Abstract
Background
Carpal tunnel syndrome (CTS) is the most frequent and well-known form of median nerve entrapment and accounts for 90% of all entrapment neuropathies. The outcomes of CTS release surgery are usually evaluated with patient-reported outcome measures. To compare the accuracy of Quick-DASH versus CTS-6 evaluation tools in assessing the outcome of surgical treatment for carpal tunnel syndrome.
Methods
We conducted a study involving 60 cases undergoing carpal tunnel release at our institute to consider the accuracy of QUICK-DASH and CTS-6 evaluation tools. The results were assessed by self-administering questionnaires filled by patients on 3 different occasions: pre-operatively, 1 month, and 6 months post-operatively. The accuracy of QUICK-DASH and CTS-6 assessment tools was analyzed independently using the dependent t-test and Wilcoxon matched pairs test.
Results
A positive correlation was found between the mean change in both Quick-DASH and CTS-6 scoring systems. However, CTS-6 showed higher responsiveness to changes from baseline to 1 month and 6 months respectively compared to Quick-DASH.
Conclusions
Quick-DASH and the CTS-6 evaluation tools both are highly responsive to change after surgery for carpal tunnel syndrome and reflect the clinical improvement in terms of disabilities and symptoms respectively. The higher responsiveness to CTS-6 could be attributed to the fact that the CTS-6 is a disease-specific measure of symptoms, whereas the Quick-DASH is a region-specific measure of function. Our study provides additional support for CTS-6 given accessing treatment outcomes, as it is easier and less time-consuming to adapt.
Level of evidence: Not gradable
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) remains a common disabling condition frequently encountered by hand surgeons. It is defined as symptomatic compression neuropathy of the median nerve at the level of the wrist caused by mechanical distortion due to a compressive force [1]. It is the most frequent and well-known form of median nerve entrapment and accounts for 90% of all entrapment neuropathies [1, 2]. The characteristic symptoms are numbness, tingling, and pain in the hand, and difficulties in performing daily and work-related activities [3] CTS affects 3–4% of the general population and is more common in middle-aged and obese patients with a female preponderance [4]. Diseases like hypothyroidism, diabetes, and rheumatoid arthritis also carry an increased risk of developing carpal tunnel syndrome [4]. Open carpal tunnel release is considered as a gold standard method and known to give good-to-excellent results for treating the majority of the patients [4].
Outcomes of CTS release surgery are usually evaluated with patient-reported outcome measures [3]. An ideal evaluation tool needs are reproducible, valid, with internal consistency, and able to respond to clinical changes [5]. The carpal tunnel syndrome (CTS-6) and the Disabilities of the Arm, Shoulder, and Hand (QUICK-DASH) are the most representative specific instruments used in hand surgery. CTS-6 is a disease-specific measure of symptoms, whereas the QUICK-DASH is a region-specific measure of function [3]. Previous literature has studied these two assessment tools independently but very few studies have compared these two tools and their results are conflicting. We conducted this study intending to assess the responsiveness of QUICK-DASH and CTS-6 in evaluating the outcome after carpal tunnel release and compare them.
Materials and methods
Study population
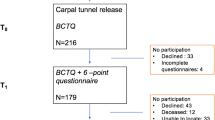
We conducted this prospective study over 2 years: July 2016 to August 2018 at our tertiary care center. Sixty patients undergoing carpal tunnel release by the open method were included in the study.
-
Inclusion criteria: Patients undergoing open carpal tunnel release surgery
-
Exclusion criteria: Patients with known neurological diseases and known bone disorders with affected upper limb.
The diagnosis was based on clinical evaluation and ultrasonography. Before surgery, all patients completed a questionnaire consisting of the Quick-DASH and the CTS-6. A follow-up questionnaire consisting of the Quick-DASH and the CTS-6 tools reflecting the post-operative change in clinical symptoms and functional status of hand were filled at the interval of 1 month and 6 months respectively.
