Nalón River sediments were 3–4 orders of magnitude lower than the reproduction CBR50 values reported for the same species in Cd-spiked sediment bioassays (13.5–29.54 µmol Cd g−1 dw [Méndez-Fernández et al. 2013] and 30.38–32.18 µmol Cd g−1 dw [Gillis et al. 2002] ). With respect to Ni, Borgmann et al. (2001) found that Ni-ERs for growth and survival in Hyalella azteca varied between 0.12 and 0.19 μmol Ni g−1 dw (4- to 10-week sediment exposure), whereas worms exposed to Nalón River T sites showed a lower tissue concentration (0.07 ± 0.03 μmol Ni g−1 dw). Cu critical tissue concentrations reported from other studies were usually greater than maximum tissue residues measured in present study: For instance, reproduction CBR50 values ranged from 3.88 to 4.47 µmol g−1 dw for T. tubifex in laboratory Cu-spiked sediment bioassays (Méndez-Fernández et al. 2013), and CBR50 values estimated in relation to field benthic community alterations were 5.5 µmol Cu g−1 dw in Rhithrogena sp. (De Jonge et al. 2013) and 2.68 µmol Cu g−1 dw in H siltalai (Rainbow et al. 2012). These data can explain in part the classification of sites affected by Cu mining works (N3–N7) as NT and may support the statement that historical Cu mining activity in Asturias represents a moderate and local environmental problem (<1 km from mine facilities [Loredo et al. 2007] ).
Some differences between field and laboratory data can possibly be discussed in terms of metal bioavailability that may change through the processing of sediment samples (i.e., sieving). Nevertheless, unsieved sediment has been showed to cause “false positives” in sediment bioassays (Reynoldson et al. 1994), and sediment sieving was preferred over heating, freezing, or drying to remove competing or predating resident invertebrates (Day et al. 1995). One of the most important factors controlling metal availability in anoxic sediments has been the amount of acid-volatile sulfides (AVS). However, in the studied area AVS probably were not of concern because the water column was well mixed and oxygenated. Moreover, De Jonge et al. (2009, 2010, 2011) found that an excess of AVS was not an important factor in determining metal bioaccumulation in field-collected benthic invertebrates (Chironomus gr. thummi and Tubifex sp.). However, De Jonge et al. (2012) showed that increased oxygen concentrations in overlaying surface can directly enhance metal accumulation and toxicity in some invertebrates (namely, Asellus aquaticus and Daphnia magna), which could also explain some differences between laboratory and field data. Variability may also be expected from different populations or genetic strains of the same species (Reynoldson et al. 1996; Sturmbauer et al. 1999).
Finally, although toxicity and bioaccumulation in the field cannot be readily implied from laboratory studies, results from the present study show that polluted sediments in Hg mining areas entail a high risk that programs that ignore sediment ecotoxicity and bioaccumulation, such as the European Water Framework Directive, fail to meet their own objectives of attaining Good ecological status (Byrne et al. 2012) due to sediment pollution.
Conclusions
Sediments downstream of Hg mines showed impairment of survival and reproduction in T. tubifex bioassays related to sediment metal pollution and As and Hg bioaccumulation. This fact provides information on metal bioavailability and evidence of metal transfer from the sediment to the food web. Results suggest the existence of an important environmental problem in the study area, where there is a long history of mining activities, and demand effective remediation plans to decrease runoff and other sources of metal pollution that contaminate the river sediments below abandoned Hg mine facilities. Comprehensive and long-term studies on sediment toxicity, bioaccumulation, and field community alterations are necessary for a sound environmental risk assessment of water courses in the mining districts of Asturias.
