Abstract
Purpose
Recurrent kidney stones are associated with bone mineral density loss, altered bone remodeling markers, hypercalciuria and increased in fasting calcium/creatinine ratio. The objective was to determine biochemical alterations in urine in patients with osteopenia/osteoporosis without calcium kidney stones compared with patients with calcium kidney stones.
Methods
This is a cross-sectional study including 142 patients who were divided in two groups: Group 1 (patients with recurrent calcium kidney stones) and Group 2 (patients with osteopenia/osteoporosis in the lumbar spine or hip). Analyses of bone mineral density, calcium–phosphorous and bone metabolism and lithogenic risk factors in fasting urine samples and 24-h urine samples were performed. Statistical analysis was carried out with SPSS 17.0. A p ≤ 0.05 was considered statistically significant.
Results
Patients in Group 2 presented greater loss of bone mineral density and more elevated alkaline phosphatase, iPTH, phosphorous and β-crosslaps levels, as compared to patients in Group 1. However, Group 1 presented greater urine calcium, oxalate and uric acid and a higher proportion of hypocitraturia, hypercalciuria and hyperoxaluria, as compared to Group 2. Multivariate analysis revealed that advanced age and β-crosslaps levels are risk factors for bone mineral density loss, while low urinary calcium excretion was protective against bone demineralization.
Conclusion
Patients with osteopenia/osteoporosis without lithiasis present some urinary biochemical alterations. This would explain the lack of lithogenic activity, although low calcium excretion in 24-h urine samples is a protective factor against the loss of bone mineral density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic calcium kidney stones are associated with hypercalciuria and produce bone mineral density loss in a significant proportion of patients [1]. The factor most related with the bone mineral density loss and with alterations in bone remodeling markers is fasting hypercalciuria, defined as a fasting calcium/creatinine ratio greater than 0.11 [2]. Up to 54 % of patients with calcium stones present osteopenia, while 14 % present osteoporosis [3]. The risk of osteoporotic bone fracture is increased with the presence of stones. Therefore, calcium kidney stones can be considered a risk factor for osteoporotic fracture [4]. The diagnosis of bone mineral density loss in patients with lithiasis can measure using dual-energy X-ray absorptiometry that calculate bone density, although bone remodeling marker levels—which can be measured in urine or blood samples—can be used to follow up these patients due to bone densitometry is recommended every 2 years. The bone remodeling markers have been elevated in patients with osteopenia/osteoporosis and stones; however, dual-energy X-ray absorptiometry is the gold standard test to diagnose bone mineral density loss [5–9].
A previous study demonstrated that patients with recurrent kidney stones and severe lithogenic activity presented a high percentage of bone mineral density loss, which can reach 50 % of osteopenia in the hip and up to 70 % in the lumbar spine [10]. The bone resorption marker, β-crosslaps and fasting calcium/creatinine ratio are generally elevated in these patients [9, 11]. We observed in other study that a high proportion of patients with lithiasis and osteopenia/osteoporosis presented hypercalciuria and an elevated fasting calcium/creatinine ratio [10]. This is consistent with previous studies that indicated a fasting calcium/creatinine ratio above 0.25 represents a 3.8-fold increase in the risk of bone mineral density loss in patients with kidney stones [7].
Therefore, it seems to be a clear relationship between recurrent calcium kidney stones and bone mineral density loss. However, patients with bone mineral density loss do not necessarily develop stones, which led us to analyze and compare the essential biochemical differences between a group of patients with calcium kidney stones versus a group of patients with osteopenia/osteoporosis in the lumbar spine or hip.
The objective was to determine biochemical alterations in urine in patients with osteopenia/osteoporosis without calcium kidney stones compared with patients with calcium kidney stones.
Materials and methods
Study groups
A cross-sectional study was performed from January 2013 to December 2013 including 142 patients from Eastern Andalusia, Spain, who were divided in two groups:
-
Group 1: A total of 75 patients with a diagnosis of recurrent kidney stones aged 18–70 years.
-
Group 2: A total of 67 patients with a diagnosis of osteopenia/osteoporosis aged 18–70 years.
