Abstract
A critical examination of data in the literature and in as yet unpublished laboratory records on the possible role of so-called inhibitors of crystallisation in preventing the formation of calcium-containing kidney stones leads to the following conclusions. So-called inhibitors of spontaneous “self-nucleation” are unlikely to play any role in the initiation of the crystallisation of CaOx or CaP in urine because excessive urinary supersaturation of urine with respect to these salts dominates the onset of “self-nucleation” within the normal time frame of the transit of tubular fluid through the nephron (3–4 min). Inhibitors of the crystal growth of CaOx crystals may or may not play a significant role in the prevention of CaOx stone-formation since once again excessive supersaturation of urine can overwhelm any potential effect of the inhibitors on the growth process. However, they may play a role as inhibitors of crystal growth at lower levels of metastable supersaturation when the balance between supersaturation and inhibitors is more equal. Inhibitors of CaOx crystal aggregation may play a significant role in the prevention of stones, since they do not appear to be strongly affected by excessive supersaturation, either in vitro or in vivo. Inhibitors of CaOx crystal binding to renal tubular epithelium may exist but further studies are necessary to elucidate their importance in reducing the risk of initiating stones in the renal tubules. Inhibitors of CaOx crystal binding to Randall’s Plaques and Randall’s Plugs may exist but further studies are necessary to elucidate their importance in reducing the risk of initiating stones on renal papillae. There may be an alternative explanation other than a deficiency in the excretion of inhibitors for the observations that there is a difference between CaOx crystal size and degree of aggregation in the fresh, warm urines of normal subjects compared those in urine from patients with recurrent CaOx stones. This difference may depend more on the site of “self-nucleation” of CaOx crystals in the renal tubule rather than on a deficiency in the excretion of so-called inhibitors of crystallisation by patients with CaOx stones. The claim that administration of potassium citrate, potassium magnesium citrate or magnesium hydroxide reduces the rate of stone recurrence may be due to the effect of these forms of medication on the supersaturation of urine with respect to CaOx and CaP rather than to any increase in “inhibitory activity” attributed to these forms of treatment. In summary, there is a competition between supersaturation and so-called inhibitors of crystallisation which ultimately determines the pattern of crystalluria in stone-formers and normals. If the supersaturation of urine with respect to CaOx reaches or exceeds the 3–4 min formation product of that salt, then it dominates the crystallisation process both in terms of “self-nucleation” and crystal growth but appears to have little or no effect on the degree of aggregation of the crystals produced. At supersaturation levels of urine with respect to CaOx well below the 3–4 min formation product of that salt, the influence of inhibitors increases and some may affect not only the degree of aggregation but also the crystal growth of any pre-formed crystals of CaOx at these lower levels of metastability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 50 years or so, much has been written on the topic of inhibitors of the crystallisation of calcium salts in urine claiming that they may protect the individual against the formation of calcium-containing kidney stones. The wide range of candidates for this role includes magnesium [1], citrate [2], pyrophosphate [3, 4] adenosine diphosphate [5], adenosine triphosphate [5], two phosphopeptides [6], various glycosaminoglycans [7, 8], non-polymerised Tamm–Horsfall protein (also known as uromodulin) [9, 10], nephrocalcin (now known to be the same as bikunin) [11–14], albumin [15], osteopontin (also known as uropontin) [16, 17], calgranulin [18], α-1-microglobulin [19], β-2-microglobulin [20], matrix Gla protein [21], urinary prothrombin fragment 1 [22, 23], human urinary trefoil factor 1 (THF1) [24] and inter-α-trypsin inhibitor (bikunin light chain) [25, 26]. Many of the macromolecules in this list appear to act by binding to calcium sites on growing crystals through polyanionic sites such as the gamma-carboxyglutamic (GLA) domain in their protein structure. Of the above list, only citrate and magnesium have been found to be excreted in lower amounts by some calcium stone-formers than by normal subjects [27]. There have also been occasional claims that stone-formers may excrete lower amounts than normal subjects of some of the macromolecular members of the above list [28]. However, the excretion of none of these ions and macromolecules has been found to discriminate exclusively between stone-formers and normal subjects.
Other urinary constituents have been identified that are claimed to be promoters of the crystallisation process. These include matrix substance A [29, 30], various uncharacterized urinary proteins and glycoproteins [31–33], polymerised Tamm–Horsfall protein (also known as uromucoid) [34, 35], albumin [15, 36, 37], lysozyme [38] and lactoferrin [38]. For a comprehensive review of the various constituents in urine which have been claimed to inhibit or promote the formation of calcium-containing stones in the urinary tract, the reader is referred to an excellent article by Aggarwal et al. [39] and to other articles in this Special Issue [40, 41] which deal in more detail with the identification of individual urinary inhibitors and promoters of crystallisation and their claimed effect on the crystallisation of calcium salts in urine. Instead, this current article will attempt to answer the more general, but nevertheless important, question—do any of these so-called “inhibitors of crystallisation” actually play a role in the prevention of stone-formation based on evidence contained in the literature and in previously unpublished data from a number of laboratory records?
Originally, many of the above urinary constituents were shown to modify one or more of the steps involved in the crystallisation of both of these salts solely on the basis of in vitro studies. These test systems generally employed relatively simple supersaturated solutions which bore little or no resemblance to the overall composition of urine [1–9]. Eventually, test systems were devised which utilised more complex multi-component solutions, such as those used in constant composition [42, 43], mixed-suspension-mixed-product-removal (MSMPR) (the latter sometimes referred to as continuous crystallisers) [35, 44–47] and reverse osmosis systems [48]. It was then assumed that these inhibitory factors played a similar role in “whole” urine. I will be the first to admit that I originally fell into this trap myself [7] but have since realised that there are many more factors to be considered before it can be accepted that these apparent in vitro “inhibitors” actually play a role in stone prevention. Subsequent studies have been carried out on the effects of some of these substances in more “urine-like” solutions [44–48] and even in urine itself [48, 49] but there are many problems associated with both the theory behind the test systems employed and the practical aspects of the tests themselves.
It has been claimed that the various so-called “inhibitors of crystallisation” identified using the above test systems can act on a number of processes in the overall course of crystallisation. These include (1) inhibition of what may be termed “crystal self-nucleation”,Footnote 1 (2) inhibition of crystal growth, (3) inhibition of crystal aggregation, (4) inhibition of the binding of calcium-containing crystals to tubular cell epithelium or (5) inhibition of crystal growth on Randall’s Plaques or Randall’s Plugs.
But what is the evidence then that so-called “inhibitors of crystallisation” play any role in inhibiting one or more of the above steps in the crystallisation process or in preventing the formation of stones in the urinary tract? Since there are few, if any, data in the literature on urinary inhibitors of the crystallisation of cystine, uric acid or magnesium ammonium phosphate, the remainder of this article will concern itself with the various ions and macromolecules which have been proposed to play a role in preventing the formation of stones consisting of either calcium oxalate (CaOx) or calcium phosphate (CaP) or both.