References
American Society for Testing and Materials (2005) Standard test method for measuring the toxicity of sediments-associated contaminants with freshwater invertebrates. ASTM E1706-05. ATSM, Philadelphia
AQEM Consortium (2002) Manual for the application of the AQEM system. A comprehensive method to assess European streams using benthic macroinvertebrates. Developed for the purpose of the Water Framework Directive. Version 1.0, February 2002
Borgmann U, Norwood WP, Reynoldson TB, Rosa F (2001) Identifying cause in sediment assessments: bioavailability and the sediment quality triad. Can J Fish Aquat Sci 58:950–960
Bouché ML, Habets F, Biagianti-Risbourg S, Vernet G (2000) Toxic effects and bioaccumulation of cadmium in the aquatic oligochaete Tubifex tubifex. Ecotoxicol Environ Saf 46(3):246–251
Bryan GW, Langston WJ, Hummer-Stone LG, Burt GR (1985) A guide to the assessment of heavy metal contamination in estuaries using biological indicators. J Mar Biol Ass UK Occasional Publication No. 4
Burton GA Jr, Batley GE, Chapman PM, Forbes VE, Smith EP, Reynoldson TB et al (2002) A weight-of-evidence framework for assessing sediment (or other) contamination: improving certainty in the decision-making process. Hum Ecol Risk Assess 8:1675–1696
Byrne P, Wood PJ, Reid I (2012) The impairment of river systems by metal mine contamination: a review including remediation options. Crit Rev Environ Sci Technol 42(19):2017–2077
Cammuso M, Polesello S, Valsecchi S, Vignati DAL (2012) Importance of dietary uptake of trace elements in the benthic deposit-feeding Lumbriculus variegatus. Trends Anal Chem 36:103–112
Carère M, Dulio V, Hanke G, Polesello S (2012) Guidance for sediment and biota monitoring under the common implementation strategy for the Water Framework Directive. Trend Anal Chem 36:15–24
Casado-Martínez MC, Smith BD, Luoma SN, Rainbow PS (2010) Metal toxicity in sediment-dwelling polychaete: threshold body concentrations or overwhelming accumulation rates? Environ Pollut 158:3071–3076
Casado-Martínez MC, Duncan E, Smith BD, Maher WA, Rainbow PS (2012) Arsenic toxicity in a sediment-dwelling polychaete: detoxification and arsenic metabolism. Ecotoxicology 21:576–590
Chapman PM (2001) Utility and relevance of aquatic oligochaetes in ecological risk assessment. Hydrobiologia 463:149–169
Chapman PM, McDonald BG (2005) Using the sediment quality triad (SQT) in ecological risk assessment. In: Blaise C, Férard JF (eds) Small-scale freshwater toxicity investigations, vol 2, 1st edn. Kluwer Academic, Norwell, pp 305–329
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley R (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Clements WH (1994) Integrated laboratory and field approach for assessing impacts of heavy metals at the Arkansas River, Colorado. Environ Toxicol Chem 13:397–404
Crommentuijn T, Polder M, van de Plassche E (1997) Maximum permissible concentrations and negligible concentrations for metals, taking background concentrations into account [RIVM Report 601501 001]. National Institute of Public Health and the Environment, Bilthoven, Netherlands. Available at: http://www.rivm.nl/bibliotheek/rapporten/601501001.html. Accessed 3 Nov 2014
Dawson TD, Lott KG, Leonard EN, Mount D (2003) Time course of metal loss in Lumbriculus variegatus following sediment exposure. Environ Toxicol Chem 22:886–889
Day KE, Kirby RS, Reynoldson TB (1995) The effect of manipulation of freshwater sediments on responses of benthic invertebrates in whole-sediment toxicity tests. Environ Toxicol Chem 14:1333–1343
De Blas Cortina MA (1996) La primera minería metálica del N. Penínsular: Las indicaciones del C-14 y la cronología prehistórica de las explotaciones cupriferas del Áramo y El Milagro. Comphaun Extra 6(I):217–226
De Blas Cortina MA, Suárez Fernández M (2009) Investigaciones arqueológicas de 2005 y 2006 en las minas de cobre prehistóricas de la sierra del Aramo, Texéu (Riosa). Excavaciones arqueológicas en Asturias 2003–2006. Consejería de Cultura y Turismo del Gobierno del Principado de Asturias
De Cooman W, Florus M, Vangheluwe ML, Janssen CR, Heylen S, DePauw N et al. (1999) Sediment characterisation of rivers in Flanders. The triad approach. In: Proceedings of CATS 4: Characterisation and treatment of sediments, 15–17 September, Antwerp, Netherlands, pp 351–367