Inclusion criteria: Men and women aged 18–70 years (calcium stones is more frequent in this range of age) with recurrent calcium lithiasis or osteopenia/osteoporosis. Recurrent calcium stones are related to bone mineral density loss and with bone metabolic disorders. Recurrent calcium stones are considered patients with two or more episode of calcium stones in different period of time.
Exclusion criteria: Patients aged <18 or >70 years, congenital bone disease, congenital kidney disease, hyperparathyroidism, inflammatory bowel disease, renal tubular acidosis, biphosphonate treatment, hormone replacement therapy, thiazide therapy, potassium citrate therapy, corticosteroid therapy and calcium and Vitamin D therapy or any possible situation which may interfere calcium metabolism (e.g., immobilization syndrome).
Methods
All patients were submitted to anamnesis, physical examination and measurement of weight, height, BMI and blood pressure. Bone mineral density was assessed by dual-energy X-ray absorptiometry.
Subsequently, serum and urinary biochemical analyses were performed along with bone densitometry of the femur and lumbar spine.
-
Blood tests: Creatinine, uric acid, sodium, potassium, chlorine, calcium, phosphorous, intact parathyroid hormone (PTHi), alkaline phosphatase, osteocalcinFootnote 1 and β-crosslaps.Footnote 2
-
Fasting urine tests: Density, pH, calcium, creatinine, oxalate, citrate, uric acid, calcium/creatinine.
-
24-h urine tests: Diuresis volume, creatinine, urea, uric acid, sodium, potassium, chlorine, calcium, phosphorous, citrate, oxalate, calcium/creatinine, calcium/citrate. Hypercalciuria defined as urinary excretion of more than 260 mg per day; hypocitraturia defined as urinary excretion of less than 320 mg per day; hyperoxaluria defined as urinary excretion of more than 40 mg per day; and hyperuricosuria defined as urinary excretion of more than 750 mg per day.
-
Bone densitometry by dual-energy X-ray absorptiometry using Hologic QDR 4500 equipment.
Statistical analysis
Student’s t test was performed for qualitative–quantitative variable analysis. Chi-square test and binary logistic regression were performed for qualitative variable analysis. Results were obtained by OR, with a 95 % confidence interval. The Kolmogorov–Smirnov test was performed to attest the normality of the variables; variance analysis was performed by Levene’s test. A p ≤ 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS 17.0 for Windows.
Ethical considerations
Informed consent was obtained from all patients. This study was approved by the Ethics Committee of the San Cecilio University Hospital, Granada, Spain, and was performed with the financial support of the Fundación Progreso y Salud (Andalusian Regional Government, Spain).
Results
The mean age was 43.08 ± 11.59 in Group 1 and 51.88 ± 11.27 in Group 2 (p = 0.0001). Men and women were similarly distributed (39 men/36 women in Group 1 and 35 men/32 women in Group 2).
Bone densitometry (see Table 1) revealed a greater loss of bone mineral density either in the femur or in the lumbar spine in Group 2 versus Group 1. In relation to serum calcium–phosphorous metabolism, we observed that patients in Group 2 presented higher alkaline phosphatase, PTHi, β-crosslaps levels and phosphorous levels, compared with Group 1 (Table 1).
In the 24-h urine samples, a higher urine calcium, oxalate and uric acid excretion in Group 1 versus Group 2 was observed (Table 2). In fasting urine samples, higher urine oxalate excretion and lower citrate excretion in Group 1 versus Group 2 were observed (Table 2).
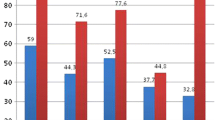
In relation to biochemical alterations in urine, according to Table 3, Group 1 presented a higher percentage of hypercalciuria, hyperoxaluria and hypocitraturia than Group 2 (Fig. 1).
A sub-analysis was performed in group 1 dividing patients according to the presence or not of osteopenia/osteoporosis (T score < −1), noting that patients with osteopenia/osteoporosis with kidney stones have higher urinary calcium levels versus patients with kidney stones without osteopenia/osteoporosis (283.3 ± 112.3 vs. 224.5 ± 127.3, respectively; p = 0.04). There have been no statistically significant differences in levels of citrate, oxalate and urinary uric.
Multivariate binary logistic regression analysis revealed that increased β-crosslap levels and an advanced age are independently related with the bone mineral density loss, while a low 24-h urine calcium excretion is protective against bone demineralization.