Inhibitors of crystallisation of calcium salts
As mentioned above, there are three main steps in the crystallisation of calcium salts in urine plus two other mechanisms by which crystallisation might take place where the so-called inhibitors might play a role in delaying or blocking the process. Let us look at the concepts involved in these various stages and the evidence that these so-called inhibitors play any role in modifying them:
Inhibitors of spontaneous “self-nucleation”
Since the late 1960s, many studies have been carried out on so-called “inhibitors of spontaneous self-nucleation”. Most have been concerned with measuring the effect of these substances on some empirically determined upper limit of supersaturation at which crystal nucleation appears to take place, sometimes referred to as the formation product (FP) or metastable limit (ML) or upper limit of metastability (ULM) of the calcium salt concerned. The problem is that many of the studies were carried out under conditions incompatible with those involved during the elaboration of urine in the renal tubules. Most importantly, the majority employed incubation times which were quite unrealistic when compared with the actual transit time of urine through the renal tubules (about 3–4 min). The importance of time of incubation in determining whether or not a given so-called “inhibitor of self-nucleation” is relevant to the initiation of stones within the transit time of urine through the renal tubules can be seen from the studies in Figs. 1, 2, 3, 4 and 5, which show the effect of time of incubation and inhibitor concentration on the nucleation of crystallisation of CaOx and CaP in simple metastable solutions (Figs. 1, 2, 3, 4) and in urine (Fig. 5).
The effect of time of incubation on the formation product of calcium oxalate (CaOx) from various studies compared with the 4-min formation product of CaOx determined by Robertson [50]
First, and most importantly, the FP/ML/ULM values for both CaOx and CaP in simple metastable solutions are time-dependent (Figs. 1, 2) i.e. the longer one leaves a given metastable solution, the more likely it is to crystallise out. This is simple school chemistry! The Figures include data from my own PhD studies [50, 51] but also data from other workers in the field who have determined FP/ML/ULM at various incubation times ranging from a few hours to 3 days or even longer [3, 4, 52–54]. These contrast with the very short-time FP/ML/ULM values for these salts obtained by myself when I attempted to simulate the supersaturation conditions that would be necessary to cause the “self-nucleation” of CaOx and CaP within the transit time of urine through the nephron (3–4 min) [50, 51]. For these short-term studies, CaOx was nucleated by titration of metastable solutions of CaOx with sodium oxalate and CaP nucleated by titration of metastable solutions of CaP with sodium hydroxide. The data from these studies show that the supersaturation levels required to initiate the crystallisation of these salts within 3–4 min are much higher than those required at the 6-, 20-h and 3-day incubation times variously employed by other workers in the field [3, 4, 52–54]. Since urine rarely, if ever, remains in the renal tubules or even in the renal calyces for such long times of incubation, the FP/ML/ULM values obtained at these relatively long time periods would seem to be totally irrelevant to the stone initiation process and, perhaps, should be disregarded in any consideration of the “free-particle” model of stone-initiation. It might be argued that incubation times up to a few hours might be relevant to the initiation of the crystallisation of calcium salts in the bladder but, as far as the more proximal sections of the urinary tract are concerned, they would only apply to that small number of cases who develop urinary stasis in the renal pelvis and/or ureter caused by some anatomical abnormality.
The second piece of evidence for questioning the early claim for the existence of so-called “inhibitors of self-nucleation” is shown in Figs. 3 and 4. These show the effects of pyrophosphate and other modifiers of crystallisation on the FP/ML/ULM of CaOx and CaP at time intervals ranging between 6 and 24 h or more [3, 4, 55, 56]. These are shown in relation to the 3–4 min FP values for these salts in the absence of inhibitors as determined by Robertson [50]. This shows that the maximum effect of these inhibitors on the FP/ML/ULM of these salts at 20–24 h only takes the FP/ML/ULM value up to the 3–4 min FP values of Robertson but never exceeds them. This suggests that any potential effect of these inhibitors on spontaneous “self-nucleation” at periods longer than 3–4 min can be overwhelmed if the supersaturation of urine reaches or exceeds the 3–4 min FP value of the salt concerned. In other words, these so-called inhibitors of “self-nucleation” become ineffective at the levels of supersaturation that lead to spontaneous rapid nucleation of calcium salts in urine.
This hypothesis is further supported by the data in Fig. 5 which shows the results of short-term studies on CaOx nucleation in whole urine which had been freshly collected from normal subjects and filtered through a 2 µm grade filter to remove any pre-existing particulate material [50]. Presumably, these urine samples still contained most, if not all, of the above inhibitors claimed to be of importance in the prevention of crystal nucleation. Figure 5 shows that the 3–4 min FP/ML/ULM of CaOx in these urines is close to that found in simple inorganic solutions, once the actual supersaturation of urine is taken into account. The supersaturation levels of the urine samples were calculated using the updated version of SUPERSAT program [57] (an early predecessor of EQUIL 2 [58]). This supports the above suggestion that any inhibitors that were present in the urine, including all of those mentioned in “Introduction”, do not have the ability to increase the 3–4 min FP/ML/ULM of CaOx, once the potential complexation of calcium and oxalate to these constituents has been taken into account in the calculation of supersaturation. Therefore, in terms of short-term (i.e. 3–4 min) “self-nucleation” of calcium salts in urine, supersaturation is by far the dominant factor in the process and inhibitors appear to play little, if any, part in delaying the onset of nucleation at these high levels of supersaturation.
Figures 3, 4 and 5 add further support to the overall hypothesis that studies purporting to show that certain constituents of urine can inhibit the “self-nucleation” of calcium salts are irrelevant because they were either carried out using unrealistic incubation times or did not take into account the fact that supersaturation may overwhelm any potential inhibitory effect of these compounds. The conclusion from these observations is that claims that these compounds (a) affect the upper limit of metastability or the metastable limit of calcium salts in urine and (b) are important in preventing the initiation of stones appear to be unsupported.
The important question then remaining is—does tubular fluid ever actually reach the 3–4 min FP values for CaOx and CaP within its transit time through the nephron? Recent calculations using a computer model of tubular fluid composition as it progresses through the nephron have shown that this is possible following transient increases either in plasma oxalate, e.g. after ingestion of a meal high in oxalate (see on “Inhibitors of crystal aggregation” below) in the case of calcium oxalate crystallisation, or following a small increase in either plasma phosphate or plasma pH in the case of calcium phosphate crystallisation [59].
If inhibitors are unlikely to influence the “self-nucleation” of calcium salts within the transit time of urine through the nephron, is it possible that they may have an effect on the subsequent crystal growth and/or agglomeration of crystal nuclei once formed? The answer to this may be “Yes” but, as will be shown below, excessive supersaturation can frequently overwhelm the effect of these inhibitors at least on the crystal growth phase.
