Abstract
Metabolic syndrome (MS) individuals have a higher risk of developing chronic kidney disease through unclear pathogenic mechanisms. MS has been also related with higher nephrolithiasis prevalence. To establish the influence of MS on renal function, we designed a murine model of combined metabolic syndrome and hyperoxaluria. Four groups of male Sprague–Dawley rats were established: (1) control group (n = 10) fed with standard chow; (2) stone former group (SF) (n = 10) fed with standard chow plus 0.75% ethylene glycol administered in the drinking water; (3) metabolic syndrome group (MS) (n = 10), fed with 60% fructose diet; (4) metabolic syndrome + stone former group (MS + SF) (n = 10), 60% fructose diet and 0.75% EG in the drinking water. MS group showed a significant injury to renal function when hyperoxaluria was induced. It was demonstrated by a significant decrease of creatinine clearance (p < 0.001), with higher tubular damage (34.3%, CI 95% 23.9–44.7, p < 0.001), produced by deposition of crystals, and increased tubular synthesis of osteopontin as a response to tubular damage. Induction of hyperoxaluria in rats with MS causes severe morphological alterations with a significant impairment of renal function. This impairment is not produced in rats without MS. Therefore, this model can be useful for the study of the influence of MS in stone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MS) is defined as the presence of hyperinsulinemia or insulin resistance in the same individual along with certain vascular risk factors such as dyslipidemia, hypertension or central obesity. These factors are often associated with hypercoagulability, endothelial dysfunction, and inflammation [1].
Among the 3 most accepted characterizations of MS is one of the American Heart Association (AHA/NHLBI) 2005 [2], which comes from the one made by the NCEP ATP III in 2001. It includes five criteria, three of which are required for the diagnosis of MS: a waist circumference greater than 102 cm in men and 88 in women, hypertriglyceridemia, hypercholesterolemia hypertension, and hyperglycemia.
The estimation of MS prevalence is complex because of these different definitions. It is estimated at 11.7% for men and 7.5% for women. Nevertheless, the most modern definitions are associated with higher rates [3, 4].
The relationship between cardiovascular morbidity and mortality, cerebrovascular disease, coronary disease and MS has been well demonstrated. However, in addition to cardiovascular involvement, other organs such as liver, pancreas, smooth muscle, or kidney has been also implicated [5].
Lipotoxicity, associated with MS, is caused by the abnormal storage of fatty acids in non-fatty tissues. This fact triggers a phenomenon of apoptosis in the aforementioned target organs. Diseases associated with this process such as type 2 diabetes or kidney failure can have their origin in these phenomena [6].
The kidney, as target organ of lipotoxicity, presents lesions in the glomerulus as well as in the proximal tubule, mainly. An accumulation of intracellular fatty acids, the mitochondrial metabolism of which produces metabolites such as ceramides or diacylglycerol. Thus, generation of intracellular reactive oxygen species (ROS) damages cell organelles, alters intracellular signal mechanisms, releases pro-inflammatory factors and produces lipid-induced apoptosis [7].
Some epidemiological studies have shown a higher incidence of kidney stone disease in patients with MS, with an OR ranging between 1.25 and 2.2 [8,9,10].
However, the specific pathogenic mechanisms that facilitate kidney stone formation in patients with MS are not yet known. There are several studies that analyze the composition of calculi in relation to obesity. Chou et al. described a higher prevalence of calcium oxalate (CaOx) and uric acid in 907 obese patients [11].
In the present study, we investigated whether MS promotes the formation of CaOx stones, the most common composition of renal lithiasis, using an established animal-diet induced MS model. This rat model has been extensively used for the study of MS because it displays numerous features of this disease such as insulin resistance or acquired systolic hypertension.
Materials and methods
We compared ethylene glycol (EG)-induced CaOx renal crystal deposition between 60% fructose diet rats and normal chow-fed rats, and examined plasma and urinary biochemistry, pathological studies and the expression of a major modulator of kidney stone formation, OPN.
Experimental methods
The animals were handled according to the European and Spanish regulations for protection of experimental animals (2010/63/UE and RD 53/2013). The institutional ethic committee for animal research approved the study.
