Abstract
Environmental and genetic factors are important in development of nephrolithiasis. In a recent study, it has been demonstrated that hepatocyte growth factor (HGF) has an anti-apoptotic effect and thus can reduce the adhesion of calcium oxalate monohydrate crystals to renal epithelial cells. The aim of this study was to evaluate the HGF serum levels and its two gene polymorphisms and possible association of the two in patients with nephrolithiasis. One hundred and five patients with nephrolithiasis and 70 healthy volunteers with similar demographic features were included in this study. Serum HGF levels were measured, and HGF intron 13 C>A (in 102 stone patients and 68 healthy subjects) and intron 14 T>C (in 99 stone patients and 56 healthy subjects) polymorphisms were determined using real-time polymerase chain reaction with TaqMan allelic discrimination method. There were no statistically significant differences in HGF intron 13 C>A and intron 14 T>C polymorphisms between the control and patient groups (X 2 = 1.72 df = 2; p = 0.42, and X 2 = 0.68 df = 2; p = 0.71, respectively). Mean serum HGF concentration was significantly lower in the stone disease patients than in the control subjects (1.05 ± 0.63 pg/mL and 1.35 ± 0.58 ng/mL respectively, p = 0.0001). When allele distribution frequency between stone patients and healthy subjects was compared, there were no significant differences in intron 13 and intron 14 allele distributions between two groups (p = 0.43 and p = 0.44, respectively). It may be concluded from the findings that decrease in HGF levels may play a role in renal stone formation, independent from gene polymorphisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract stone disease is a complex, multifactorial disorder with an incidence of >10 % in general population and the incidence may have some geographical variations [1]. The anatomic, genetic and environmental factors, which include dietary intake of salt, protein, calcium and other nutrients, fluid intake, urinary tract infections, socioeconomic status of the individual, lifestyle and climate, are important in development of this disease [2]. The prevalence of symptomatic kidney stone formation is 10 % for men and 5 % for women. After a first stone, the risk of recurrence is 40 % by 5 years, and 75 % by 20 years [3]. Calcium is a major component of 85 % of kidney stones, largely as either calcium oxalate or calcium phosphate [3, 4].
The stone formation mechanism is not yet clearly understood. It is thought that adhesion of calcium oxalate monohydrate (COM) crystals to the surface of renal tubular cells and the subsequent cellular response may constitute critical pathogenic steps in the development of nephrolithiasis [5]. High levels of oxalate and COM crystals lead to injury and apoptosis in renal tubular cells, which thus increases crystal adhesion to the renal epithelial cells [5–7].
Although initially thought to be a liver-specific mitogen, hepatocyte growth factor (HGF) was later reported to have mitogenic, motogenic (enhancement of cell motility), morphogenic, proliferative and anti-apoptotic activities in various cell types [5, 8–11]. HGF has protection and regeneration functions in various organs and systems such as kidney, liver, lung, gastrointestinal tissues, and cardiovascular, cutaneous and nervous systems [12, 13]. For this reason, blood and tissue levels may increase after acute injury and illness [13].
Tei et al. [5] have shown that HGF reduces cell damage and apoptosis formed by oxalate and COM crystals and inhibits adhesion of COM crystals to the cells of renal tubules in stone-forming rats. Furthermore, they determined that HGF gene transfer significantly reduced crystal deposits and the number of apoptotic cells on the renal tubules in stone-forming rat kidneys [5].
Studies have shown that stone disease is a polygenic disease and calcification inhibitor protein genes such as osteopontin [14], fetuin-A [15], and matrix Gla protein [16] and polymorphisms of urokinase [17] and claudin-14 gene [18] may be in important development of urinary tract stones. Similarly, some receptor single nucleotide polymorphisms associated with calcium metabolism, such as calcium-sensing receptor [19] and vitamin D receptor [20], were investigated in nephrolithiasis cases. There is no clear evidence about the role of HGF gene polymorphism in stone disease.
HGF gene has been associated with kidney stone formation in experimental rat studies [5]. The intron 13 C>A and intron 14 T>C substitutions are relatively common polymorphisms in HGF gene, and these polymorphisms have been related to several diseases including essential hypertension [21].
The aim of this study was to evaluate the possible association of hepatocyte growth factor serum levels in nephrolithiasis cases. We also examined its two polymorphisms in these patients.
Patients and methods
One hundred and five patients (68 males, 37 females) with nephrolithiasis and stone composition of COM or calcium oxalate dihydrate, who were treated at Urology Department of Ataturk University, and 70 healthy volunteers with similar demographic features, were included in the study. The control group consisted of healthy volunteers without a history of kidney stone or family history of stone disease. Stone samples were obtained either after extracorporeal shock wave lithotripsy or surgery for treatment, and all patients had stone analysis by X-ray diffraction.
