Abstract
Introduction
It has been demonstrated that rehabilitative interventions can promote motor function recovery in stroke patients. However, little is known regarding the neural mechanisms that underlie the rehabilitation treatments. The aim of this study was to investigate the plasticity of intrinsic functional connectivity patterns that are associated with rehabilitation intervention in chronic stroke patients.
Methods
Twelve chronic stroke patients with subcortical lesions in the left motor pathway participated in a 4-week rehabilitation intervention and underwent resting-state functional magnetic resonance imaging (fMRI) scanning before and after the intervention. Both functional connectivity analyses of the ipsilesional (left) primary motor cortex (M1) and measurements of the lateralization index of the connectivity patterns were performed in both the stroke patients and healthy controls (HC).
Results
Compared with the HC, the decreased connectivity of the ipsilesional M1 with the contralesional sensorimotor cortex (SMC), bilateral supplementary motor areas, and inferior parietal lobule due to stroke were remarkably restored after the intervention. More specifically, the lateralization index of the bilateral SMC tends to be the normal level. Moreover, comparing post- with pre-intervention, we observed significantly increased connectivity of ipsilesional M1 with the contralesional M1 and medial superior frontal gyrus (mSFG). Additionally, the index of pre-intervention connectivity with the contralesional mSFG was positively correlated with motor improvement.
Conclusion
The impact of rehabilitation intervention on intrinsic functional connectivity patterns throughout the brain was measurable on resting-state fMRI, and systematic assessment of resting-state functional connectivity can provide prognostic insight for later motor improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is the leading cause of long-term disability worldwide, and more than 50 % of the stroke patients are left with residual motor deficits [1] that significantly reduce the quality of daily life. Over the past few years, many studies have attempted to understand the neural mechanisms of motor recovery following stroke in the hope that understanding these mechanisms will improve our ability to accelerate the recovery process. Although it is well accepted that cortical reorganization underlies the restoration of motor function following stroke [2–6], the precise restoration mechanisms have not been understood completely. As a non-invasive in vivo neuroimaging technique, functional magnetic resonance imaging (fMRI) has undoubtedly played an important role in investigating the neural mechanisms that underlie motor recovery following stroke. Task-based fMRI studies have reported various patterns of cerebral reorganization that contribute to the restoration of motor function following stroke [7–10]. However, many factors puzzle investigators when determining the patterns of cerebral reorganization, including task-induced head motion, differences in task performance, the inhomogeneity of the patients recruited (e.g., variation in age, gender, location, and scope of the lesion), the degree of functional deficit, and the time after stroke. In particular, it is difficult for severely disabled patients to perform the selected tasks.
In recent years, there is growing interest in resting-state fMRI, which mainly focuses on low-frequency blood oxygen level-dependent (BOLD) signal fluctuations (0.01–0.08 Hz) during rest periods. These fluctuations reflect spontaneous neural activity, and temporal correlations have also been identified between functionally related regions, such as motor, default mode, language, visual, attentional, and salience networks [11–14], which are typically called intrinsic functional networks. In particular, it has been shown that alterations in these intrinsic fluctuations can be used as a marker of network dysfunction in stroke patients [15–17]. In contrast to task-based fMRI, the advantage of resting-state fMRI is that it can be performed without an overt task or external input, and thus, it provides an ideal tool for studying patients who are unwilling or unable to adhere to task paradigms.
The most often-used method for investigating intrinsic functional connectivity with resting-state fMRI is the “seed-based” approach [11]. At present, seed-based functional connectivity analysis of primary motor cortex (M1) is usually employed in stroke brain reorganization research because it can successfully identify the motor executive network [17, 18]. The previous longitudinal studies mainly focused on cerebral reorganization following spontaneous motor recovery [18, 19]. Numerous therapies have been developed for stroke-related motor deficits, such as movement training, pharmacotherapy, acupuncture, constraint-induced movement therapy (CIMT) [20], motor imagery training (MIT) [21], and so on. However, little is known concerning the neural mechanisms underlying these rehabilitation interventions. In this study, we selected a homogeneous group of chronic stroke patients and performed resting-state functional connectivity analysis of the ipsilesional M1. We hypothesized that the functional connectivity changes in motor-related network would be associated with the 4-week rehabilitation intervention in those chronic stroke patients. Furthermore, we also investigated neural correlates associated with motor recovery after intervention. This brain–behavior correspondence can make a unique contribution in the rehabilitation field and possibly help to evaluate new treatment regimens that assist motor recovery.
