Abstract
Background
The fibula free flap (FFF) constitutes the gold standard surgical approach for mandibular reconstruction. Mandible reconstruction is routinely performed in high-volume academic centers. To our best knowledge, this is the first case series exclusively dedicated on FFF conducted in community hospitals. This study evaluates our 10-year experience with FFF mandible reconstruction in two community hospitals.
Methods
This is a retrospective review of all 12 patients who underwent partial or total mandibulectomy with concomitant mandible reconstruction using FFF from September 2005 through February 2015.
Results
The majority of the patients were men (75%) with a mean age of 61 years. Eleven (91.7%) patients had malignancies of the head and neck, and 10 (83.3%) received preoperative XRT. Overall flap survival was 100%, with no arterial/venous thrombosis or malunion. Partial flap failure (with skin paddle necrosis) was reported in only 1 patient, but the bone was viable and survived. Recipient-site wound infection, hardware exposure, and orocutaneous fistula occurred among previously irradiated patients, and in those who were suffering from osteoradionecrosis.
Conclusions
Our FFF outcomes were non-inferior to those reported in specialized university hospitals and are evidence that successful results can be obtained outside of high-volume academic centers. FFF represents a reliable surgical approach for mandible reconstruction in university and community hospitals.
Level of Evidence: Level IV, therapeutic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oromandibular defect reconstruction after a mandibulectomy renders a significant challenge for the head and neck and plastic surgeons. Anterior and lateral mandibular defects result in a disruption of the muscles of mastication and create an imbalance of forces that leads to the loss of the form and function of the mandible [1]. Nowadays, the reconstruction of defects resulting from trauma, tumor ablation, or osteoradionecrosis can be achieved through a variety of surgical techniques [2]. Historically, initial attempts at such reconstruction were very simple. The mucosal defects were closed primarily or by using a skin graft, but the continuity of the mandible was not re-established with any reliability, often resulting in malocclusion and contour disfigurement [3]. Later, steel and titanium alloplasts were used to bridge mandibular defects. Nonetheless, alloplastic reconstruction had unacceptable high failure rates in up to 67% of the cases [3,4,5,6]. Pedicled pectoralis and latissimus dorsimyocutaneous flaps have been utilized in conjunction with non-vascularized bone grafts (NVBG) for mandible reconstruction [2,3,4]. The lack of blood supply in NVBG usually causes bone resorption, incomplete healing, and non-union of the mandible [2, 3, 7]. The advent of microsurgery revolutionized mandible reconstructive surgery with several options of vascularized bone flaps (VBF), such as the radius, metatarsus, rib, scapula, iliac, and fibula [1, 2, 8]. VBF contain an intrinsic blood supply and demonstrate superior cosmesis, function, bone union, and resistance to infection over NVBGs, alloplastic plates, and regional pedicled flaps [2, 4, 7, 9].
Radial forearm free flap (RFFF) has been utilized for mandible reconstruction. This flap is based on the radial artery with a caliber of 1.9 to 2.7 mm and its perforators. RFFF offers good flexibility for contouring and in setting its soft tissue into the mandibular defect. In comparison to fibula free flap (FFF), the RFFF has less amount of soft tissue and bone length (10 cm) to reconstruct the mandible [1, 4, 10].
FFF constitutes the gold-standard surgical approach for mandibular reconstruction since its introduction by Hidalgo in 1989 [2, 4, 11]. This osteomyocutaneous free flap offers a sufficient length (up to 26 cm) of dense cortical bone with the ability to contour the neomandible through several osteotomies and support osseointegrated dental implants [2, 4, 8, 12]. Its long pedicle based on peroneal vessels of a caliber of 1.5 to 3 mm allows reliable vascular anastomosis using the external carotid artery and its branches [8, 12, 13]. Large skin and muscle islands based on septocutaneous and musculocutaneous peroneal perforators can be harvested with the FFF to replace composite mandible and soft-tissue defects [2, 14, 15]. Moreover, FFF has the lowest rates of complication and donor-site morbidities, when comparing it with other osteocutaneous flap techniques used for mandible reconstruction [2, 4, 8, 16].