De Jonge M, Dreesen F, De Paepe J, Blust R, Bervoets L (2009) Do acid volatile sulfides (AVS) influence the accumulation of sediment bound metals to benthic invertebrates under natural field conditions? Environ Sci Technol 43:4510–4516
De Jonge M, Blust R, Bervoets L (2010) The relation between acid volatile sulfides (AVS) and metal accumulation in aquatic invertebrates: implications of feeding behaviour and ecology. Environ Pollut 158:1381–1391
De Jonge M, Eyckmans M, Blust R, Bervoets L (2011) Are accumulated sulfide-bound metals metabolically available in the benthic oligochaete Tubifex tubifex? Environ Sci Technol 45:3131–3137
De Jonge M, Teuchies J, Meire P, Blust R, Bervoets L (2012) The impact of increased oxygen conditions on metal-contaminated sediments part I: effects on redox status, sediment geochemistry and metal bioavailability. Water Res 46(10):3387–3397
De Jonge M, Tipping E, Lofts S, Bervoets L, Blust R (2013) The use of invertebrate body burdens to predict ecological effects of metal mixtures in mining-impacted waters. Aquat Toxicol 142:294–302
den Besten P, Deckere E, Babut MP, Power B, DelValls TA, Zago C, Oen AMP, Heise S (2003) Biological effects-based sediment quality in ecological risk assessment for European waters. J Soil Sediments 3:144–162
Eisler R (2000) Handbook of chemical risk assessment. Health hazard to humans, plants and animals, vol 1. Lewis, Boca Raton
EC, European Commission (2000) Directive 2000/60/CE of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. L327/1 (22.12.2000). Official Journal of the European Union
EC, European Commission (2008) Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/CEE, 84/491/CEE and 86/280/CEE and amending Directive 2000/60/EC of the European Parliament and of the Council. L 348/84 (24.12.2008). Official Journal of the European Union
EC, European Commission (2010) WFD-CIS Guidance Document No. 25. Guidance on chemical monitoring of sediment and biota under the Water Framework Directive. Office for Official Publications of the European Communities, Luxembourg
Gillis PL, Diener LC, Reynoldson TB, Dixon DG (2002) Cadmium induced production of a metallothionein-like protein in Tubifex tubifex (Oligochaeta) and Chironomus riparius (Diptera): correlation with whole body (reproduction and growth) endpoints of toxicity. Environ Toxicol Chem 21:1836–1844
Gillis PL, Reynoldson TB, Dixon DG (2006) Metallothionein-like protein and tissue metal concentrations in invertebrates (oligochaetes and chironomids) collected from reference and metal contaminated field sediments. J Great Lakes Res 32:565–577
Grapentine L, Anderson J, Boyd D, Burton GA, DeBarros C, Johnson G et al (2002) A decision making framework for sediment assessment development for the Great Lakes. Hum Ecol Risk Assess 8:1641–1655
Gust KA, Fleeger JW (2005) Exposure-related effects on Cd bioaccumulation explain toxicity of Cd-phenanthrene mixtures in Hyalella azteca. Environ Toxicol Chem 24:2918–2926
Hernández M, Rovira JV, Antonio MT (1988) Relationship between Cu and Pb levels in sediments and dwelling tubificidae in Jarama River (Madrid, Spain). In: Austrc M, Lester JN (eds) Heavy metals in the hydrological cycle. Selper, London, pp 531–538
Hollert H, Heise S, Pudenz S, Brüggemann R, Ahlf W, Braunbeck T (2002) Application of a sediment quality triad and different statistical approaches (Hasse diagrams and fuzzy logic) for the comparative evaluation of small streams. Ecotoxicology 11:311–321
Kaiser M, Irmer U, Weiler K (1989) Monitoring water quality: seasonal variation of heavy metals in sediments, suspended particulate water and tubificids of the Elba River. Environ Technol Lett 10:845–855
Klerks PL, Bartholomew PR (1991) Cadmium accumulation and detoxification in a Cd-resistant population of the oligochaete Limnodrilus hoffmeistieri. Aquat Toxicol 19:97–112
Krantzberg G, Reynoldson T, Jaagumagi R, Painter S, Boyd D, Bedard D et al (2000) SEDS: setting environmental decisions for sediment management. Aquat Ecosyst Health 3:387–396