Materials and methods
Participants
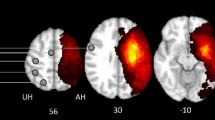
We recruited 12 subcortical stroke patients (12 males, age ± SD 58.67 ± 10.26 (years)) with pure motor deficits from both outpatient and inpatient services at Huashan Hospital, which is affiliated with Fudan University, and 12 healthy controls (HC) (9 males and 3 females, age ± SD 57.25 ± 9.37 (years)) who were from local communities. The two groups are comparable for age and gender. And, all of the subjects gave their informed consent prior to being included in this study. Both the mini-mental state examination (MMSE) and the upper limb section of the Fugl–Meyer Scale (FM-UL) were administered to all of the stroke patients. The inclusion criteria for the stroke patients were as follows: (1) only one stroke (infarct or hemorrhage); (2) the time post-stroke (≥3 and ≤6 months) prior to the study enrollment; (3) all of the participants had sufficient cognitive abilities (MMSE ≥27); (4) the ages of the participants were between 18 and 80 years; (5) pure motor hemiplegia, as evaluated according to a modified Brunnstrom classification, of both the paretic arm and hand is lower than grade IV [22]; and (6) right handed prior to stroke as determined by the Edinburgh Handedness scale [23]. The exclusion criteria were as follows: (1) any contraindications to MRI testing or claustrophobia; (2) excessive pain in the affected arm, as measured by a score of >4 on a 10-point visual analog scale; (3) excessive spasticity, defined as a score of >2 on the Modified Ashworth Spasticity Scale (4) severe aphasia, neglect, and sensory disturbances; (5) participation in any experimental rehabilitation or drug studies; (6) major artery occlusion or severe tenosis; (7) active malignant disease or renal, liver, or cardiac failure; (8) other neurological disease or depression; and (9) receiving drugs such as benzodiazepine, antidepressants, or antiepileptics. Additionally, one patient was excluded due to incomplete fMRI data. The clinical and demographic data of the remaining 11 patients are summarized in Table 1, and the lesion with the maximum area for each stroke patient is shown in Fig. 1. The protocol for this prospective study was approved by the institutional ethics committee of local university.
Behavioral assessment
The upper limb section of the Fugl–Meyer Scale [24] was used to assess motor impairment in the upper extremities before and after the 4-week rehabilitation intervention period. The FM-UL scores are acquired in the form of a 3-point ordinal scale (0 = cannot perform at all; 1 = can perform partially; 2 = can perform perfectly) that is applied to each item, and the items are summed to provide a maximum score of 66. The larger the FM-UL scores are, the better the motor function. The assessment was performed by the same experienced doctor from the Department of Rehabilitation Medicine, Huashan Hospital, who was blind to the patient’s group allocation.
Rehabilitation intervention
All 12 patients underwent a 4-week rehabilitation intervention. More specifically, 6 of 12 patients underwent only the conventional rehabilitation intervention, which included physical and occupational therapy of the upper limbs (passive or active movement), electrical stimulation, Chinese acupuncture, and massage for 3 h per day, 5 days per week, for a total of 4 weeks in addition to drug therapy every day; the other six patients underwent the above conventional rehabilitation intervention to which we also added MIT of the impaired upper extremity 30 min per day, 5 days per week, over four consecutive weeks. Therapy was administered one on one by an experienced clinical therapist in the same environment. Extended methodological details of the rehabilitation intervention can be found in previously published papers [9].
Data acquisition
Resting-state fMRI data were acquired for stroke patients before and after intervention. For healthy subjects, we obtained one-time resting-state fMRI data. All of the subjects underwent structural and functional MRI scans in a single session using a Siemens Trio 3.0 Tesla MRI scanner (Siemens, Erlangen, Germany) at Key Laboratory of Magnetic Resonance. Foam pads were used to limit the head motion and reduce the scanner noise. All of the images were acquired parallel to the anterior commissure–posterior commissure line with an auto-align technique. Whole-brain, resting-state fMRI data were collected using an echo-planar imaging (EPI) sequence: repetition time (TR) = 2000 ms, 30 axial slices, echo time (TE) = 30 ms, thickness = 4 mm, gap = 0.8 mm, matrix = 64 × 64, flip angle = 90°, and a field of view = 220 × 220 mm. During the collection of the EPI data, the participants were instructed to remain awake, remain motionless, and relax with their eyes closed and attempt not to think about anything in particular. For each participant, the scanning lasted for 8 min and 6 s; the first 6 s was consumed on the dummy scan. Thus, we collected a total of 240 image volumes. Three-dimensional structural images of T1-weighted images covering the entire brain were obtained in a sagittal orientation at the beginning of each session by employing magnetization prepared by rapid gradient echo sequence (MPRAGE): TR = 1900 ms, 192 slices per slab, TE = 3.42 ms, thickness = 1 mm, gap = 0.5 mm, field of view = 240 × 240 mm, and a matrix = 256 × 256. To identify the location of the lesion, T2-weighted images were collected using a turbo-spin-echo sequence: TR = 6000 ms, 30 axial slices, TE = 93 ms, thickness = 5 mm, no gap, field of view = 220 × 220 mm, and a matrix = 320 × 320.