Male Sprague–Dawley rats (150–200 g) were housed in standard conditions and fed a control (n = 20) or 60% fructose diet (Harlan, Madison, WI, n = 20) for 65 days. The control diet contained 46% carbohydrate, which is mainly composed of starch, whereas the fructose diet contained 60% fructose as the carbohydrate. The caloric content of these diets are 3.1 and 3.6 kcal/g, respectively.
One-half of each group: normal chow or the fructose-fed rats were administered 0.75% EG in the drinking water for an additional 3 weeks. Rats were divided into four groups of 10 rats each: control, metabolic syndrome (MS), stone forming (SF) and MS + SF.
Blood was sampled at the end of MS induction at 65th day (MS establishment), and at the time of killing (creatinine). Three weeks later, after hyperoxaluria was induced in the corresponding groups (SF or MS + SF), rats were introduced into metabolic cages, and 24-h urine collected. Finally, following anesthesia with inhaled sevoflurane vaporized in oxygen, the carotid artery was catheterized with a 24-gauge polyethylene catheter via surgical cut down for blood sampling and arterial blood pressure measurement via a calibrated pressure transducer. Urine was obtained by puncturing the bladder. The rats were then euthanized under general anesthesia with potassium chloride intravenously. Renal samples were obtained and processed for hematoxylin–eosin staining, immunofluorescence, and for RNA extraction for subsequent polymerase chain reaction in real time (RT-PCR).
Plasma and urinary biochemistry
Serum glucose, cholesterol, triglycerides, uric acid and creatinine were measured with the spectrophotometer ADVIA Chemistry Analyzer multi Siemens 2400, which quantifies the parameters after a series of enzymatic reactions.
For the observation of the punctured bladder urine, pH was measured, ultracentrifugation was performed and sediment observation optical microscope was performed, which was made by observing 10 fields at 40× magnification. Absence or presence of crystals in the sediment was reported.
Twenty-four hour urine samples were analyzed for calcium, citrate, oxalate, uric acid and creatinine.
Creatinine clearance (CrCl) was calculated from the following equation: CrCl (mL/min) = [urine creatinine (mg/dL) × urine volume (mL/24 h)]/[serum creatinine (mg/dL) × 1440 (min)].
Renal histology and immunofluorescence staining
Half the length of the kidney samples were immersion fixed in 10% phosphate-buffered formalin for light microscopy. The formalin-fixed kidney samples were sectioned at 4 mm thickness. Sections were stained with hematoxylin and eosin (HE) to count crystal deposits. Most representative fields at 100× magnification were assessed in each section, with the observer blinded to the animal groups. Crystal deposits (% in total tubules) were counted.
Immunofluorescence staining was performed to detect expression of osteopontin on paraffin sections fixed in formalin. Two slides per animal were selected, subsequently they were visualized and images were acquired with a Leica SP5 Confocal Microscope Research.
Quantitative RT-PCR
Kidney samples were preserved in RNA-later at 4° C during 1 day and frozen at −80 until RNA purification. The preparations obtained had adequate spectrophotometric quality for our purposes and the mean concentration of the samples was 376.07 ng/μl.
A relative quantification of gene expression was performed using specific probes Real Time ready for SPP1 target gene (coding for rat osteopontin) and for GAPDH gene reference.
Statistical analysis
Based on similar studies [13], the percentage of tubules affected by crystals was used as indicator variable. The number necessary to demonstrate significant differences was established in 10 animals per group.
To analyze the effect of MS on hyperoxaluria and its effect on the kidney, the four groups of animals were compared using the Chi-square test, for categorical variables (% tubular damage) and ANOVA or Kruskal–Wallis for quantitative variables, depending on whether the variables were adjusted to a normal distribution (previous KS test). Data are expressed by the mean and 95% confidence interval, establishing a level of statistical significance of 95%. SPSS statistical package 15.0® was used for the statistical analysis of the results.
Results
Metabolic syndrome establishment
Sixty-five days of treatment with 60% fructose diet was associated with a significant increase of serum triglyceride levels, reaching as much as three times in the one of the untreated rats. However, no differences were found in weight and mean arterial pressure values between the groups. Weight values were even slightly lower than those of the untreated group. Mean arterial pressure values were very similar, even when rats were posteriorly treated with EG. No differences were found in blood glucose, and cholesterol levels between the groups either, indicating that non-apparent clinical injury was still produced by lipotoxicity in targeted organs such as blood vessels or pancreas. Nevertheless, the MS group showed significantly higher levels of HDL cholesterol.