The study was approved by Institutional Ethical Committee. All the carpal tunnel release surgeries were performed by highly experienced plastic surgeons.
Assessment tools
QUICK-DASH score
This scale is an 11-item measure of upper extremity-related disability. The Quick-DASH is not disease specific but rather region specific and reflects activity limitation. According to conventional scoring, the Quick-DASH is scored from 0 (no disability) to 100 (most severe disability), with only 1 missing item response allowed [6].
CTS-6 score
This scale consists of six items that reflect severity and frequency of night and daytime numbness as well as tingling and pain. The CTS-6 measures severity of the symptoms related to CTS. The CTS-6 is scored on a scale from 1 (no symptoms) to 5 (most severe symptoms), with only 1 missing item response allowed [7].
Statistical analysis
The normality of Quick-DASH and CTS-6 tool was evaluated using Kolmogorov–Smirnov test and regression analysis. The comparison of baseline (pre-operative), 1 month, and 6 months post-operative Quick-DASH scores was done by dependent t-test while the comparison of baseline, 1 month, and 6 months post-operative CTS-6 scores was done by Wilcoxon matched pairs test. Comparison between DASH with CTS-6 scores was done by Spearman’s rank correlation coefficient method.
Results
Demographic characteristics and primary details
The mean age of participants in the study was 45.93 ± 9.56 years. The male to female ratio was 1:1.22. A total of 41 (68.33) patients presented with right-sided CTS, 17 (28.33%) presented with left-sided CTS while 2 (3.33%) patients had bilateral CTS.
Normality of Quick-DASH and CTS-6
Both the Quick-DASH and CTS-6 scores at baseline, 1 month, and 6 months followed a normal distribution (as per Kolmogorov–Smirnov test and regression analysis respectively).
Comparison of Quick-DASH scores at pre-operative, 1 month, and 6 months post-operative
There was a statistically significant change between the Quick-DASH scores at pre-operative values and 1 month and 6 months post-operative values and also between 1-month and 6-month values. As per the dependent t-test, the p-value was < 0.0001 for the comparison of Quick-DASH scores between pre-operative values versus 1 month post-operative values and pre-operative versus 6 months post-operative values (Table 1, Fig. 1).
Comparison of CTS-6 scores at pre-operative, 1 month, and 6 months post-operative
There was a statistically significant change between the CTS-6 scores at pre-operative values and 1 month and 6 months post-operative values. As per the Wilcoxon matched pairs test, the p-value was < 0.0001 for the comparison of CTS-6 scores between pre-operative values versus 1 month post-operative values and pre-operative versus 6 months post-operative values (Table 2, Fig. 2).
Comparison between the changes in Quick-DASH with CTS-6 scores pre-operative versus 1 month post-operative and pre-operative versus 6 month post-operative
The changes in the Quick-DASH and CTS-6 scores pre-operative versus 1 month post-operative and pre-operative versus 6 months post-operative were compared using Spearman’s rank correlation coefficient. Both were found to be statistically significant. However, CTS-6 was found to show higher responsiveness to the changes between pre-operative values versus 1 month and 6 months post-operative values (Table 3, Fig. 3).
Discussion
First described by Paget in 1854, 1 carpal tunnel syndrome is defined as symptomatic compression neuropathy of the median nerve at the wrist caused by mechanical distortion due to a compressive force. Affecting 3–4% of the general population, the incidence rates of up to 276: 100,000 per year are reported [1, 2]. The incidence of carpal tunnel syndrome is 1 to 3 persons per 1000 per year in the USA. Almost similar incidence is found in most of the developed countries [8]. Carpal tunnel syndrome release is the second most common musculoskeletal condition with almost eight million people undergoing carpal tunnel release surgery each year as per the Bureau of Labor and Statistics and the National Institute for Occupational Safety and Health (NIOSH) [9].