Inhibitors of crystal growth
Many studies have shown that there are numerous constituents of urine which have the ability to inhibit the rate of growth of CaOx and/or CaP crystals in vitro [2, 5] although many of the early studies were carried out using simple solutions which only faintly resembled the actual composition of urine. As mentioned above, such studies have been criticised as possibly being irrelevant to the stone-forming process since many of the so-called inhibitors used could be influenced by some of the many urinary factors omitted from the test systems employed. There are, however, some in vivo studies which claim that some of these inhibitors may have an effect on the crystal growth of calcium salts in the urine of stone patients and, in some instances, may actually lead to a reduction in stone recurrence. Principal among these are potassium citrate medication and, to a lesser extent, magnesium supplements, which have been claimed to reduce stone recurrence by increasing urinary citrate excretion [60–62] and magnesium [63–65], respectively. I have to say, however, the claimed beneficial effect of potassium citrate medication did not materialise in our study carried out in a small group of patients with recurrent CaOx stones treated in this way (Unwin and Robertson, unpublished data) and was not effective in small doses in another study [66]. In our study, potassium citrate did not increase urinary citrate and the patients were “converted” from being “pure” CaOx stone-formers to being “mixed” CaOx + CaP stone-formers as a result of the alkalinisation of their urine, a potential risk that was pointed out by Coe and his colleagues [67] and recently confirmed in a rat model by Krieger et al. [68]. Our patients continued to form stones at the same or even higher rate than before. Even if administration of citrate (either as potassium citrate or potassium magnesium citrate), does have a beneficial effect on stone recurrence in larger groups of patients, as claimed by several groups [60–62], the potential for both citrate and magnesium to reduce the level of supersaturation of urine with respect to CaOx and CaP (as a result of their ability to complex with calcium, in the case of citrate, and oxalate and phosphate, in the case of magnesium) cannot be ignored. In addition, the patients studied on these treatment modalities were frequently also advised to drink more water which would further decrease the level of supersaturation with respect to both calcium salts. So, it is difficult to disentangle the precise mechanism(s) by which these treatments may have actually worked. Given the predominance of supersaturation over inhibitory activity as a determinant of crystal nucleation, as suggested in “Introduction”, it might be that the claimed beneficial effects of potassium citrate and magnesium potassium citrate on stone recurrence rates are more related to their ability to reduce supersaturation rather than to any increase induced in the inhibitory activity of the urine concerned.
One of the earliest naturally occurring urinary inhibitors of crystallisation to be identified was pyrophosphate, the properties of which were extensively studied by Fleisch and Russell and their colleagues in Switzerland [3, 4, 69]. From the early 1960s onwards, this group conducted many in vitro studies on the inhibitory effect of pyrophosphate on the crystallisation of both CaOx and CaP as shown in Figs. 3 and 4 [3, 4]. Their findings were later supported by the results obtained from more sophisticated studies on the effects of pyrophosphate and other polyphosphates on the crystal growth of CaOx carried out by Meyer and Smith at the Mayo Clinic [2, 5]. Their in vitro studies on pyrophosphate led the Mayo Group to treat CaOx stone-formers with phosphate supplements, which are known to increase the urinary excretion of pyrophosphate by inhibiting its tubular reabsorption through competition for a common reabsorption site in the renal tubule [70]. Studies followed which showed that phosphate supplements could reduce the rate of recurrence of stone-formation in CaOx stone-formers [71–73]. However, yet again there was a dual effect of this form of treatment on urine composition since the high doses of phosphate not only increased urinary pyrophosphate excretion but also reduced the intestinal absorption of calcium by precipitating it as calcium phosphate, thereby leading to a decrease in urinary calcium and the relative supersaturation of urine with respect to CaOx. So, it is not clear that the observed increase in urinary pyrophosphate was the main or even partial cause of the observed decrease in stone recurrence. Subsequently, phosphate supplements fell out of favour as a treatment for idiopathic calcium stone-formers because of fears that the potential fall in plasma calcium, following the reduction in the intestinal absorption of calcium, might stimulate the parathyroids. However, in spite of this potential side-effect, phosphate supplements are still used in some patients with primary hyperoxaluria [74] for whom there is no alternative completely satisfactory form of prophylaxis other than a very high fluid intake or, in about 21% of cases in whom it is effective, pyridoxine supplements [75].
In an early attempt to use synthetic inhibitors of the crystallisation of CaOx to reduce stone recurrence in highly recurrent idiopathic CaOx stone-formers and based on the findings from in vitro and animal studies [76, 77] carried out by Fleisch’s group, the Leeds group gave an oral dose of ethane-1-hydroxy-1,1-diphosphonate (EHDP) for 4 weeks to a small group of such patients in order to try and reduce CaOx crystalluria by inhibiting the crystal growth and/or crystal aggregation of that salt [78]. EHDP, now known as 1-hydroxyethane-1,1-diphosphonate (HEDP) or etidronate, was one of the early bisphosphonates which was originally used in studies aimed at reducing bone resorption but which has now been superseded by more effective bisphosphonates with fewer side-effects, such as alendronate, ibandronate, risedronate and zoledronate [79]. Because EHDP could only be given orally at the time of our studies and because it is poorly absorbed by the intestine, it had to be prescribed in relatively high doses (80 µmol/kg body weight/day). Unfortunately, at this dose level it appeared to precipitate or complex with sufficient calcium in the intestine (a) to reduce the urinary excretion of calcium and (b) to free up oxalate within the contents of the intestine (oxalate which would have otherwise been precipitated with the calcium now bound to EHDP) thereby rendering this oxalate available for passive absorption in the large bowel. As a result, urinary oxalate increased by over 50% which, in turn, caused CaOx crystalluria to increase markedly, both in terms of amount and average particle size, when compared with the crystalluria observed in the same patients on their baseline diets (Fig. 6). Although many of the crystals and aggregates of CaOx were smaller than those found in the fresh, warm urines from the same group of stone-formers given a small dose of sodium oxalate to simulate the hyperoxaluric effect of the EHDP, a significant number of very large crystals and aggregates of CaOx were still frequently observed (Figs. 7, 8). The data were interpreted to show that, although for part of the time EHDP seemed to be excreted in sufficient quantities to inhibit the growth and aggregation of CaOx crystals, there were other occasions when the supersaturation of urine with respect to CaOx seemed to overwhelm the inhibitory effect of EHDP on at least on crystal growth. The data also confirmed the conclusion drawn from the in vitro studies shown in Fig. 3 that EHDP could not inhibit the initiation of CaOx crystallisation at the high levels of supersaturation observed in vivo and was not, therefore, an inhibitor of “self-nucleation” under these conditions.
The total CaOx crystalluria measured in the fresh, warm urine samples from recurrent CaOx stone-formers a on their basal diet, b on their basal diet augmented by a single dose of sodium oxalate (2.6 mmol) given before breakfast and c on their basal diet augmented by a daily dose of EHDP (80 µmol/kg body weight/day). (Dashed line upper limit of normal)
The percentage of large crystals and aggregates of CaOx measured in the fresh, warm urine samples from recurrent CaOx stone-formers a on their basal diet, b on their basal diet augmented by a single oral dose of sodium oxalate (2.6 mmol) given before breakfast and c on their basal diet augmented by a daily dose of EHDP (80 µmol/kg body weight/day). (Dashed line upper limit of normal)
Photomicrographs of CaOx crystals and aggregates measured in the fresh, warm urine samples from recurrent CaOx stone-formers a on their basal diet, b on their basal diet augmented by a single oral dose of sodium oxalate (2.6 mmol) given before breakfast and c on their basal diet augmented by a daily dose of EHDP (80 µmol/kg body weight/day)
More recently, in vitro studies on the crystallisation of CaOx using a continuous crystalliser, have shown that at least three of the above more sophisticated bisphosphonates currently used in the treatment of osteoporosis [79], slightly reduced the crystal growth of the CaOx crystals produced but had a major inhibitory effect on the aggregation of these crystals [80]. Yet again, the data confirmed that these bisphosphonates could not inhibit the spontaneous “self-nucleation” of CaOx crystallisation at the high levels of supersaturation employed in the continuous crystalliser and did not appear, therefore, to be inhibitors of “self-nucleation”. Since these bisphosphonates were shown to be effective at micromolar concentrations (concentrations which are estimated to be reproducible in urine following treatment with these forms of medication), it may be that one or more of these compounds may eventually be useful for preventing the formation of CaOx stones, a possibility supported by studies in rats [81]. Of course, the treatment would have to be limited to adults since bisphosphonates could potentially interfere with the development of bone in children.