Renal function
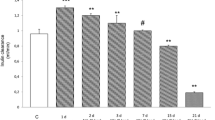
A significant impairment of renal function was observed in MS rats after 21 days of EG administration. Diuresis was much higher in this group, reaching as much as three times that of the other three groups. Serum creatinine levels endorsed the clinical evidence showing values two times higher in 0.75% EG-treated MS rats in comparison with those treated equally but fed with standard chow. This finding was still more evident in comparison with the two groups that drank normal water, reaching levels four times higher. Obviously, ClCr values were lower in the SF group, but this finding was much enhanced in MS + SF group (Fig. 1).
Microscopic evaluation of hematoxylin–eosin stained kidney sections showed significant tubular damage with CaOx crystal deposits in the corticomedullary region in 9 of the 10 rats of the MS + SF group. In the SF group, only 2 of the 10 rats were affected. None in the control or MS groups presented any damage. In addition, a higher percentage of tubules were affected in the MS + SF group in comparison with the SF group.
The crystals were located both in the cortex and the medulla. Most crystals were intraluminal aggregates. Severe damage of the tubules was also seen with atrophy of renal interstitium and collecting ducts dilation. Some of them were occupied by an inflammatory infiltrate composed by polymorphonuclear neutrophils aggregates (Fig. 2).
Urinary biochemistry
Ethylene glycol administration was associated with increased urinary excretion of oxalate in all treated animals, reaching as much as 5 times that of untreated rats after 3 weeks. Oxaluria was even higher in MS + SF rats. The urine of treated rats contained CaOx monohydrate and CaOx dihydrate crystals (Table 1). The urine of untreated rats did not contain CaOx crystals.
Urinary pH was significantly lower in rats fed with 60% fructose diet, being even lower in the MS + SF group.
In the same way, excretion of citrate decreased in both MS groups, than in normal chow-fed rats; again urinary values of citrate were lower in MS + SF group.
Urinary excretion of calcium did not show any differences between the groups. Uricosuria was significantly lower in EG-treated rats.
Osteopontin determination
Quantitative PCR showed a significant increase of OPN mRNA expression in EG-treated groups. This finding was much more evident when rats were also fed with 60% fructose diet (Table 1).
Increased osteopontin primary antibody was detected especially located in the epithelial cells of the distal tubules and collecting ducts of the rats treated with 60% fructose diet and 0.75% EG (Fig. 3).
Discussion
MS is currently one of the major public health challenges worldwide. It predisposes individuals to the development of type 2 diabetes, cardiovascular diseases, and chronic kidney disease (CKD).
The relationship between MS and urolithiasis has been well established by several epidemiological reports [8, 9–10]. At present, the specific pathogenic mechanisms of this fact are unknown.
Two murine models for explaining the higher incidence of urolithiasis have been reported. Rofe [12] reported the effect of administering refined carbohydrates in the diet on CaOx deposition in the kidney of rats given 1% EG in their drinking water. They suggested that when sucrose, other refined sugars or sugar alcohols were included in the diet of EG-treated rats, large deposits of CaOx were observed in the renal cortex and medulla. The increase in plasma cholesterol with increasing sucrose consumption and a small but significant increase in creatinine values was reported. In contrast, the changes in urinary biochemical parameters, due to increasing sucrose consumption were more marked. In this model, the sucrose group had reduced urinary outputs, urinary pH, creatinine, urate and phosphate. Sucrose-treated rats kidneys were bigger and heavier, and the surface of the kidneys was mottled and dimpled. Unfortunately, only calcium content was reported, without histological or molecular descriptions.
Okamoto [13] reported in a genetic model of MS that EG form more CaOx crystal deposits in MS strain rats than controls, suggesting that MS promotes the formation of CaOx kidney stones. This model also showed a slight deterioration of renal function when hyperoxaluria was associated with MS.
Both genetic and environmental factors contribute to the development of MS although the exact cause of this disorder remains uncertain. However, diet is a major factor that triggers MS in humans. That is the reason we chose a MS diet induction model.