In complex and extensive oromandibular defects that are not amenable to a single osteocutaneous free flap reconstruction, the flow-through sequentially linked free flaps can be considered as an alternative. Using this technique, the surgeon couples a fasciocutaneous RFFF in sequence to a osteocutaneous FFF. The distal stumps of the radial artery and cephalic vein are sutured, under magnification, end-to-end to the vascular pedicle of the FFF. The proximal stumps of the radial artery and cephalic vein are anastomosed to the external carotid artery or its branches and to the deep jugular vein, respectively. Combining the fasciocutaneous RFFF and the osteocutaneous FFF provides the availability of two large well-vascularized skin surface areas and sufficient bone length to reconstruct compound mandibular defects [17].
Mandible reconstruction using VBF is routinely performed in high-volume academic centers. There is very scant literature on overall free tissue transfer operated in community-based hospitals [18, 19]. To the best of our knowledge, this is the first case series exclusively dedicated on FFF mandible reconstruction in community-based hospitals. This study evaluates our 10-year experience with FFF mandible reconstruction in two community hospitals and compares the cases we saw in that time with those of historical controls.
Methods
After Institutional Review Board approval, a retrospective review was conducted on all the patients presenting from September 2005 through February 2015 at San Lucas Hospital in Ponce, Puerto Rico, USA, and HIMA-San Pablo Hospital in Caguas, Puerto Rico, USA, for mandibular reconstruction. A total of 12 patients were identified. They all underwent partial or total mandibulectomy with concomitant mandible reconstruction using the microvascular FFF technique. The surgeries were performed using a simultaneous 2-team approach on all 12 patients. The head and neck surgeon performed the ablative surgery of the mandible while the plastic surgeon harvested the FFF and eventually recreated the neomandible. No routine preoperative angiography was ordered. The preoperative patency of the posterior tibial and that of the dorsalis pedis vessels was assessed and confirmed clinically with palpable pulses. All surgical procedures were performed by the same team of surgeons, led by the senior author, in the above-named community hospitals.
Right after FFF reconstruction, patients were admitted to intensive care unit (ICU) for close flap monitoring. The FFF blood flow, capillary refill, and skin flap appearance were thoroughly assessed every hour for the first 2 days, then three times per shift, using a hand-held Doppler and serial clinical exams. Nurse staff had no previous experience with this type of surgical procedure. Therefore, they were trained to identify warning signs of flap failure: loss of Doppler blood flow signal, cool to touch or pale flap, congested and edematous flap with a cyanotic appearance, absent or delayed capillary refill more than 2 s. If any of these signs were detected by a nurse, the plastic surgeon was called immediately. The plastic surgeon examined the patient and determined whether an exploration was needed. A team of surgical technicians and nurses were routinely in house, and an anesthesiologist was on call available for any kind of surgical emergency that requires to take a patient to the operating room. Hence, the access to the operating theater was instantaneous upon request for the takebacks.
Patient demographics, preoperative medical status, surgical indication, tumor characteristics, the location of the tumor, neoadjuvant/adjuvant therapy, the extension of the defect, anesthesia time, ischemia time, cervical recipient vessels in which anastomoses were performed, flap failure, recipient- and donor-site complications, and length of stay (LOS) were evaluated. Finally, the outcomes of this study were compared with the data found in the literature.
Statistical analysis was performed using Epi Info7® software produced by the Centers for Disease Control (CDC). Descriptive variables were calculated. Continuous variables were reported as means and standard deviations. Categorical variables were reported with frequencies and percentages. The results were compared with the ranges of the same outcomes reported in the literature.
Results
From September 2005 through February 2015, a total of 12 patients underwent partial or total mandibulectomy and concomitant reconstruction with FFF in the already mentioned community-based hospitals. The mean age of the patients was 61 years (median = 65.5; SD = ± 12.6 years; range = 35–74). Sex distribution was 8 men (75%) and 4 women (25%). Table 1 describes the various comorbid conditions of the patients prior to their surgeries. The most common characteristic among the patients was preoperative radiotherapy (XRT). Ten out of 12 (83.3%) patients received neoadjuvant XRT. In the pre-anesthesia evaluation, 9 of the 12 (75%) patients were classified as having an ASA score of 3, and 3 out of the 12 (25%) had an ASA score of 2.