Inhibitors of crystal aggregation
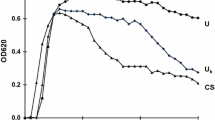
Attention was first drawn to the potential role of crystal aggregation as a risk factor for stones in the late 1960s [82, 83] and this led to many subsequent quantitative studies on the possible role of crystalluria and particle size in the initiation of stones [84–86]. As a result of these studies, many workers, although not all, became convinced that crystal aggregation is a vital step in the initiation and continued growth of stones [87, 88]. Since crystal growth is a relatively slow process, it was readily appreciated that the most rapid way to increase the particle size of incipient crystals of calcium salts was through crystal aggregation and not through crystal growth per se. The importance of crystal aggregation was given further support when studies with fresh, warm urine from recurrent CaOx stone-formers and normal subjects showed that the urine from patients often contained large crystals and aggregates of CaOx whereas that from normal subjects never contained such large particles although they occasionally passed small individual crystals of CaOx when their urine exceeded the 3–4 min FP of that salt. Figure 9 shows the percentage of large crystals and aggregates in the fresh, warm urine samples passed by a group of recurrent idiopathic CaOx stone-formers and in the urines of normal subjects in relation to the level of supersaturation of urine with respect to CaOx. This shows that, as mentioned above, the stone-formers passed much larger crystals and aggregates of CaOx than did the normal subjects at all levels of supersaturation above the 3–4 min FP of CaOx. These data were originally interpreted to show that the stone-formers had less inhibitory activity towards the growth and aggregation of CaOx than did the normal subjects and this led myself and many others to study inhibitors (and also promoters) of crystallisation for several years. Many urinary constituents were shown to inhibit not only the crystal growth of CaOx and CaP, as mentioned above, but also, more importantly, the aggregation of these crystals. As a result, a deficiency in the urinary excretion of inhibitors of growth and aggregation by stone-formers compared with normal subjects was suggested to be the cause of the difference in crystalluria patterns between the two groups [85, 86].
The percentage of large crystals and aggregates of CaOx measured in the fresh, warm urine samples from recurrent CaOx stone-formers (RSF) and normal subjects (N) in relation to the relative supersaturation (RSS) of urine with respect to CaOx. The data are shown in relation to the Solubility Product (SP) and 4-min formation product (FP) of CaOx [50]
More recently, however, I have come to an alternative possible interpretation of the data in Fig. 9. A second explanation for the difference between the CaOx crystal sizes excreted by normals and recurrent stone-formers emerges from a computer model of the functioning of the renal tubule, which shows that there are two possible points in the nephron where CaOx crystallisation may be initiated, particularly in the tubular fluid of recurrent stone-formers [59]. The first occurs at the end of the descending limb of the loop of Henle (DLH), following a small transient increase in plasma oxalate and secretion of oxalate into the lumen towards the end of the proximal tubule. The second occurs in the latter part of the collecting duct (CD) where supersaturation increases for a second time following the final adjustment of water reabsorption which occurs throughout the CD section of the tubule. Those crystals initiated at the end of the DLH by spontaneous “self-nucleation” may be delayed in transit through the renal tubule by various physical and hydrodynamic mechanisms described in the paper [59] and grow into the large crystals and aggregates frequently observed in fresh, warm urine collected from recurrent stone-formers. In contrast, the model shows that those crystals initiated in the CD will not grow fast enough in the remaining transit time of fluid through the latter part of the CD and will be excreted as relatively small crystals measuring only a few microns in diameter, such as observed in the urines of many recurrent stone-formers and occasionally in the urines of some normal subjects [83]. Thus, the model predicts that normal urine should contain a unimodal peak of small crystals of CaOx and that the urine from recurrent stone-formers should show a bimodal distribution of particle size consisting of an initial peak of small crystals and a second of much larger crystals and aggregates of that salt.
These predictions from the model are supported by the findings from an in vivo study, carried out in the early 1970s, in which six idiopathic calcium recurrent stone-formers (RSF) and six normal control subjects (N) were fed a fixed basal diet for 4 days [84]. On the second day of the study, urine samples from both groups were collected into warm vacuum flasks at 09.00 (immediately before breakfast) and then at 3-h intervals over the following 12 h and finally at 09.00 the next morning. The size profiles of CaOx crystals and aggregates contained in the urine samples (maintained at 37°) were measured within 10 min of voiding using a Coulter Counter. The size and appearance of the crystals and aggregates were also checked by light microscopy immediately after voiding. On the fourth day of the study, a small oral dose of sodium oxalate (2.6 mmol—equivalent to the quantity contained in about 30 g of dry spinach or 40 g of boiled spinach) was administered to the patients and control subjects immediately before breakfast at 09.00 and fresh, warm urine samples collected according to the above protocol. Again, the size profiles of CaOx crystals in the urine samples were measured within 10 min of voiding using a Coulter Counter and the size and appearance of the crystals and aggregates of CaOx checked by light microscopy on a warmed microscope stage.
Figure 10 shows the mean size distributions of CaOx crystals and aggregates in the fresh, warm urine from the normals and the RSF at 12.00 on the second day of the basal diet and Fig. 11 shows the corresponding data on Day 4 of the study, 3 h after the sodium oxalate bolus was given. On the basal diet, both groups produced a peak of small CaOx crystals (mean diameter ~6 µm) in the urine samples taken at 12.00 although the peak in the urine from the RSF was much higher than that in the urines from the normals. However, the urines from the RSF exhibited a second peak of much larger CaOx crystals and aggregates (mean diameter ~25 µm, but some as large as 50 µm, which was the upper limit of measurement in the Coulter Counter system used at that time). In fact, the light microscopy studies showed that some of the particles were much larger than 50 µm and these particles frequently blocked the 65 µm aperture of the Coulter Counter probe. After the oxalate load, the normals showed an increase in the peak of small crystals of CaOx but still did not pass any large crystals or aggregates in their urines at that point or during the remainder of the day. The RSF, on the other hand, showed a marked increase in the peak of large crystals and aggregates and 3 out of the 6 patients had attacks of renal colic within a few hours following the oxalate load. Urinary oxalate excretion increased within the first 3 h in both groups but slightly more so in the RSF than in the normals. Furthermore, the normals had a slight diuresis, which helped to moderate the increase in oxalate concentration in their urine (0.22–0.28 mmol/l) caused by the ingestion of the sodium oxalate. The RSF, on the other hand, had little or no diuresis and their urinary oxalate concentrations increased from 0.30 mmol/l to 0.52 mmol/l. The reason for this difference in the diuretic response to the oxalate load between the two groups is not clear but may be worthwhile investigating. In this connection, it is interesting that dandelions, which are known in France as “pis-en-lit” (literally piss-in-the-bed), have a high content of oxalate and are often used as a diuretic in homeopathic medicine. Perhaps, some stone-formers have a reduced ability to exhibit this “protective” diuretic response to an oxalate load relative to that shown by normal subjects and this exacerbates their risk of forming CaOx-containing stones after a meal high in oxalate?