The pathophysiology of CTS involves a combination of mechanical trauma, increased pressure, and ischemic injury to the median nerve within the carpal tunnel. Repetitive mechanical forces activate a vicious cycle of venous congestion, ischemia, and local metabolic alterations. This eventually leads to demyelination, axonal degeneration, macrophage attraction and activation, the release of inflammatory cytokines, and fibrosis in the chronic setting of the nerve into neurapraxia [10, 11]. The common symptom is a “pins-and-needles” sensation in the radial three fingers and nocturnal pain. As the condition progresses, intermittent pain and numbness arise during daytime activities. For some, this pain eventually becomes constant, and patients begin to report swelling of the affected hand, motor control difficulties, and, in some cases of the late disease, weakness due to thenar atrophy [12]. Two-point discrimination and pinprick testing are often also used to elicit sensory deficits in the median distribution. Consequently, many patients develop symptoms in all of the fingers and the entirety of the hand, forearm, arm, or shoulder [13].
The treatment of CTS is conservative or surgical. Conservative treatment includes oral and local corticosteroids, vitamins B6 and B12, nonsteroidal anti-inflammatory drugs (NSAIDs), diuretics, low-dose laser, ultrasound, physiotherapy, carpal bone mobilization, and the use of hand splints [14, 15]. In a review, the surgical release provides the most effective outcomes in the medium and long term [16]. The two approaches that have resulted in the most consistent satisfaction are steroid injection and carpal tunnel release [13]. Open CTS release is widely considered as the gold standard [17].
Various instruments have been proposed to assess symptoms, functionality in daily life, and outcomes of surgery in CTS patients. While these instruments have been evaluated based on reliability and validity, studies still seek the ideal post-surgery outcome scoring method. As comprehensive assessment tools, self-administered questionnaires are effective and sensitive to change in function and provide physical measures of recovery [18]. Clinically useful and easily administered written questionnaires are preferred for determining a patient’s perception of difficulties in daily activities and hand functionality. An ideal evaluation tool should be reproducible, valid, with internal consistency and test–retest reliability, good validity, ability to respond to clinical changes, and with ease for transcultural adaptation [19].
The Quick-DASH is reliable and valid for patients with CTS and easy to administer. In a comparative review by Yucel et al. of the Boston Carpal Tunnel Questionnaire (BCTQ), the Michigan Hand Outcome Questionnaire (MHQ) and the quick form of the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire were well correlated with pain and paraesthesia, and appear to be more practical and effective [18]. The Quick-DASH is the shorter version of the DASH PRO tool [12] developed for measuring “upper extremity disability.” It consists of 11 items and it is scored from 0 (best) to 100 (worst). At least 10 of the 11 items must be completed for a score to be calculated. Each item is scored 1 to 5 and the assigned values for all completed items are summed and averaged, producing a score of 1 to 5. This value is then transformed to a score of 0 to 100 by subtracting one and multiplying by 25. This adaptation is done to make the score easier to compare to other measures scaled on a 0–100 scale [7]. The Quick-DASH mainly emphasizes functional domains rather than symptoms and is intended to measure disability related to any upper extremity condition [3].
The CTS PRO tool developed by Levine et al. [20] has been among the most widely used during the last two decades. It has two domains: symptoms severity (SS) (11 items) and functional status (FS). Atroshi et al. [21], using factor analysis and Items Response Theory methodology, developed a shorter version—“CTS-6-scale” consisting of 6 items to reduce respondent burden while maintaining its properties. The CTS-6 measures symptoms severity related to CTS. Five of the 6 items in the CTS-6 have similar item text as the corresponding items in the original 11-item symptom severity scale and the remaining item (the result of the merger of 2 symptom severity scale items) has text from the 2 items. The scoring is similar to that for the 11-item symptom severity scale; for each patient, the item responses are scored from 1 (best) to 5 (worst) and then averaged for the 6 items to yield a CTS-6 score (only 1 missing item response is allowed). The CTS-6 tool has demonstrated a good level of reliability, validity, and responsiveness [3, 21].