Loredo J, Pereira A, Ordóñez A (2003) Untreated abandoned mercury mining works in a scenic area of Asturias (Spain). Environ Int 29:481–491
Loredo J, Álvarez R, Ordóñez A (2005) Release of toxic metals and metalloids from Los Rueldos mercury mine (Asturias, Spain). Sci Total Environ 340:247–260
Loredo J, Ordóñez A, Álvarez R (2006) Environmental impact of toxic metals and metalloids from the Muñón Cimero mercury-mining area (Asturias, Spain). J Hazard Mater 136:455–467
Loredo J, Álvarez R, Ordóñez A, Bros T (2007) Mineralogy and geochemistry of the Texeo Cu–Co mine site (NW Spain): screening tools for environmental assessment. Environ Geol 55:1299–1310
Loredo J, Petit-Domínguez MD, Ordoñez A, Galán MP, Fernández-Martínez R, Alvarez R et al (2010) Surface water monitoring in the mercury mining district of Asturias (Spain). J Hazard Mater 176:323–332
Luoma SN, Cain DJ, Rainbow PS (2010) Calibrating biomonitors to ecological disturbance: a new technique for explaining metal effects in natural waters. Integr Environ Assess Manag 6(2):199–209
Lyytikäinen M, Sormunen A, Peräniemi S, Kukkonen JVK (2001) Environmental fate and bioavailability of wood preservatives in freshwater sediments near and old sawmill site. Chemosphere 44:341–350
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Maestre Z, Martinez-Madrid M, Rodriguez P, Reynoldson T (2007) Ecotoxicity assessment of river sediments and a critical evaluation of some of the procedures used in the aquatic oligochaete Tubifex tubifex chronic bioassay. Arch Environ Contam Toxicol 53:559–570
Maestre Z, Rodriguez P, Martinez-Madrid M (2009) Application of the sediment quality TRIAD to rivers in northern Spain. In: Santos EB (ed) Ecotoxicology research development. Nova Science, New York, pp 205–224
Martinez-Madrid M, Rodriguez P, Pérez-Iglesias JI, Navarro E (1999) Sediment toxicity bioassays for assessment of contaminated sites in the Nervión River (Northern Spain). 2. Tubifex tubifex reproduction sediment bioassay. Ecotoxicology 8:111–124
Meador JP, Adams WJ, Escher BI, McCarty LS, McElroy AE, Sappington KG (2011) The tissue residue approach for toxicity assessment: findings and critical reviews from a Society of Environmental Toxicology and Chemistry Pellston Workshop. Integr Environ Assess Manag 7(1):2–6
Méndez-Fernández L (2013) Metal toxicity and bioaccumulation in Tubifex tubifex (Müller) (Annelida) exposed to river sediments from Northern Spain. Dissertation, University of Basque Country, Bilbao, Spain
Méndez-Fernández L, Martínez-Madrid M, Rodriguez P (2013) Toxicity and critical body residues of Cd, Cu and Cr in the aquatic oligochaete Tubifex tubifex (Müller) based on lethal and sublethal effects. Ecotoxicology 22(10):1445–1460
Mudroch A, Azcue JM (1995) Manual of aquatic sediment sampling. Lewis, Boca Raton
Organization for Economic Co-operation and Development (2008) Guidelines for testing of chemicals no. 315: bioaccumulation in Sediment-dwelling benthic oligochaetes. OECD, Paris
Protano C, Zinnà L, Giampaoli S, Romano-Spica V, Chiavarini S, Vitali M (2014) Heavy metal pollution and potential ecological risks in rivers: a case study from southern Italy. Bull Environ Contam Toxicol 92:75–80
Rainbow PS, Hildrew AG, Smith BD, Geatches T, Luoma SN (2012) Caddisflies as biomonitors identifying thresholds of toxic metal bioavailability that affect the stream benthos. Environ Pollut 166:196–207
Reynoldson TB, Thompson SP, Bampsey JL (1991) A sediment bioassay using the tubificid oligochaete worm Tubifex tubifex. Environ Toxicol Chem 10:1061–1072
Reynoldson TB, Day KE, Clarke C, Milani D (1994) Effects of indigenous animals on chronic endpoints in freshwater sediment toxicity tests. Environ Toxicol Chem 13:973–977
Reynoldson TB, Bailey RC, Day KE, Norris RH (1995) Biological guidelines for freshwater sediment based on BEnthic Assessment of Sediment (the BEAST) using a multivariante approach for predicting biological state. Aust J Ecol 20:198–219
Reynoldson TB, Rodriguez P, Martinez-Madrid M (1996) A comparison of reproduction, growth and acute toxicity in two populations of Tubifex tubifex (Müller, 1774) from the North American Great Lakes and Northern Spain. Hydrobiologia 334(1–3):199–206