The study protocol was approved by the local institutional ethics board, and written informed consent was obtained from all subjects before participation. Before any intervention, blood samples were taken after an overnight fasting. Serum samples obtained from blood were stored at −80 °C until HGF assay. Genomic DNA was extracted from blood samples in EDTA tubes using a commercial spin column kit (PureLink Genomic DNA Mini Kit, Invitrogen, Inc. Carlsbad, CA).
Analyses of the HGF gene C>A polymorphism (rs2074725) in intron 13 and T>C polymorphism (rs2074724) in intron 14 were performed by TaqMan allelic discrimination method using real-time polymerase chain reaction (Applied Biosystems 7300). Allele specific TaqMan probes that utilized in analysis process were as follows: for intron 13 C/A of HGF gene 5′-FAM-GCACAAATTATAGTCCAGAGCTTACcGTCTGGCAAGCAGATGTGATCAGCT-Tamra-3′ and 5′-VICGCACAAATTATAGTCCAGAGCTTACaGTCTGGCAAGCAGATGTGATC AGCT-Tamra-3′; forward primer 5′-CTACCTCTGGAGGCACAAATTA-3′ and reverse primer 5′-GGGTACAACCTTCAGGACCA-3′ and for intron 14 T/C 5′-FAM-CTACAGGAGAAAGAAGTAGTGAGGAtTGAAAAAAGCCTATTGACAATTTAG-Tamra-3′ and 5′-VIC-CTACAGGAGAAAGAAGTAGTGAGGAcTGAAAAAAGCCTATTGACA ATTTAG-Tamra-3′.
HGF in serum samples were measured using an ELISA (BioVendor, GmbH, Heidelberg, Germany) according to the manufacturer’s standard protocol. Intra-assay and inter-assay variations were 4.8 % and 5.4 %, respectively. Serum HGF levels were calculated as pg/mL.
Statistical analysis
Statistical analysis was done using SPSS version 11.5 (SPSS Inc, Chicago, USA). Distribution of continuous variables was determined with Kolmogorov–Smirnov test. Comparison of HGF levels was analyzed between the groups using Mann–Whitney U test and Kruskal–Wallis test. Chi-squared test was used for comparison of the distribution of HGF gene polymorphism. p value <0.05 was regarded the statistically significant.
Results
A total of 105 patient samples and 70 control samples were analyzed for serum HGF levels. Mean serum HGF concentration was significantly lower in the stone disease patients than in control subjects (1.05 ± 0.63 ng/mL for patient group and 1.35 ± 0.58 ng/mL for control group, p = 0.0001).
Genotype distributions at HGF intron 13 C>A and intron 14 T>C gene polymorphisms in control and patient groups are shown in Table 1. There were no statistically significant differences in these gene polymorphisms between the control and patient groups (p = 0.42, and p = 0.71, respectively). When the allele distribution frequency between stone patients and healthy subjects were compared, there were no significant differences in intron 13 and intron 14 allele distributions between two groups (p = 0.43 and p = 0.44, respectively) (Table 2). For HGF intron 13 C>A, genotype could not be detected in three patients and two controls, thus the results of 102 patients and 68 control subjects were statistically analyzed. Also, intron 14 T>C genotype could be determined in 99 patients and 56 control subjects and statistical evaluation was done in these subjects.
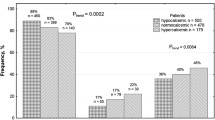
In addition, the effect of HGF intron 13 C>A and HGF intron 14 T>C genotypes on serum levels of HGF was investigated. For HGF intron 13 C>A, serum HGF levels were not significantly different between CC, CA and AA genotype carriers among healthy subjects and patients with urinary tract stones (p = 0.78 for the control group, p = 0.44 for the patient group). In both groups, the serum HGF levels were not significantly different among TT, CT and CC genotype carriers for HGF intron 14 T>C genotypes (p = 0.22 for the control group, p = 0.12 for the patient group) (Fig. 1).
Further, comparison of CC genotype patients with A allele carriers at rs2074725 polymorphism in HGF intron 13 showed no significant difference in terms of serum HGF levels (p = 0.42, Z = −0.79). Also comparison of TT genotype patients with the C allele carriers at rs2074724 polymorphism in HGF intron 14 showed no significant difference in terms of serum HGF levels (p = 0.60, Z = −0.51) (Fig. 2; Table 3).
Discussion
In the present study, we investigated the importance of C>A polymorphism in HGF gene intron 13 and T>C polymorphism in intron 14 in patients with urinary tract stone disease. We detected that C>A polymorphism in HGF gene intron 13 and T>C polymorphism in intron 14 did not correlate with urinary tract stone disease. Additionally, A and C allele frequency for intron 13 and C and T allele for intron 14 in HGF gene of patients was not different from healthy controls. Furthermore, in patients with nephrolithiasis, serum HGF concentrations were significantly lower when compared to the control group. In addition, we found that HGF intron 13 and 14 genotypes had no effect on serum HGF levels.