Based on the patterns of CaOx supersaturation along the renal tubule, one explanation of these data is that, on the basal diet, the tubular oxalate concentrations at the end of the DLH were likely to be too low in the normals to cause CaOx crystals to “self-nucleate” at that point. Any crystals that might form in normal tubular fluid would only do so towards the end of the CD if CaOx supersaturation in the tubular fluid reached the critical level for “self-nucleation” FP of CaOx. However, any crystals so-formed would not have had time to grow before reaching the end of the CD and would, therefore, be small and easily excreted through the Ducts of Bellini. Even after the oxalate load, the CaOx supersaturation levels in the normals would still be unlikely to reach the FP of CaOx at the end of the DLH and would only become high enough to nucleate spontaneously in the CD. Based on the above reasoning, this would only lead to an increased number of small crystals in the urines of the normals, as observed in Fig. 11. In the RSF, however, because of their higher intestinal absorption of oxalate and subsequently higher urinary oxalate concentrations, CaOx nucleation would be likely to be increased both at the end of the DLH and in the CD. Those crystals initiated at the end of the DLH could potentially be delayed by the various physical and hydrodynamic mechanisms described in the model [59] and under the conditions of the increased tubular concentration of oxalate in these patients give rise to the formation of many more large individual crystals and aggregates, such as observed in the RSF urines after the oxalate load. The much higher RSS CaOx values in the CD of the RSF compared with those in the CD of the normals would also result in a higher crystal growth rate of any small crystals formed at that site and would lead to fewer small crystals actually being excreted compared with the numbers formed on the basal diet as shown in Figs. 10 and 11. Whether or not inhibitors of crystallisation play any role under the conditions of high supersaturation predicted by the model in the transit time between the end of the DLH and the ducts of Bellini, is open to question. Clearly, from the evidence in the “Introduction”, they do not affect “self-nucleation” but it is possible that they might influence crystal growth during the passage of crystals through the distal tubule (DT), where supersaturation decreases slightly [59] before it finally increases again in the CD, or it might affect crystal aggregation at any point in the CD section of the nephron, as predicted from the continuous crystalliser studies described below.
Various other polyanionic compounds have been shown to be effective as inhibitors of CaOx crystal aggregation in the continuous crystalliser system for studying the factors affecting the crystallisation of CaOx [35, 45–47]. These include glycosaminoglycans, ribonucleic acid, the non-polymerised form of Tamm–Horsfall glycoprotein [45–48] and pentosan polysulphate [89]. All of these work by increasing the negative zeta potential on the surface of the CaOx crystals produced, thereby leading to crystal–crystal repulsion and a decrease in crystal aggregation [45–47, 89, 90]. This may also explain the inhibition of aggregation observed in the studies involving pyrophosphate and bisphosphonates [86].
In summary, some polyanionic inhibitors may be important in inhibiting the aggregation of CaOx crystals in urine since they act by changing the charge on the crystal surface, a process that is less likely to be affected by high levels of urinary supersaturation.
Inhibitors of crystal binding to tubular cell epithelium
The interaction between crystals and cells became a popular line of research in the 1990s and 2000s [91–96] and still occupies some workers to this day [97]. It was originally hypothesised by Finlayson and Reid that it was unlikely that CaOx crystals could be initiated and grow sufficiently large to become trapped within the lumen of the tubule within the transit time of urine through the nephron [98] and that stone-formation could only occur on crystals already anchored to the renal epithelium for some reason. However, this assertion has been challenged by a number of workers who have further developed the original kidney model of Finlayson and Reid to incorporate various physical and hydrodynamic factors omitted by them in their original model. This has resulted in an alternative conclusion on the possibility of crystals being trapped in the renal tubules to that drawn by the original authors [59, 88]. Their theory was that crystals formed spontaneously in the tubular fluid had to become attached to the epithelium lower down in the nephron by some unspecified mechanism and, once anchored there, could continue to grow and form the nucleus of an actual stone. Many studies followed using procedures involving layers of various types of tubular cell grown by tissue culture which showed that, under certain conditions, these cell lines could bind crystals of CaOx and that some urinary constituents could inhibit this binding process [91–96]. The most potent inhibitors detected so far are various polyanionic molecules, such as glycosaminoglycans, glycoproteins and citrate [99]. The studies on crystal–cell interactions and the factors influencing these are covered in various excellent reviews on this topic by Khan [97, 100] and I will not elaborate on these now. It is not yet clear if the findings from these studies can be developed into a treatment for preventing CaOx stone-formation.
Inhibitors of crystal growth on randall’s plaques
Since the advent of renewed interest in the role of Randall’s Plaques and Randall’s Plugs in the initiation of calcium-containing stones, much has been written on this topic [101, 102]. However, there has been little research on the possible role of inhibitors of crystallisation in preventing the growth of these entities into stones. Presumably, the same arguments will apply as in “Inhibitors of crystallisation of calcium salts”, “Discussion” and “Conclusions” above but it remains to be seen if there are some more specific factors in urine, other than those already identified, which can influence crystal growth and aggregation on these calcific deposits in the renal papillae.
Discussion
This review clearly demonstrates that excessive supersaturation sufficient to cause the “self-nucleation” of CaOx and CaP in urine or “urine-like” solutions seems to dominate this step in the crystallisation of these salts within the normal 3–4 min transit time of tubular fluid between the glomerulus and the ducts of Bellini. It is also clear that the claims that this step can be inhibited by certain constituents of urine are incorrect since all of the test systems used involved incubation times incompatible with the transit time of tubular fluid through the nephron. At best, some of the inhibitors identified might inhibit the further crystal growth and/or aggregation of the nuclei at lower levels of supersaturation. Thus, the “discovery” of many of these so-called “inhibitors of nucleation” appears to have been made based on a false premise and it is perhaps fortuitous that some of them eventually turned out to be inhibitors of later steps in the crystallisation process.
Unquestionably, urine does possess a certain ability to modify the rate of crystal growth and/or aggregation of calcium oxalate and calcium phosphate crystals and may also contain factors that influence the binding of these crystals to renal epithelial cells. Currently, however, it would seem that the crystal growth and aggregation modifying activity of some of these ions and macromolecules is unlikely to be attributable to one single magic factor X but is probably due to the net effect of all the above promoters and inhibitors (and probably others not yet identified). None of the above factors appears to dominate the kinetics of crystal growth, crystal aggregation and binding of crystals to cells, and none has yet been universally accepted as being uniquely different, either quantitatively or qualitatively, between stone-formers and normal subjects. Therefore, the assertion that a deficiency in one particular inhibitor or an excess of one particular promoter is THE cause of stone formation remains open to question. In the final analysis, stone-formation is probably due to the abnormal combination of factors that influence both the thermodynamic driving force (supersaturation) and the kinetic (rate-controlling) processes involved in the crystal growth and aggregation of the various stone-forming minerals. For some types of stone-formation, such as cystine, xanthine, 2,8-dihydroxyadenine, uric acid and magnesium ammonium phosphate, the thermodynamic factors appear to dominate the process; for calcium-containing stones both sets of factors may be involved but the effectiveness of the inhibitors on crystal growth appear to be largely dependent on the level of supersaturation of the urine with respect to the calcium salt concerned. However, inhibitors may play a role in influencing the degree of aggregation of the crystals produced which is independent of the level of supersaturation.