Reynoldson TB, Norris RH, Resh VH, Day KE, Rosenberg DM (1997) The reference condition: a comparison of multimetric and multivariate approaches to assess water-quality impairment using benthic macroinvertebrates. J N Am Benthol Soc 16:833–852
Riba I, Forja JM, Gómez-Parra A, DelValls TA (2004) Sediment quality in littoral regions of the Gulf of Cádiz: a triad approach to address the influence of mining activities. Environ Pollut 132:341–353
Ritz C, Streibeig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22
Rodriguez P, Reynoldson TB (2011) The pollution biology of aquatic oligochaetes. Springer, Dordrecht
Rodriguez P, Maestre Z, Martinez-Madrid M, Reynoldson TB (2011) Evaluating the type II error rate in a sediment toxicity classification using the reference condition approach. Aquat Toxicol 101:207–213
Rodríguez-Terente LM, Luque-Caval C, Gutiérrez-Claverol M (2006) Los resgistros mineros para sustancias metálicas en Asturias. Trabajos de Geología, Universidad de Oviedo 26:19–55
Rosen G, Lotufo GR (2005) Toxicity and fate of two munitions constituents in spiked sediment exposures with the marine amphipod Eohaustorius estuaries. Environ Toxicol Chem 24:2887–2897
Say PJ, Giani N (1981) The Riou Mort, a tributary to the river Lot polluted by heavy metals. II. Accumulation of zinc by oligochaetes and chironomids. Acta Oecol 2:339–355
Singh RK, Chavan SL, Sapkale PH (2007) Heavy metal concentrations in water, sediments and body tissues of red worms (Tubifex spp.) collected from natural habitats in Mumbai, India. Environ Monit Assess 129:471–481
Solá C, Burgos M, Plazuelo A, Toja J, Plans M, Prat N (2004) Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage an accidental spell (Guadiamar River, SW Spain). Sci Total Environ 333:109–126
Steen-Redeker E, Bervoets L, Blust R (2004) Dynamic model for the accumulation of cadmium and zinc from water and sediment by the aquatic oligochaete Tubifex tubifex. Environ Sci Technol 38(23):6193–6200
Sturmbauer C, Opadiya GB, Niederstätter H, Riedmann A, Dallinger R (1999) Mitochondrial DNA reveals cryptic oligochaete species differing in cadmium resistance. Mol Biol Evol 16(7):967–974
Teruggi ME (1982) Diccionario sedimentológico. Volúmen I. Rocas Clásticas y Piroclásticas. Ed. Cient. Arg. (LIBRART). Buenos Aires
United States Environmental Protection Agency (1990) Macroinvertebrate field and laboratory methods for evaluating the biological integrity of surface waters. Selection of sampling stations. EPA 600/4-90/030
Winger PV, Laiser PJ, White DH, Seginal JT (2000) Effects of contaminants in dredge material from the lower Savannah River. Arch Environ Contam Toxicol 38:128–136
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, Upper Saddle River, pp 226–227
Zuur AF, Ieno EN, Smith GM (2007) Analyzing ecological data. Springer, New York
Acknowledgments
This work was possible thanks to the support from the Education and Science Ministry research project (MEC CGL2008-04502/BOS) and from the Basque Government (IT-405-10). L. Méndez-Fernández was assisted in this work by a doctoral grant from the Basque Government. We gratefully acknowledge Dr. Ordóñez for help identifying metal mines location in Asturias and Dr. Miranda for providing information on surveillance nets. Our thanks also go to B. Arce from Analytical Services of Sosprocan Unit (University of Cantabria) for technical and human support provided. We thank Keith Somers of the Ontario Department of Environment (Canada) for providing the Excel macro for constructing the ellipses and also P. Markaide for the valuable contribution on the custom made R script for dose–response model analysis. We also gratefully acknowledge the anonymous reviewers who helped to improve the manuscript with useful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Figs. 4, 5 and Tables 5, 6, and 7 in Appendix.
Rights and permissions
About this article
Cite this article
Méndez-Fernández, L., Rodríguez, P. & Martínez-Madrid, M. Sediment Toxicity and Bioaccumulation Assessment in Abandoned Copper and Mercury Mining Areas of the Nalón River Basin (Spain). Arch Environ Contam Toxicol 68, 107–123 (2015). https://doi.org/10.1007/s00244-014-0093-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0093-8