Rats fed with a high-fructose diet represent an animal model of MS associated with insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension. Fructose, unlike glucose, does not stimulate insulin and leptin secretion, which are involved in the long-term regulation of energy homeostasis. Indeed, fructose is more lipogenic than glucose [14].
In our model, 65 days of treatment with 60% fructose diet was associated with a significant increase of serum triglyceride levels; nevertheless, no changes in weight, mean arterial pressure or glucose values were observed. Pokrywczynska and colleagues [15] also observed a slight decrease in weight after 8 weeks of 60% fructose diet, in a murine model for the evaluation of the impact of fructose diet on renal function. Sanchez-Lozada [16] did not observe significant weight gain or a raise in glucose levels, although observed a significant elevation of the MAP. Interestingly, no changes were observed between 60% fructose diet or 10% fructose solution in drinking water.
Uniformly, in our model and the two other similar models, serum triglyceride levels reached as much as three times those of the control group. Fructose metabolism occurs through an insulin-independent mechanism. After ingestion, fructose is rapidly absorbed from the intestine via the fructose-specific glucose transporter. In the liver, fructose is phosphorylated by a highly expressed fructose-specific enzyme, fructokinase, to form fructose 1-phosphate (fructose 1-P), which is further converted to three-carbon phosphate intermediates glyceraldehyde, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate (glyceraldehyde 3-P). Some of these intermediates enter the gluconeogenic pathway and are metabolized to form lactate or triglycerides [14].
Ethylene glycol administration has been extensively used as a model for development of CaOx nephrolithiasis promoted in rats by induction of hyperoxaluria. Chronic hyperoxaluria induced by the administration of 0.75% EG to male rats produces crystalluria, which is followed by CaOx nephrolithiasis. It takes approximately 12 days of chronic administration of EG alone to show persistent crystalluria and about 3 weeks to start depositing crystals in the kidneys [17].
With this information, we decided to perform an established MS model with 9 weeks of 60% fructose diet, and posteriorly to induce CaOx nephrolithiasis, just at the moment crystals were beginning to deposit in the kidneys, so that we could demonstrate the influence of MS on nephrolithiasis formation.
It has been shown that fructose consumption contributes to the development and progression of chronic kidney disease. High-fructose diet increases kidney weight and causes mild tubulointerstitial injury in the normal kidney. Moreover, fructose diet induces renal microvascular disease and glomerular hypertension and cortical vasoconstriction and significant decrease in single-nephron glomerular filtration rate [15].
In our study, the administration of 0.75% EG in the drinking water to the high-fructose diet rats group triggered a significant impairment of renal function compared with the other groups. Interestingly, a significant difference has been obtained over the 0.75% EG with normal diet group, thus MS looks like responsible for this circumstance. Renal failure has been characterized by significant hyperfiltration resulting in higher urine output, as much as three times that in the other three groups. Sever damage has been also seen microscopically, with destruction and dilation of tubules and with intraluminal crystal aggregates. An inflammatory response is also observed with intratubular occupation of polymorphonuclear cells.
The explanation of an increased renal disease associated with MS can be first in the urinary parameters. The two groups fed with 60% fructose diet showed low urinary pH. These results are consistent with previous reports that obesity and type 2 diabetes are associated with low urine pH [18]. Also uricosuria was higher when hyperoxaluria was induced. Both circumstances could promote uric crystals aggregation and CaOx formation through heterogeneous nucleation [19].
Excretion of urinary citrate also decreased significantly in both our MS groups. This finding is also described in other models of metabolic syndrome as OLEFT rats. The exact mechanism in which insulin resistance leads to low urinary citrate is not described. Insulin has been shown to promote ammonia genesis from the substrate glutamine and to stimulate Na+/H+ exchanger in the proximal tubule [5]. Urinary citrate is a potent inhibitor of nucleation, aggregation and crystallization processes of CaOx stone formation. The urinary excretion of citrate was found to be significantly lower in patients with CaOx stone disease as compared with normal subjects, and about 30% of the calcium stone formers can be considered as hypocitraturic. The heterogeneous growth of CaOx on calcium phosphate is also counteracted by citrate [20].