Pre-processing of the functional MRI data
Image pre-processing was performed using statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) implemented in a MATLAB suite (Mathworks, Inc., Natick, Massachusetts). The first 10 volumes of each function time series were discarded because of the instability of the initial MRI signal and the adaptation of the subjects to the circumstances, thus leaving 230 images that were corrected for the acquisition delay between the slices and for head motion. Motion was estimated by the values of translation and rotation for each of the 230 volumes. Excessive motion was defined as more than 2.5 mm of translation or greater than a 2.5° rotation in any direction. Following the motion correction, each of the participant’s structural images was co-registered to the mean functional image using a linear transformation. Each structural image was then segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) images using the SPM8 “new segment” tool, and the deformation field was calculated. This deformation field was applied to each of the functional images to transform them into Montreal Neurological Institute (MNI) standard space and then resampled to a 3-mm isotropic voxel. Finally, the normalized images were smoothed using an isotropic Gaussian filter at full width at a half maximum (FWHM) of 4 mm.
Functional connectivity analysis
Following pre-processing, the smoothed images of all of the remaining 11 patients were further processed using the Resting-State fMRI Data Analysis Toolkit (REST, http://restfmri.net) [25]. First, we removed the linear trend and conducted the temporal band pass (0.01–0.08 Hz) filtering to reduce the low-frequency drift and high-frequency respiratory and cardiac noise [26]. Subsequently, we selected the seed region of interest (ROI) in the ipsilesional M1 (left side in our study) at the MNI coordinate of −38, −22, 56, as used in previous report [27]. The radius of 6 mm was used to define the spatial extent of the ROI. We then used the seed ROI to perform functional connectivity analysis for both the stroke patients and healthy controls.
The functional connectivity of each individual participant was calculated as follows. First, several sources of variance for the resting-state data were obtained as follows: (a) six head motion parameters, (b) global mean signal, (c) WM signal, and (d) CSF signal. Second, the reference time course was calculated by averaging the time series of all of the voxels in the seed ROI. Third, Pearson correlation analysis was performed based on the voxel between the reference time course and the time series of each voxel in the brain using the global signal, WM signal, CSF signal, and the six parameters of head motion as nuisance covariates. Finally, the resulting correlation coefficients were transformed into z-scores using Fisher’s z-transformation in such a way that their distributions could better satisfy normality [28]. Subsequently, a two-tailed, one sample t test was conducted on the z-maps of stroke patients (pre- and post-intervention) and HC, respectively, and two sample t tests were then conducted to compare the z-FC maps between the stroke patients (pre- and post-intervention) and HC. Paired t tests were also performed to identify changes between pre- and post-intervention. Multiple comparisons correction was performed using AlphaSim program in AFNI (see AlphaSim in AFNI, http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), and a corrected threshold of p < 0.05 was utilized, with a combined cutoff value of p < 0.01 and a minimum cluster size of 486 mm3 (18 voxels). The statistically significant differences in the functional connectivity between each pair of stroke patients (pre- and post-intervention) and HC overlapped on the rendered views.
Lateralization index
As a quantitative measure, the lateralization index (LI) was calculated to provide a specific description of the symmetry of the functional connectivity pattern. Previous studies have suggested that stroke recovery mostly focused on the bilateral sensorimotor cortex (SMC) [29–31]. The SMC was defined as the combination of the pre-central and post-central gyri. Therefore, the LI of bilateral SMC was calculated for each correlation map in our study, according to the following definition: (number of connected voxels in the ipsilesional SMC − number of connected voxels in the contralesional SMC) / (number of connected voxels in the ipsilesional SMC + number of connected voxels in the contralesional SMC). If the functional connectivity of the ipsilesional M1 with any voxel of bilateral SMC had a p value <0.001 [32], then the voxel was considered to be connected. Thus, the LI could range from −1.0 (contralesional connectivity only) to 1.0 (ipsilesional connectivity only), and values close to 0 referred to the symmetrical connectivity. The LI of the patients (pre- and post-intervention) was assessed and compared with that of the HC.