Discussion
Recurrent calcium kidney stones are associated with bone mineral density loss [1], altered bone remodeling markers, hypercalciuria [12], hypocitraturia and an increased calcium/creatinine ratio in 24-h urine samples [13]. Some studies [6] indicate that there is a statistically significant negative correlation between urine calcium excretion and Z score densitometry changes in the femoral neck after a 3-year follow-up. This confirms that hypercalciuria might be a predictive factor of bone mineral density loss in the femoral neck. This is consistent with the results obtained in our study due to multivariate analysis revealed that a low urine calcium excretion is a protective factor against the bone mineral density loss. Therefore, calciuria plays an essential role in the pathogenesis of osteopenia/osteoporosis and should be considered in the diagnosis of patients although they have no calcium kidney stones. It is evident that the presence of osteopenia/osteoporosis—which is present in a significant proportion of recurrent calcium stones patients with hypercalciuria [14]—does not determine lithogenesis by itself. In this study, patients in Group 2 presented a lower proportion of alterations in lithogenic urinary factors, versus Group 1. Hypercalciuria was found in 50.4 % of patients in Group 1, while it was present in 25 % of patients in Group 2, and it was the most frequent lithogenic risk factor presented. It is notable that we are talking about fasting hypercalciuria, due to the increased fasting calcium/creatinine ratio in both groups. This is associated in most cases with an intrinsic bone disorder that may represent a fourfold increase in the risk of fracture in patients with hypercalciuria and lithiasis [15]. Consequently, hypercalciuria is the most important lithogenic factor, and it is associated with the bone mineral density loss when it is not related with diet [16, 17]. In agreement with previous studies performed by our research group [1, 18], we did not observe a clear and direct influence of iPTH and Vitamin D on calcium stones or bone mineral density. Nevertheless, iPTH was slightly higher in Group 2, but within normal limits. However, bone remodeling markers—especially the resorption marker β-crosslaps—were increased in both groups above the threshold (0.311 ng/ml) established in other studies [19]. So, although we found no alterations in Vitamin D levels, we cannot deny that there might be an increase in Vitamin D receptors’ sensitivity, as other studies indicate [20–22]. This sensitivity would condition the loss of bone mineral density due to increased bone resorption. Hypocitraturia is another risk factor that affects 25 % of patients with calcium kidney stones. However, it is only found in 7.5 % of patients with osteopenia/osteoporosis. We have observed in other studies [13] that low urine citrate levels are an important determinant of bone mineral density loss and lithiasis. This is probably due to a latent state of metabolic acidosis that produces citrate consumption as a buffer effect. Hyperoxaluria is the third most frequent metabolic alteration in urine in Group 1 patients, where it is increased in 21 % of patients, versus 9 % in Group 2 patients. Finally, we observed that patients in Group 2 presented fewer alterations in the urine metabolic analysis, with a low percentage of hypercalciuria and hyperoxaluria, and a very low percentage of hypocitraturia. This means that we found no important urinary alterations that conditioned the presence of stones. However, multivariate analysis revealed that low urine calcium excretion is a protective factor against bone mineral density loss; therefore, it should be considered at diagnosis, due to increased calciuria, and its ratios (calcium/creatinine and calcium/citrate) may produce the appearance of stones and the gradual bone mineral density loss. This study revealed the importance of calciuria in patients with osteopenia/osteoporosis without stones, because, probably the hypercalciuria was the first phenomenon previously to appear the calcium stones. However, is necessary to perform a prolonged follow-up to determine exactly whether osteopenia/osteoporosis, hypercalciuria and later stones are the correct order about the physiopathology of calcium stones related to bone mineral density loss.
Conclusion
Patients with osteopenia/osteoporosis without lithiasis present some urinary biochemical alterations. This would explain the lack of lithogenic activity, although low calcium excretion in 24-h urine samples is a protective factor against the loss of bone mineral density.
Notes
Determined by schimioiluminescence using the automatic analyzer LIAISON-Osteocalcin (DIASORIN).
Determined by schimioiluminescence immunoassay “ECLIA” using the automatic analyzer Elecsys MODULAR ANALYTICS E170—(Roche Diagnostic).