Conclusions
The evidence examined in this critique would suggest the following:
-
1.
So-called inhibitors of “self-nucleation” do not play any role in preventing the initiation of the crystallisation of CaOx or CaP in urine because excessive urinary supersaturation can overwhelm their effect on the “self-nucleation” of these salts within the normal time frame of the transit of urine through the nephron (3–4 min).
-
2.
Inhibitors of the crystal growth of CaOx crystals may or may not play a significant role in the prevention of CaOx stone-formation since once again excessive urinary supersaturation can overwhelm the effect of the inhibitors on the growth process. However, they may play a role as inhibitors of crystal growth at lower levels of metastable supersaturation where the balance between supersaturation and inhibitors as competitive driving forces in the crystal growth process is more even.
-
3.
Inhibitors of CaOx crystal aggregation may play a significant role in the prevention of stones, since they do not seem to be strongly affected by excessive supersaturation, at least in vitro. There is also some evidence that at least some of these inhibitors may not be affected by excessive supersaturation in vivo.
-
4.
Inhibitors of CaOx crystal binding to renal tubular epithelium may exist but further studies are necessary to elucidate their importance in the actual formation of stones in the urinary tract.
-
5.
There may be an alternative explanation than a deficiency in the excretion of inhibitors for the observations in those studies which have shown a difference in the size and degree of aggregation of CaOx crystals in the fresh, warm urines collected from normal subjects and patients with recurrent CaOx stones. This may depend more on the site of “self-nucleation” of CaOx crystals in the renal tubule rather than to differences in the excretion of so-called inhibitors of crystallisation between the two groups.
-
6.
The claims that administration of potassium citrate, potassium magnesium citrate or magnesium hydroxide reduces the rate of stone recurrence may be more due to the effect of these forms of medication on the supersaturation of urine with respect to CaOx and CaP than to any increase in “inhibitory activity” attributed to these treatments.
-
7.
In summary, there is a competition between supersaturation and so-called inhibitors of crystallisation which ultimately determines the pattern of crystalluria in stone-formers and normals. If the supersaturation of urine with respect to CaOx reaches or exceeds the 3–4 min formation product of that salt, then it dominates the crystallisation process both in terms of “self-nucleation” and crystal growth but may have little or no effect on the degree of aggregation of the crystals produced. As the level of supersaturation of urine with respect to CaOx decreases within the metastable region, the influence of inhibitors increases and some may affect both the crystal growth and aggregation of any existing crystals of CaOx.
Notes
By “crystal self-nucleation”, is meant the type of spontaneous nucleation that has not been initiated either by pre-existing crystals of the salt itself or heterogeneously by pre-existing crystals of other salts or by the surfaces of epithelial cells derived from various sections of the tubular lumen or by pre-existing Randall's Plaques or Randall's Plugs.
Abbreviations
- CaOx:
-
Calcium oxalate
- CaP:
-
Calcium phosphate
- MSMPR:
-
Mixed-suspension-mixed-product-removal
- FP:
-
Formation product
- ML:
-
Metastable limit
- ULM:
-
Upper limit of Metastability
- SP:
-
Solubility product
- RSS:
-
Relative supersaturation
- EHDP:
-
Ethane-1-hydroxy-1,1-diphosphonate
- HEDP:
-
1-Hydroxyethane-1,1-diphosphonate
- Cl2MDP:
-
Dichloromethylenediphosphonate
- DLH:
-
Descending limb of the loop of Henle
- CD:
-
Collecting duct
- RSF:
-
Recurrent stone-formers
- N:
-
Normal controls
References
Borden TA, Lyon ES (1969) The effects of magnesium and pH on experimental calcium oxalate stone disease. Invest Urol 6:412–422
Meyer JL, Smith LH (1975) Growth of calcium oxalate crystals. II. Inhibition by natural urinary crystal growth inhibitors. Invest Urol 13:36–39
Fleisch H, Bisaz S (1962) Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol 203:671–675
Fleisch H, Bisaz S (1964) The inhibitory effect of pyrophosphate on calcium oxalate precipitation and its relation to urolithiasis. Experientia 20:276–277
Meyer JL, McCall JT, Smith LH (1974) Inhibition of calcium phosphate crystallization by nucleoside phosphates. Calcif Tissue Res 15:289–293
Howard JE, Thomas WC, Barker LM, Smith LH, Wadkins CL (1967) The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopkins Med J 120:119–136
Robertson WG, Peacock M, Nordin BEC (1973) Inhibitors of the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta 43:31–37
Ryall RL, Harnett RM, Marshall VR (1981) The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta 112:349–356
Robertson WG, Scurr DS, Bridge CM (1981) Factors influencing the crystallization of calcium oxalate in urine—a critique. J Crystal Growth 53:182–194
Worcester EM, Nakagawa Y, Coe FL (1987) Glycoprotein calcium oxalate crystal growth inhibitor in urine. Miner Electrolyte Metab 13:267–272
Worcester EM, Nakagawa Y, Wabner CL, Kumar S, Coe FL (1988) Crystal adsorption and growth slowing by nephrocalcin, albumin and Tamm–Horsfall protein. Am J Physiol 255:F1197–F1205
Nakagawa Y, Ahmed MA, Hall SL, Deganello S, Coe FL (1987) Isolation from human calcium oxalate stones of nephrocalcin, a glycoprotein inhibitor of calcium oxalate crystal growth. Evidence that nephrocalcin from patients with calcium oxalate nephrolithiasis is deficient in gamma-carboxyglutamic acid. J Clin Invest 79:1782–1787
Hess B, Nakagawa Y, Coe FL (1989) Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol 257:F99–F106
Coe FL, Parks JH (1990) Defenses of an unstable compromise: crystallization inhibitors and the kidney’s role in mineral regulation. Kidney Int 38:625–631
Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P (1995) Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res 23:231–238
Shiraga H, Min W, Van Dusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG (1992) Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci USA 89:426–430
Tsuji H, Tohru U, Hirotsugu U, Masanori I, Yuji H, Takashi K (2007) Urinary concentration of osteopontin and association with urinary supersaturation and crystal formation. Int J Urol 14:630–634
Pillay SN, Asplin JR, Coe FL (1998) Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol 275:F255–F261
Tardivel S, Médétognon J, Randoux C, Kébédé M, Drüeke T, Daudon M, Hennequin C, Lacour B (1999) Alpha-1-microglobulin: inhibitory effect on calcium oxakate crystallization in vitro and decreased urinary excretion in calcium oxalate stone formers. Urol Res 27:243–249