It has been suggested that HGF’s anti-apoptotic effect might result in the reduction of adhesion of COM crystals [5]. Furthermore, patients with nephrolithiasis had lower levels of serum HGF than the healthy control group in our study. It may be speculated that decrease in HGF production may be important factor in the formation of stone in kidneys.
Recent studies have stated that HGF is a multifunctional molecule [22–24]. HGF gene polymorphism studies are limited in the literature. Polymorphism studies were performed in diseases such as hypertension [21], myopia [25, 26], autism [27] and retinopathy of prematurity [28], and some HGF gene region polymorphisms were found to be associated with these diseases. For example, Motone et al. [24] studied HGF gene C/A substitution in intron 13, T/C substitution in intron 14 and T/A substitution in intron 8 in patients with essential hypertension. They found that genotype distributions are 83 % for CC, 16 % for CA and 1 % for AA in HGF gene intron 13 C/A. They have suggested that the prevalence of hypertension was significantly higher in individuals with the CC genotype, and the presence of A allele reduced the risk of essential hypertension in lean or female subjects. In the same study, authors could not find any significant association between the distributions of allele with hypertension. Additionally in some studies, it has been shown that serum HGF concentration was significantly higher in the hypertensive subjects than in the normotensive ones [29, 30]. HGF gene polymorphisms did not affect the risk of development of autism in many regions of the intron from 1 to 15 is shown by the Toyoda et al. [27].
HGF seems to function in protection of the myocardium with preventing apoptosis of myocardial cells and by increasing the formation of a blood vessel in ischemic field [31]. Studies have shown that serum HGF levels were markedly elevated in patients with acute myocardial infarction [31, 32]. Chen et al. [33] showed that HGF gene transfer before ischemia reduced the myocardial infarct size and protected left ventricular function via multiple beneficial actions, such as antioxidant, anti-apoptotic, and anti-fibrotic actions, and increased expression of bcl-2 and angiogenesis.
It may be concluded from our findings that decrease in HGF levels has a role in renal stone formation independently from gene polymorphisms. We think that decrease of HGF levels may be a cause of the crystal precipitation via increase of apoptosis in kidney cells. Further studies investigating polymorphism of HGF gene regions different from this study are need.
Abbreviations
- COM:
-
Calcium oxalate monohydrate
- HGF:
-
Hepatocyte growth factor
References
Tiselius HG (2003) Epidemiology and medical management of stone disease. BJU Int 91(8):758–767
Stechman MJ, Loh NY, Thakker RV (2009) Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol 24(12):2321–2332
Worcester EM, Coe FL (2010) Clinical Practice Calcium Kidney Stones. N Engl J Med 363(10):954–963
Favus MJ (2011) The risk of kidney stone formation: the form of calcium matters. Am J Clin Nutr 94(1):5–6
Tei N, Tsujihata M, Tsujikawa K, Yoshimura K, Nonomura N, Okuyama A (2006) Hepatocyte growth factor has protective effects on crystal-cell interaction and crystal deposits. Urology 67(4):864–869
Khan SR, Byer KJ, Thamilselvan S, Hackett RL, McCormack WT, Benson NA, Vaughn KL, Erdos GW (1999) Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol. Suppl 14:457–463
Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M (2005) Oxalate toxicity in renal cells. Urol Res 33(5):329–339
Madonna R, Cevik C, Nasser M, De Caterina R (2012) Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb Haemost 107(4):656–661
Nakamura T, Nawa K, Ichihara A (1984) Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 122(3):1450–1459
Aoki S, Takahashi K, Matsumoto K, Nakamura T (1996) Molecular cloning of the Xenopus c-met/hepatocyte growth factor receptor and its regional expression during early development. J Biochem 120(5):961–968
Boros P, Miller CM (1995) Hepatocyte growth factor: a multifunctional cytokine. Lancet 345(8945):293–295
Nakamura T, Sakai K, Nakamura T, Matsumoto K (2011) Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol 26(suppl 1):188–202
Matsumoto K, Nakamura T (2001) Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int 59(6):2023–2038
Gao B, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K (2005) A polymorphism of the osteopontin gene is related to urinary calcium stones. J Urol. 174(4Pt 1):1472–1476