Our analysis indicated that both Quick-DASH and CTS-6 scores reduced significantly post-operatively at 1 month and 6 months compared to the pre-operative values. Both the assessment tools were highly responsive to change after surgery for carpal tunnel syndrome and reflect the clinical improvement in terms of disabilities and symptoms, respectively. However, CTS-6 was found to show higher responsiveness to the changes between pre-operative values versus 1 month and 6 months post-operative values which could be attributed to the fact that the CTS-6 is a disease-specific measure of symptoms, whereas the Quick-DASH is a region-specific measure of function. The study provides additional support for CTS-6 given accessing treatment outcomes, as it is easier and less time-consuming to adapt.
The most appropriate scoring method for patients should be chosen depending on the advantages and disadvantages of these instruments. We believe that the use of simpler means of scoring will aid in studies to reduce the time and effort required for assessment.
Conclusions
Both Quick-DASH and the CTS-6 assessment tools are responsive to change after surgery for CTS. In addition, the scales also effectively reflect the perceived change after surgery and level of treatment satisfaction. The higher responsiveness of the CTS-6 compared with that of the Quick-DASH can be attributed to the fact that the CTS-6 is a disease-specific measure of symptoms, whereas the Quick-DASH is a region-specific measure of function. Our study provides additional support for CTS-6 for the assessment of treatment outcome, as it is convenient to administer and less time-consuming.
References
Ibrahim I, Khan WS, Goddard N, Smitham P (2012) Carpal tunnel syndrome: a review of the recent literature. Open Orthop J 6:69–76. https://doi.org/10.2174/1874325001206010069
İlhan D, Toker S, Kilincioğlu V, Gülcan E (2008) Assessment of the Boston Questionnaire in diagnosis of idiopathic carpal tunnel syndrome: comparing scores with clinical and neurophysiological findings. Düzce Tıp Fakültesi Derg 10(3):4–9
Lyrén P-E, Atroshi I (2012) Using item response theory improved responsiveness of patient-reported outcomes measures in carpal tunnel syndrome. J Clin Epidemiol 65(3):325–334. https://doi.org/10.1016/j.jclinepi.2011.08.009
Rosales RS, Delgado EB, Díez de la Lastra-Bosch I (2002) Evaluation of the Spanish version of the DASH and carpal tunnel syndrome health-related quality-of-life instruments: cross-cultural adaptation process and reliability. J Hand Surg Am 27(2):334–343. https://doi.org/10.1053/jhsu.2002.30059
Tunnel C (2006) Evaluation of boston questionnaire applied at late post-operative period of carpal tunnel syndrome operated with the paine retinaculatome through palmar port. Acta Ortop Bras 14(3):126
Imaeda T, Toh S, Wada T et al (2006) Validation of the Japanese Society for Surgery of the Hand Version of the Quick Disability of the Arm, Shoulder, and Hand (QuickDASH-JSSH) questionnaire. J Orthop Sci Off J Jpn Orthop Assoc 11(3):248–253. https://doi.org/10.1007/s00776-006-1013-1
Rosales RS, Martin-Hidalgo Y, Reboso-Morales L, Atroshi I (2016) Reliability and construct validity of the Spanish version of the 6-item CTS symptoms scale for outcomes assessment in carpal tunnel syndrome. BMC Musculoskelet Disord 17:115. https://doi.org/10.1186/s12891-016-0963-5
Hegmann KT, Merryweather A, Thiese MS et al (2018) Median nerve symptoms, signs, and electrodiagnostic abnormalities among working adults. J Am Acad Orthop Surg 26(16):576–584. https://doi.org/10.5435/JAAOS-D-17-00034