Dussol B, Geider S, Lilova A, Leonetti F, Dupuy P, Daudon M, Berland Y, Dagorn JC, Verdier JM (1995) Analysis of the soluble matrix of five morphologically different kidney stones. Urol Res 23:45–51
Goiko M, Dierolf J, Gleberzon JS, Liao Y, Grohe B, Goldberg HA, de Bruyn JR, Hunter GK (2011) Peptides of Matrix Gla protein inhibit nucleation and growth of hydroxyapatite and calcium oxalate monohydrate crystals. PLoS One 8(11):e80344. doi:10.1371/journal.pone.0080344
Stapleton AM, Dawson CJ, Grover PK, Hohmann A, Comacchio R, Boswarva V, Tang Y, Ryall RL (1996) Further evidence linking urolithiasis and blood coagulation: urinary prothrombin fragment 1 is present in stone matrix. Kidney Int 49:880–888
Grover PK, Ryall RL (1999) Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro: relationship between protein structure and inhibitory activity. Eur J Biochem 263:50–56
Thongboonkerd V, Chutipongtanate S, Semangoen T, Malasit P (2008) Urinary trefoil factor 1 is a novel potent inhibitor of calcium oxalate crystal growth and aggregation. J Urol 179:1615–1619
Dawson CJ, Grover PK, Ryall RL (1998) Inter-alpha-inhibitor in urine and calcium oxalate urinary crystals. Br J Urol 81:20–26
Evan AP, Bledsoe S, Worcester EM, Coe FL, Lingeman JE, Bergsland KJ (2007) Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int 72:1503–1511
Robertson WG (2003) A risk factor model of stone-formation. Front Biosci 8:1330–1338
Nishio S, Hatanaka M, Takeda H, Aoki K, Iseda T, Iwata H, Yokoyama M (2000) Calcium phosphate crystal-associated proteins: alpha2-HS-glycoprotein, prothrombin F1, and osteopontin. Mol Urol 4:383–390
Boyce WH, King JS (1963) Present concepts concerning the origin of matrix and stones. Ann N Y Acad Sci 104:563–578
Morse RM, Resnick MI (1988) A new approach to the study of urinary macromolecules as a participant in calcium oxalate crystallization. J Urol 139:869–873
Spector AR, Gray A, Prien EL (1976) Kidney stone matrix. Differences in acidic protein composition. Invest Urol 13:387–389
Lian JB, Prien EL, Glimcher MJ, Gallop PM (1977) The presence of protein-bound c-carboxyglutamic acid in calcium-containing renal calculi. J Clin Invest 59:1151–1157
Jones WT, Resnick MI (1990) The characterization of soluble matrix proteins in selected human renal calculi using two-dimensional polyacrylamide gel electrophoresis. J Urol 144:1010–1014
Rose GA, Sulaiman S (1982) Tamm–Horsfall mucoproteins promote calcium oxalate crystal formation in urine: quantitative studies. J Urol 127:177–179
Scurr DS, Robertson WG (1986) Modifiers of calcium oxalate crystallization found in urine. III. Studies on the role of Tamm–Horsfall mucoprotein and of ionic strength. J Urol 136:505–507
Ebrahimpour A, Perez L, Nancollas GH (1991) Induced crystal growth of calcium oxalate monohydrate at hydroxyapatite surfaces. The influence of human serum albumin, citrate and magnesium. Langmuir 7:577–583
Cerini C, Geider S, Dussol B, Hennequin C, Daudon M, Veesler S, Nitsche S, Boistelle R, Berthézène P, Dupuy P, Vazi A, Berland Y, Dagorn JC, Verdier JM (1999) Nucleation of calcium oxalate crystals by albumin: involvement in the prevention of stones. Kidney Int 55:1776–1786
Farmanesh S, Chung J, Sosa RD, Kwak JH, Karande P, Rimer JD (2014) Natural promoters of calcium oxalate monohydrate crystallization. J Am Chem Soc 136:12648–12657. doi:10.1021/ja505402r
Aggarwal KP, Narula S, Kakkar M, Tandon C (2013) Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int. doi:10.1155/2013/292953
Kok DJ, Boellaard W, Ridwan Y, Levchenko VA (2017) Timelines of the “free-particle” and “fixed-particle” models of stone-formation: theoretical and experimental investigations. Urolithiasis (this issue)
Rimer JD, Kolbach-Mandel AM, Ward MD, Wesson JA (2017) The role of macromolecules in the formation of kidney stones. Urolithiasis (this issue)
Sheehan ME, Nancollas GH (1980) Calcium oxalate crystal growth. A new constant composition method for modelling urinary stone formation. Invest Urol 17:446–450
Koutsoukos P, Amjad Z, Tomson MB, Nancollas GH (1980) Crystallization of calcium phosphates. A constant composition study. J Am Chem Soc 102:1553–1557
Finlayson B (1972) The concept of a continuous crystallizer. Its theory and application to in vivo and in vitro urinary tract models. Invest Urol 9:258–263
Drach GW, Kraljevich Z, Randolph AD (1982) Effects of high molecular weight urinary macromolecules on crystallization of calcium oxalate dihydrate. J Urol 127:805–810
Robertson WG, Scurr DS (1986) Modifiers of calcium oxalate crystallization found in urine. I. Studies with a continuous crystallizer using an artificial urine. J Urol 135:1322–1326
Scurr DS, Robertson WG (1986) Modifiers of calcium oxalate crystallization found in urine. II. Studies on their mode of action in an artificial urine. J Urol 136:128–131
Azoury R, Garside J, Robertson WG (1986) Habit modifiers of calcium oxalate crystals precipitated in a reverse osmosis system. J Cryst Growth 76:259–262
Grover PK, Thurgood LA, Wang T, Ryall RL (2010) The effects of intra-crystalline and surface-bound proteins on the attachment of calcium oxalate monohydrate crystals to renal cells in undiluted human urine. BJU Int 105:708–715
Robertson WG (1969) Physico-chemical aspects of renal stone-formation. PhD Thesis, University of Leeds, Leeds, UK
Robertson WG (1973) Factors affecting the precipitation of calcium phosphate in vitro. Calcif Tissue Res 11:311–322
Pak CY, Eanes ED, Ruskin B (1971) Spontaneous precipitation of brushite in urine: evidence that brushite is the nidus of renal stones originating as calcium phosphate. Proc Natl Acad Sci USA 68:1456–1460
Pak CY, Ohata M, Holt K (1975) Effect of diphosphonate on crystallization of calcium oxalate in vitro. Kidney Int 7:154–160
Bouropoulos N, Bouropoulos C, Klepetsanis PG, Melekos M, Barbalias G, Koutsoukos PG (1996) A model system for the investigation of urinary stone formation. Br J Urol 78:169–175
Fleisch H, Russell RG, Bisaz S, Casey PA, Mühlbauer RC (1968) The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution. Calcif Tissue Res 2(Suppl):10–10a
Robertson WG, Fleisch H (1970) Imidodiphosphate: an inhibitor of in vitro precipitation and the dissolution of calcium phosphate. Biochim Biophys Acta 222:677–680
Robertson WG (1969) Measurement of ionized calcium in biological fluids. Clin Chim Acta 24:149–157
Werness PG, Brown CM, Smith LH, Finlayson B (1985) EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol 134:1242–1244
Robertson WG (2015) Potential role of fluctuations in the composition of renal tubular fluid through the nephron in the initiation of Randall’s Plugs and calcium oxalate crystalluria in a computer model of renal function. Urolithiasis 43(Suppl 1):S93–S107
Pak CY, Sakhaee K, Fuller CJ (1983) Physiological and physiochemical correction and prevention of calcium stone formation by potassium citrate therapy. Trans Assoc Am Physicians 96:294–305