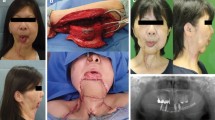
Indications for surgery included the mandibular infiltration of squamous cell carcinoma (SCC) in the oral cavity/neck in nine patients (75%), osteoradionecrosis of the mandible (in two patients [16.7%]) after XRT for SCC, and destruction of the mandible bone by fungal osteomyelitis (in one patient [8.3%], after a dental procedure). No preoperative or postoperative evidence of malignancy was found in the patient with osteomyelitis. Of the nine patients who had SCC, five were diagnosed with SCC of the retromolar trigone, three had SCC in the floor of mouth and base of tongue, and one had SCC of the neck (see Fig. 1). Of the two patients who had osteoradionecrosis of the mandible, one had previously been diagnosed with adenoid cystic carcinoma of the submandibular gland and the other with SCC of the tongue. They both had undergone oncological resection and had full courses of XRT that eventually resulted in osteoradionecrosis of the mandible.
All 12 mandibular defects occurred after ablative surgery of the oral cavity that resulted in significant bone loss and adjacent soft-tissue deficit. The average bone loss was 12.6 cm. Ten out of the 12 patients (83.3%) underwent partial mandibulectomy. The remaining two patients (16.7%) had a total mandibulectomy with an average mandibular bone defect of 20 cm.
FFF was the selected reconstructive technique utilized in all 12 patients. A standard lateral approach was used to harvest the FFF and skin paddle as previously described by Hidalgo and Gilbert [11, 20]. The vessels taken from the donor site for later microvascular anastomosis were the peroneal vessels, in all the cases. On the recipient site, the facial artery was used in all the cases for the arterial anastomosis. The arterial anastomosis was performed end-to-end in 11 (91.7%) cases and end-to-side in 1 (8.3%) case. For the venous anastomosis, the facial vein was used in 8 of the 12 patients, followed by the internal jugular vein in 4 of the 12 cases. Venous end-to-end anastomosis was done in 8 out of the 12 (66.7%) and end-to-side in the remaining 4 cases (33.3%). The ischemia time of the bone graft and its skin paddle reached an average of 104.7 min (median = 91.0; SD = ± 45.5 min; range = 40–165) (see Table 2). Mean LOS was 19.5 days (range = 8–30), as shown in Table 3.
Considering that bone survival was the main objective, an overall flap survival rate of 100% was achieved in this series. No total flap loss occurred. One patient (8.3%) showed partial flap failure (due to skin paddle necrosis), but the bone was viable and survived. The patient who suffered a partial flap failure had previous osteoradionecrosis of the mandible, undergone total mandibulectomy, and suffered a recipient-site wound infection and plate exposure that required a return to the operating room.
In this series, only 4 out of 12 patients (33.3%) had postoperative complications. These 4 patients needed to return to the operating room in order to manage their complications. Recipient-site wound infection occurred in all of the 4 patients mentioned above (4/12, 33.3%). Bar/plate exposure arose in 3 of these 4 patients (3/12, 25.0%), and orocutaneous fistula in 2 of these 4 patients (2/12, 16.7%). There was no arterial or venous thrombosis seen in any of the flaps. No donor-site morbidity occurred. All FFF mandible reconstruction had adequate bone union. Table 4 shows the rates of the significant clinical outcomes and postoperative complications previously mentioned.
Discussion
Despite that this is a small case series, the current study suggests that successful results can be obtained outside high-volume academic centers. Our flap survival was 100%, with no arterial/venous thrombosis and no malunion. These results were non-inferior to those reported in the literature. Published flap survival ranges are from 91.2 to 98.3%; venous or arterial flap thrombosis reaches 4.76%, and malunion varies from 1.7 to 13.7% [1, 2, 8, 12, 13, 21]. Partial flap failure occurred in only one of our patients (8.3%), which failure was due to skin paddle necrosis. Our percentage remains within the partial flap failure rate of 2.6 to 12% described in literature [2, 8, 12, 21]. The patient who suffered the partial flap failure had preoperative osteoradionecrosis of the mandible and postoperatively, suffered a recipient-site wound infection with plate exposure and orocutaneous fistula that required a return to the operating room. Nonetheless, the bone was viable and survived.