Worker Health Information from the National Health Interview Survey. In: https://www.cdc.gov/niosh/topics/nhis/data2015.html. Accessed 6 Jun 2021
Alfonso C, Jann S, Massa R, Torreggiani A (2010) Diagnosis, treatment and follow-up of the carpal tunnel syndrome: a review. Neurol Sci 31(3):243–252. https://doi.org/10.1007/s10072-009-0213-9
MacDermid JC, Doherty T (2004) Clinical and electrodiagnostic testing of carpal tunnel syndrome: a narrative review. J Orthop Sports Phys Ther 34(10):565–588. https://doi.org/10.2519/jospt.2004.34.10.565
Urits I, Gress K, Charipova K, Orhurhu V, Kaye AD, Viswanath O (2019) Recent advances in the understanding and management of carpal tunnel syndrome: a comprehensive review. Curr Pain Headache Rep 23(10):70. https://doi.org/10.1007/s11916-019-0811-z
Wang L (2018) Guiding treatment for carpal tunnel syndrome. Phys Med Rehabil Clin N Am 29(4):751–760. https://doi.org/10.1016/j.pmr.2018.06.009
Prime MS, Palmer J, Khan WS, Goddard NJ (2010) Is there light at the end of the tunnel? Controversies in the diagnosis and management of carpal tunnel syndrome. Hand (N Y) 5(4):354–360. https://doi.org/10.1007/s11552-010-9263-y
Martins RS, Siqueira MG (2017) Conservative therapeutic management of carpal tunnel syndrome. Arq Neuro-Psiquiatria 75:819–824
Huisstede BM, van den Brink J, Randsdorp MS, Geelen SJ, Koes BW (2018) Effectiveness of surgical and postsurgical interventions for carpal tunnel syndrome-a systematic review. Arch Phys Med Rehabil 99(8):1660-1680.e21. https://doi.org/10.1016/j.apmr.2017.04.024
Kaplan J, Roth C, Melillo A, Koko E, Fuller D, Perry A (2020) Analysis of surgical options for patients with bilateral carpal tunnel syndrome. J Orthop 22:86–89. https://doi.org/10.1016/j.jor.2020.03.060
Yücel H, Seyithanoğlu H (2015) Choosing the most efficacious scoring method for carpal tunnel syndrome. Acta Orthop Traumatol Turc 49(1):23–29. https://doi.org/10.3944/AOTT.2015.13.0162
de Campos CC, Manzano GM, de Andrade LB, CasteloFilho A, Nóbrega JAM (2003) Tradução e validação do questionário de avaliação de gravidade dos sintomas e do estado funcional na síndrome do túnel do carpo. Arq Neuro-Psiquiatria 61:51–55
Levine DW, Simmons BP, Koris MJ et al (1993) A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am 75(11):1585–1592. https://doi.org/10.2106/00004623-199311000-00002
Atroshi I, Lyrén P-E, Gummesson C (2009) The 6-item CTS symptoms scale: a brief outcomes measure for carpal tunnel syndrome. Qual Life Res 18(3):347–358. http://www.jstor.org/stable/40302504. Accessed 6 Jun 2021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. This is an observational study with no intervention on patients. The Kaher’s Jawaharlal Nehru Medical College Ethics Committee has confirmed that no ethical approval is required.
Informed consent
Informed consent was obtained from all individual participants included in the study. Patients were explained regarding masking the identification details and only the data will be used for the statistical analysis.
Conflict of interest
Rajesh S. Powar and Kiran S. Mahapure declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presentation: The manuscript was presented at the Annual Conference of Indian Association of Plastic Surgeons, APSICON, November 2018 at Lucknow, India (selected under J. L. Gupta Award Category).
Rights and permissions
About this article
Cite this article
Powar, R.S., Mahapure, K.S. Accuracy of Quick-DASH tool versus CTS-6 tool in evaluating the outcome of carpal tunnel release. Eur J Plast Surg 45, 315–320 (2022). https://doi.org/10.1007/s00238-021-01880-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-021-01880-8