Aksoy H, Aksoy Y, Ozturk N, Aydin HR, Yildirim AK, Akçay F (2010) Fetuin-A gene polymorphism in patients with calcium oxalate stone disease. Urology 75(4):928–932
Gao B, Yasui T, Itoh Y, Tozawa K, Hayashi Y, Kohri K (2007) A polymorphism of matrix Gla protein gene is associated with kidney stones. J Urol 177(6):2361–2365
Ozturk M, Kordan Y, Cangul H, Dogan HS, Kilicarslan H, Vuruskan H, Oktay B (2008) Association of urokinase gene 3′-UTR T/C polymorphism with calcium oxalate urolithiasis in children. Int Urol Nephrol 40(3):563–568
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF et al (2009) Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41(8):926–930. doi:10.1038/ng.404
Besiroglu H, Sahin S, Otunctemur A, Ozbek E (2014) Calcium-sensing receptor gene polymorphisms in patients with calcium urolithiasis: a systematic review. Ren Fail 36(8):1187–1192. doi:10.3109/0886022X.2014.937673
Liu W, Chen M, Li M, Ma H, Tong S, Lei Y, Qi L (2014) Vitamin D receptor gene (VDR) polymorphisms and the urolithiasis risk: an updated meta-analysis based on 20 case-control studies. Urolithiasis 42:45–52. doi:10.1007/s00240-013-0619-y
Motone M, Katsuya T, Ishikawa K, Iwashima Y, Sugimoto K, Yamamoto K, Fu Y, Matsuo A, Ohishi M, Rakugi H, Ogihara T (2004) Association between hepatocyte growth factor gene polymorphism and essential hypertension. Hypertens Res 27(4):247–251
Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV (2008) A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab 294(2):336–344
Mizuno S, Matsumoto K, Nakamura T (2008) HGF as a renotrophic and anti-fibrotic regulator in chronic renal disease. Front Biosci 1(13):7072–7086
Zhou D, Tan RJ, Lin L, Zhou L, Liu Y (2013) Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 84(3):509–520
Han W, Yap MK, Wang J, Yip SP (2006) Family-based association analysis of hepatocyte growth factor (HGF) gene polymorphisms in high myopia. Invest Ophthalmol Vis Sci 47(6):2291–2299
Yanovitch T, Li YJ, Metlapally R, Abbott D, Viet KN, Young TL (2009) Hepatocyte growth factor and myopia: genetic association analyses in a Caucasian population. Mol Vis. 20(15):1028–1035
Toyoda T, Nakamura K, Yamada K, Thanseem I, Anitha A, Suda S, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, Iwata Y, Suzuki K, Matsuzaki H, Kawai M, Sekine Y, Tsuchiya K, Sugihara G, Ouchi Y, Sugiyama T, Takei N, Yoshikawa T, Mori N (2007) SNP analyses of growth factor genes EGF, TGFbeta-1, and HGF reveal haplotypic association of EGF with autism. Biochem Biophys Res Commun. 360(4):715–720
Kaya M, Çokakli M, Berk AT, Yaman A, Yesilirmak D, Kumral A, Atabey N (2013) Associations of VEGF/VEGF-receptor and HGF/c-Met promoter polymorphisms with progression/regression of retinopathy of prematurity. Curr Eye Res 38(1):137–142
Nakamura S, Moriguchi A, Morishita R, Aoki M, Yo Y, Hayashi S, Nakano N, Katsuya T, Nakata S, Takami S, Matsumoto K, Nakamura T, Higaki J, Ogihara T (1998) A novel vascular modulator, hepatocyte growth factor (HGF), as a potential index of the severity of hypertension. Biochem Biophys Res Commun. 242(1):238–243
Nakamura Y, Morishita R, Nakamura S, Aoki M, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T (1996) A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension 28(3):409–413
Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H, Nakamura T (2000) Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 106(12):1511–1519
Matsumori A, Furukawa Y, Hashimoto T, Ono K, Shioi T, Okada M, Iwasaki A, Nishio R, Sasayama S (1996) Increased circulating hepatocyte growth factor in the early stage of acute myocardial infarction. Biochem Biophys Res Commun 221(2):391–395
Chen XH, Minatoguchi S, Kosai K, Yuge K, Takahashi T, Arai M, Wang N, Misao Y, Lu C, Onogi H, Kobayashi H, Yasuda S, Ezaki M, Ushikoshi H, Takemura G, Fujiwara T, Fujiwara H (2007) In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J Card Fail 13(10):874–883
Acknowledgments
This study was financially supported by a research fund from the Scientific and Technological Research Council of Turkey (TUBITAK) (Project number: 107S273). This study has been accepted as a poster presentation in the “American Association for Clinical Chemistry (AACC) Annual Meeting on July 25–29, 2010, California, USA”.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozturk, N., Aksoy, H., Aksoy, Y. et al. The low levels of circulating hepatocyte growth factor in nephrolithiasis cases: independent from gene polymorphism. Urolithiasis 43, 427–432 (2015). https://doi.org/10.1007/s00240-015-0793-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0793-1