Pak CY, Fuller C, Sakhaee K, Preminger GM, Britton F (1985) Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol 134:11–19
Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A (1997) Potassium–magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 158:2069–2073
Johansson G, Backman U, Danielson BG, Fellström B, Ljunghall S, Wikström B (1980) Biochemical and clinical effects of the prophylactic treatment of renal calcium stones with magnesium hydroxide. J Urol 124:770–774
Lindberg J, Harvey J, Pak CYC (1990) Effect of magnesium citrate and magnesium oxide on the crystallization of calcium salts in urine: changes produced by food–magnesium interaction. J Urol 143:248–251
Eisner BH, Sheth S, Dretler SP, Herrick B, Pais VM Jr (2012) High dietary magnesium intake decreases hyperoxaluria in patients with nephrolithiasis. Urology 80:780–783
Jendle-Bengten C, Tiselius HG (2000) Long-term follow-up of stone-formers treated with a low dose of sodium potassium citrate. Scand J Urol Nephrol 34:36–41
Coe FL, Evan A, Worcester (2011) Pathophysiology-based treatment of idiopathic calcium kidney stones. Clin J Am Soc Nephrol 6:2083–2092
Krieger NS, Asplin JR, Frick KK, Granja I, Culbertson CD, Ng A, Grynpas MD, Bushinsky DA (2015) Effect of potassium citrate on calcium phosphate stones in a model of hypercalciuria. J Am Soc Nephrol 26:3001–3008
Fleisch H, Russell RG, Straumann F (1966) Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 212:901–903
Russell RG, Bisaz S, Fleisch H (1976) The influence of orthophosphate on the renal handling of inorganic pyrophosphate in man and dog. Clin Sci Mol Med 51:435–443
Smith LH, Werness PG, Van Den Berg CJ, Wilson DM (1980) Orthophosphate treatment in calcium urolithiasis. Scand J Urol Nephrol Suppl 53:253–263
Van Den Berg CJ, Kumar R, Wilson DM, Heath H 3rd, Smith LH (1980) Orthophosphate therapy decreases urinary calcium excretion and serum 1,25-dihydroxyvitamin D concentrations in idiopathic hypercalciuria. J Clin Endocrinol Metab 51:998–1001
Heyburn PJ, Robertson WG, Peacock M (1982) Phosphate treatment of recurrent calcium stone disease. Nephron 32:314–319
Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH (1994) Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med 331:1553–1558
Hoppe B, Latta K, von Schnakenburg C, Kemper MJ (2005) Primary hyperoxaluria—the German experience. Am J Nephrol 25:276–281
Fraser D, Russell RGG, Pohler P, Robertson WG, Fleisch H (1972) The influence of a diphosphonate (ethane-1-hydroxy-1,1-diphosphonate) on the development of experimentally-induced urinary stones in rats. Clin Sci 42:197–207
Russell RGG, Robertson WG, Fleisch H (1973) Inhibitors of mineralisation. In: Zipkin I (ed) Biological mineralisation. Wiley, New York, pp 807–825
Robertson WG, Peacock M, Marshall RW, Knowles F (1974) The effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) on calcium oxalate crystalluria in recurrent renal stone-formers. Clin Sci Mol Med 47:13–22
Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, Sadler S, Wong R, Campbell F, Stevenson M, Strong M, Selby P, Gittoes N (2016) A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess 20:1–406
Allen SE, Choong S, Fry C, Robertson WG (2006) The inhibitory effect of bisphosphonates on calcium oxalate crystal formation in vitro. Proc Physiol Soc 3:PC17
Basok EK, Basaran A, Atsu N, Yildirim A, Tokuc R (2008) Are new-generation bisphosphonates effective for the inhibition of calcium oxalate stone formation in a rat model? Urol Int 81:325–329
Dyer R, Nordin BEC (1967) Urinary crystals and their relation to stone formation. Nature 215:751–752
Robertson WG, Peacock M, Nordin BEC (1969) Calcium crystalluria in recurrent renal stone-formers. Lancet 2:21–24
Robertson WG, Peacock M, Nordin BEC (1971) Calcium oxalate crystalluria and urine saturation in recurrent stone-formers. Clin Sci 40:365–374
Robertson WG, Peacock M (1972) Calcium oxalate crystalluria and inhibitors of crystallisation in recurrent renal stone-formers. Clin Sci 43:499–506
Robertson WG, Peacock M, Nordin BEC (1973) Inhibitors of the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta 43:31–37
Kok DJ, Papapoulos SE, Bijvoet OLM (1990) Crystal agglomeration is a major element in calcium oxalate urinary stone-formation. Kidney Int 37:51–56
Kok DJ, Khan SR (1994) Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int 46:847–854
Norman RW, Scurr DS, Robertson WG, Peacock M (1985) Sodium pentosan polysulphate as a polyanionic inhibitor of calcium oxalate crystallization in vitro and in vivo. Clin Sci 68:369–371
Norman RW, Scurr DS, Robertson WG, Peacock M (1984) Inhibition of calcium oxalate crystallization by pentosan polysulphate in control subjects and stone-formers. Br J Urol 56:595–598
Kumar S, Sigmon D, Miller T, Carpenter B, Khan S, Malhotra R, Scheid C, Menon M (1991) A new model of nephrolithiasis involving tubular dysfunction/injury. J Urol 146:1384–1389
Lieske JC, Leonard R, Swift HS, Toback FG (1996) Adhesion of calcium oxalate monohydrate crystals to anionic sites of renal epithelial cells. Am J Physiol 270:F192–F199
Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M (1996) Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int 49:413–419
Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS (1998) Calcium oxalate crystal attachment to cultured kidney epithelial cell lines. J Urol 160:1528–1532
Verkoelen CF, van der Boom BG, Houtsmuller AB, Schröder FH, Romijn JC (1998) Increased calcium oxalate monohydrate crystal binding to injured tubular epithelial cells in culture. Am J Physiol 274:F958–F965
Rabinovich YI, Esayanur M, Daosukho S, Byer KJ, El-Shall HE, Khan SR (2006) Adhesion force between calcium oxalate monohydrate crystal and kidney epithelial cells and possible relevance for kidney stone formation. J Colloid Interface Sci 300:131–140
Khan SR (2011) Crystal/cell interaction and nephrolithiasis. Arch Ital Urol Androl 83:1–5
Finlayson B, Reid F (1978) The expectation of free and fixed particles in urinary stone disease. Invest Urol 15:442–448
Lieske JC, Leonard R, Toback FG (1995) Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am J Physiol 268:F604–F612
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33:349–357
Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111:607–616
Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M (2005) Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 67:576–591
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interests relating to this work.
Rights and permissions
About this article
Cite this article
Robertson, W.G. Do “inhibitors of crystallisation” play any role in the prevention of kidney stones? A critique. Urolithiasis 45, 43–56 (2017). https://doi.org/10.1007/s00240-016-0953-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0953-y