Osteopontin is a potent inhibitor of crystallization of calcium phosphate and CaOx, the two most common crystals found in urine and kidney stones. Osteopontin expression is enhanced in the kidneys of rats with experimentally induced CaOx nephrolithiasis. It is limited to the thin limbs of the loop of Henle and surface epithelium of the papillae in the area of the caliceal fornix [21].
Hyperoxaluria showed, in our case, an enhanced expression of mRNA-OPN expression located in the tubular cells. This finding was much more evident when associated with MS. Expression of osteopontin is most probably the result of stimulating epithelial cells of the distal tubules and collecting ducts by the deposit of crystals and reveals differences in renal responses after exposure to MS without induction of hyperoxaluria.
The renal impairment produced by MS when hyperoxaluria is induced in rats, seen in our model, is secondary to a higher deposition of intratubular CaOx crystals. Further studies are necessary to find the biochemical pathways for explaining an enhanced inflammatory tubular cells response to MS, and must be based on this model, and focused in oxidative stress.
Conclusions
Induction of hyperoxaluria in rats with MS causes severe morphological alterations with a significant impairment of renal function. This impairment is not produced in rats without MS. Therefore, this model may be useful to study the influence of MS in stone formation.
MS triggers a special susceptibility situation for formation of kidney stones. This situation is probably produced by an inflammatory status secondary to OS.
References
Guize L, Pannier B, Thomas F, Bean K, Jego B, Benetos A (2008) Recent advances in metabolic syndrome and cardiovascular disease. Arch Cardiovasc Dis 101:577–583
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol. 4:198–203
Pannier B, Thomas F, Eschwege E, Bean K, Benetos A, Leocmach Y et al (2006) Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the “SYMFONIE” study. Diabetes Metab. 32:467–474
Guize L, Thomas F, Pannier B, Bean K, Danchin N, Benetos A (2006) Metabolic syndrome: prevalence, risk factors and mortality in a French population of 62 000 subjects. Bull Acad Natl Med. 190:685–697 (discussion 697)
Bobulescu IA (2010) Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens 19:393–402
Unger RH (2002) Lipotoxic diseases. Annu Rev Med 53:319–336
Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M (2005) Oxalate toxicity in renal cells. Urol Res 33:329–339
West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H (2008) Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am J Kidney Dis 51:741–747
Rendina D, Mossetti G, De Filippo G, Benvenuto D, Vivona CL, Imbroinise A et al (2009) Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant 24:900–906
Jeong IG, Kang T, Bang JK, Park J, Kim W, Hwang SS et al (2011) Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis 58:383–388
Chou YH, Su CM, Li CC, Liu CC, Liu ME, Wu WJ et al (2011) Difference in urinary stone components between obese and non-obese patients. Urol Res 39:283–287
Rofe AM, Bais R, Conyers RA (1986) The effect of dietary refined sugars and sugar alcohols on renal calcium oxalate deposition in ethylene glycol-treated rats. Food Chem Toxicol 24:397–403
Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T (2010) Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol 17:996–1003
Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332:145–159
Pokrywczynska M, Flisinski M, Jundzill A, Krzyzanowska S, Brymora A, Deptula A et al (2014) Impact of fructose diet and renal failure on the function of pancreatic islets. Pancreas 43:801–808
Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T et al (2007) Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 292:F423–F429
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15:236–243
Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY (2004) Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65:1422–1425
Coe FL, Strauss AL, Tembe V, Le Dun S (1980) Uric acid saturation in calcium nephrolithiasis. Kidney Int 17:662–668
Tiselius HG, Berg C, Fornander AM, Nilsson MA (1993) Effects of citrate on the different phases of calcium oxalate crystallization. Scanning Microsc. 7:381–389 (discussion 389)
Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA (2002) Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 168:1173–1181
Acknowledgements
The Urological Society from Madrid, SUM Research Grant 2013 and Astellas Pharma España are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Urological Society from Madrid. Research Grant 2013 and Astellas Pharma España are acknowledged.
Conflict of interest
Sáenz-Medina J., Jorge E., Corbacho C., Santos M., Sánchez A., Soblechero P., Virumbrales E., Ramil E., Coronado M.J., Castillón I., Carballido J. declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sáenz-Medina, J., Jorge, E., Corbacho, C. et al. Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 46, 179–186 (2018). https://doi.org/10.1007/s00240-017-0979-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-0979-9