We had high rates of recipient-site wound infection (33.3%), plate exposure (25%), and orocutaneous fistulas (16.7%). Overall, reported recipient-site wound infection reaches 2.6 to 13% [1, 8, 13, 20] and plate exposure, 7.1 to 13.7% [8, 13, 21], whereas orocutaneous fistulas range from 3.9 to 36.4% [1, 8, 12, 13, 22, 23]. In our cohort, 11 of 12 (91.7%) patients had malignancy of the head and neck, and 10 of 12 (83.3%) received preoperative XRT, in contrast to the 27 to 33% who receive preoperative XRT in literature [1, 2, 8, 12]. Higher complication rates are experienced when reconstructed tissues have been previously exposed to XRT [13]. Radiation arteritis develops, and it leads to a hypocellular, hypovascular, and hypoxic environment that compromises the integrity of recipient vessels and impairs wound healing [13, 24]. As a result, the most common complications of XRT are orocutaneous fistulas, hardware plate exposure, and flap wound infection [13]. In fact, in our study, the majority of the recipient-site wound infections (3/4, 75%) and hardware plate exposure (2/3, 66.7%) occurred in previously irradiated patients. Moreover, all (2/2) the orocutaneous fistulas arose in previously irradiated patients with osteoradionecrosis.
The average anesthesia time (17.02 h) and mandibular bone defect (12.6 cm) were higher than what has been reported in the literature: 9.57 to 14.54 h and 5.0 to 11.7 cm, respectively [8, 12, 21, 23, 25]. Performing an oncological resection with resultant long mandibular defects and reconstructing them in previously irradiated fields requires more time in the operating room than is the case in virgin tissues with smaller bone defects. Nonetheless, our ischemia time (104.7 min) and length of stay (19.5 days) concur with those in the literature: 82.9 to 109.7 min and 9.8 to 22.2 days, respectively [12, 21, 23].
Conclusions
FFF is a reliable procedure for mandible reconstruction. Our outcomes were non-inferior to those reported in specialized university hospitals. Successful results can be obtained outside high-volume academic centers [18, 19]. The current study suggests that FFF mandible reconstructions can be performed in a community hospital with the same degree of safety as in a high-volume university hospital. An experienced reconstructive surgeon and his or her surgical team, an operating microscope, an intensive care unit, a hand-held Doppler, a trained nursing staff (the members of which can recognize the indicators of flap failure), and the facility to promptly return to the operating room in the event of a complication are the important requirements a community hospital must meet prior to embarking on the practice of FFF mandible reconstruction.
References
Dean NR, Wax MK, Virgin FW, Magnuson JS, Carroll WR, Rosenthal EL (2012) Free flap reconstruction of lateral mandibular defects: indications and outcomes. Otolaryngol Head Neck Surg 146(4):547–552
Kokosis G, Schmitz R, Powers DB, Erdmann D (2016) Mandibular reconstruction using the free vascularized fibula graft: an overview of different modifications. Arch Plast Surg 43(1):3–9
Emerick KS, Teknos TN (2007) State-of-the-art mandible reconstruction using revascularized free-tissue transfer. Expert Rev Anticancer Ther 7(12):1781–1788
Schrag C, Chang YM, Tsai CY, Wei FC (2006) Complete rehabilitation of the mandible following segmental resection. J Surg Oncol 94(6):538–545
Wei FC, Celik N, Yang WG, Chen IH, Chang YM, Chen HC (2003) Complications after reconstruction by plate and soft-tissue free flap in composite mandibular defects and secondary salvage reconstruction with osteocutaneous flap. Plast Reconstr Surg 112(1):37–42
Spencer KR, Sizeland A, Taylor GI, Wiesenfeld D (1999) The use of titanium mandibular reconstruction plates in patients with oral cancer. Int J Oral Maxillofac Surg 28(4):288–290
Foster RD, Anthony JP, Sharma A, Pogrel MA (1999) Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck 21(1):66–71