References
Arrabal Polo MA, Arrabal Martín M, De Haro Muñoz T et al (2011) Mineral density and bone remodeling markers in patients with calcium lithiasis. BJU Int 108:1903–1908
Tasca A, Cacciola A, Ferrarese P et al (2002) Bone alterations in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Urology 59:865–869
Caudarella R, Vescini F, Buffa A et al (2003) Bone mass loss in calcium stone disease: focus on hypercalciuria and metabolic factors. J Nephrol 16:260–266
Lauderdale DS, Thisted RA, Wen M et al (2001) Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res 16:1893–1898
Kuczera M, Wiecek A, Kokot F (1997) Markers of bone tumover in patients with nephrolithiasis. Int Urol Nephrol 29:523–529
Asplin JR, Donahue S, Kinder J et al (2006) Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int 70:1463–1467
Letavernier E, Traxer O, Daudon M et al (2011) Determinants of osteopenia in male renal-stone–disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol 6:1149–1154
Arrabal Polo MA, Arrabal Martín M, De Haro Muñoz T et al (2012) Biochemical determinants of severe lithogenic activity in patients with idiopathic calcium nephrolithiasis. Urology 79:48–54
Arrabal Polo MA, Arrabal Martin M, Poyatos Andujar A et al (2012) Is the fasting calcium/creatinine a bone resorption marker in patients with calcium renal stones? Urol Res 40:243–245
Arrabal Polo MA, Arrabal Martín M, Giron Prieto MS et al (2012) Osteopenia/osteoporosis in patients with calcium nephrolithiasis. Urol Res 40:709–716
Arrabal Polo MA, Arias Santiago S, Arrabal Martín M (2012) What is the value of bone remodeling markers in patients with calcium stones? Urol Res 40:803
Arrabal-Polo MÁ, Sierra Girón-Prieto M, Orgaz-Molina J et al (2013) Calcium renal lithiasis and bone mineral density. Importance of bone metabolism in urinary lithiasis. Actas Urol Esp 37:362–367
Arrabal Polo MA, Arrabal Martin M, Arias Santiago S et al (2013) Importance of citrate and the calcium: citrate ratio in patients with calcium renal lithiasis and severe lithogenesis. BJU Int 111:622–627
Caudarella R, Vescini F, Buffa A et al (2004) Osteoporosis and Urolithiasis. Urol Int 72(suppl 1):17–19
Ryan LE, Ing SW (2012) Idiopathic hypercalciuria and bone health. Curr Osteoporos Rep 10:286–295
Asplin JR, Bauer KA, Kinder J et al (2003) Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int 63:662–669
Tsuji H, Umekawa T, Kurita T et al (2005) Analysis of bone mineral density in urolithiasis patients. Int J Urol 12:335–339
Arrabal Polo MA, Arrabal Martin M, Arias Santiago S et al (2012) Metabolic-mineral study in patients with renal calcium lithiasis, severe lithogenic activity and loss of bone mineral density. Singap Med J 53:808–813
Arrabal Polo MA, Arrabal Martin M, Giron Prieto MS et al (2013) Association of severe calcium lithogenic activity and bone remodeling markers. Urology 82:16–21
Moyano MJ, Gómez de Tejada MJ, García Lozano R et al (2007) Alteraciones en el metabolismo mineral óseo en pacientes con urolitiasis de repetición y polimorfismos del gen del receptor de la vitamina D. Resultados preliminares. Nefrologia 27:694–703
Bilic-Curcic I, Milas-Ahic J, Smolic M et al (2009) Urolithiasis and osteoporosis: clinical relevance and therapeutic implications. Coll Antropol 33(2):189–192
Krieger NS, Bushinsky DA (2013) The relation between bone and stone formation. Calcif Tissue Int 93:374–381
Acknowledgments
This article is part of Doctoral Thesis of María de la Sierra Giron-Prieto. This article and investigation has been funded by Fundación Progreso y Salud. Junta de Andalucía. PI 0766/2013.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arrabal-Martin, M., Poyatos-Andujar, A., del Carmen Cano-García, M. et al. The importance of calciuria as lithogenic factors in patients with osteopenia/osteoporosis. Int Urol Nephrol 47, 445–449 (2015). https://doi.org/10.1007/s11255-015-0918-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0918-z