González-García R, Naval-Gías L, Rodríguez-Campo FJ, Muñoz-Guerra MF, Sastre-Pérez J (2008) Vascularized free fibular flap for the reconstruction of mandibular defects: clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(2):191–202
King TW, Gallas MT, Robb GL, Lalani Z, Miller MJ (2002) Aesthetic and functional outcomes using osseous or soft-tissue free flaps. J Reconstr Microsurg 18(5):365–371
Virgin FW, Iseli TA, Iseli CE et al (2010) Functional outcomes of fibula and osteocutaneous forearm free flap reconstruction for segmental mandibular defects. Laryngoscope 120(4):663–667
Hidalgo DA (1989) Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg 84(1):71–79
Hidalgo DA, Rekow A (1995) A review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg 96(3):585–596
Lee M, Chin RY, Eslick GD, Sritharan N, Paramaesvaran S (2015) Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: a systematic review. J Craniomaxillofac Surg 43(10):2026–2033
Daya M (2008) Peroneal artery perforator chimeric flap: changing the perspective in free fibula flap use in complex oromandibular reconstruction. J Reconstr Microsurg 24(6):413–418
Cheng MH, Saint-Cyr M, Ali RS, Chang KP, Hao SP, Wei FC (2009) Osteomyocutaneous peroneal artery-based combined flap for reconstruction of composite and en bloc mandibular defects. Head Neck 31(3):361–370
Hayden RE, Mullin DP, Patel AK (2012) Reconstruction of the segmental mandibular defect: current state of the art. Curr Opin Otolaryngol Head Neck Surg 20(4):231–236
Costa H, Zenha H, Azevedo L, Rios L, Barroso ML, Cunha C (2012) Flow through sequentially linked free flaps in head and neck reconstruction. Eur J Plast Surg 1:31–41
Gusenoff JA, Vega SJ, Jiang S et al (2006) Free tissue transfer: comparison between university hospitals and community hospitals. Plast Reconstr Surg 118(3):671–675
de Wildt RP, Sawor JH, Fresow RN et al (2012) The unilateral deep inferior epigastric perforator flap: comparing university to community hospital. J PlastSurg Hand Surg 46(3–4):159–162
Gilbert A (1979) Vascularized transfer of the fibula shaft. Int J Microsurg 1:100
López-Arcas JM, Arias J, Del Castillo JL et al (2010) The fibula osteomyocutaneous flap for mandible reconstruction: a 15 year experience. J Oral Maxillofac Surg 68(10):2377–2384
Baumann DP, Yu P, Hanasono MM, Skoracki RJ (2011) Free flap reconstruction of osteoradionecrosis of the mandible: a 10-year review and defect classification. Head Neck 33(6):800–807
Alam D, Nuara M, Christian J (2009) Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngolog Head Neck Surg 141(2):196–201
Buchbinder D, St Hilaire H (2006) The use of free tissue transfer in advanced osteoradionecrosis of the mandible. J Oral Maxillofac Surg 64(6):961–964
González-García R, Naval-Gías L, Rodríguez-Campo FJ, Román-Romero L (2009) Reconstruction of oromandibular defects by vascularized free flaps: the radial forearm free flap and fibular free flap as major donor sites. J Oral Maxillofac Surg 67(7):1473–1477
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Luis E. IV Santaliz-Ruiz, Mark D. Rivera-Morales, Ilaria Giudiceandrea, Federica Franceschi, Edgar Domenech-Fagundo, Carlos Pérez-Mitchell, Diana Avilés, Roberto Pérez-Nieves declare that they have no conflict of interest.
Ethical approval
The project was submitted to the Institutional Review Board (IRB) and was reviewed and approved by the committee before starting the investigation.
Informed consent
This study is a retrospective review of charts. This study meets the exemption criteria for waiver of informed consent, as evaluated and approved by the IRB committee.
Funding
None.
Rights and permissions
About this article
Cite this article
Santaliz-Ruiz, L.E., Rivera-Morales, M.D., Giudiceandrea, I. et al. Fibula osteomyocutaneous free flap in mandibular reconstruction: clinical experience in a community-based hospital. Eur J Plast Surg 41, 409–414 (2018). https://doi.org/10.1007/s00238-017-1377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-017-1377-9