Correlation analysis between the index of functional connectivity and FM-UL scores
Recent findings have indicated that there is an association between the index of function connectivity and upper extremity control in patients with stroke [33], which indicated making two correlations during the analyses. The first action was to detect whether the increments in the index of function connectivity (ΔFC) varies with increments in FM-UL scores (ΔFM-UL), and the second action was to assess whether the pre-intervention function connectivity can predict motor improvement after intervention. The two regions (including the contralesional M1 and medial superior frontal gyrus (mSFG)) that showed significant differences in connectivity with the ipsilesional M1 between pre- and post-intervention were examined. The correlation analysis steps were as follows: The mean of the functional connectivity of the ipsilesional M1 with all of the voxels of the contralesional identified clusters was extracted from each pre- and post-intervention stroke patient. Subsequently, ΔFC and ΔFM-UL across all of the stroke patients after intervention made a non-parametric Spearman correlation for the first detection; the index of pre-intervention function connectivity and ΔFM-UL after intervention also made a non-parametric Spearman correlation for the second detection. We determined the significance using a p value <0.05 (uncorrected).
Results
Clinical status and motor recovery
We found no significant differences in age between the stroke patients and HC (stroke patients, 58.36 ± 10.71 and HC, 57.25 ± 9.37, p = 0.793). In addition, we observed significant improvement in the FM-UL scores after a 4-week rehabilitation intervention (post-intervention, 33.27 ± 13.63 vs. pre-intervention, 22.0 ± 15.49, p = 0.001).
Comparison of the functional connectivity of the ipsilesional M1 between the patients and HC
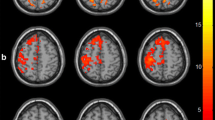
Compared with the HC, the patients at pre-intervention displayed reduced connectivity of the ipsilesional M1 with the bilateral supplementary motor area (SMA), bilateral inferior parietal lobule (IPL), contralesional SMC, cerebellum, posterior cingulate gyrus, and middle occipital gyrus. After intervention, the connectivity with the bilateral SMA and IPL was largely restored, and with contralesional SMC, it was partially restored. On the other hand, the stroke patients displayed increased connectivity with regions of the bilateral middle frontal gyrus, middle temporal gyrus, ipsilesional basal ganglia, and thalamus before intervention, compared with the HC. After the intervention, there was increased connectivity of the ipsilesional M1 with the ipsilesional superior and inferior frontal gyrus, middle temporal gyrus, basal ganglia and thalamus, and contralesional cerebellum in comparison with the HC. Furthermore, we detected significantly increased connectivity with the contralesional M1 and mSFG after intervention compared with pre-intervention in the stroke patients. In addition, no regions were observed to have reduced connectivity with ipsilesional M1 after intervention compared with pre-intervention (Fig. 2, Fig. S1, and Table 2).
Comparison of the functional connectivity of the ipsilesional M1 between stroke patients (at both pre- and post-intervention) and HC. a PRE versus POST, b PRE versus HC, and c POST versus HC. The threshold was set at a combined cutoff value of p < 0.01 and a minimum cluster size of 486 mm3 (18 voxels) to yield a corrected threshold of p < 0.05 (AlphaSim corrected). Clusters with significant differences overlapped on rendered views (posterior-anterior (row 1), right-left (row 2), inferior-superior (row 3)), Color scale = t values. HC healthy controls, PRE pre-intervention, POST post-intervention
Changes in the lateralization index
Figure 3 showed the changes in the LI together with corresponding maps of the functional connectivity. The functional connectivity of the ipsilesional M1 with the bilateral SMC was almost symmetrical for HC (the mean LI was 0.08 ± 0.09, which was close to 0). Before the intervention, the LI of the patients was dramatically increased and the standard deviation is huge (the mean LI was 0.35 ± 0.32), in such a way that it differentiates significantly from that of HC. In contrast, it decreased, with a tendency to that of HC (the mean LI was 0.15 ± 0.21) after intervention.
The lateralization index (LI) of SMC changed in a resting-state functional connectivity pattern. The LI was compared between stroke patients (both at pre- and post-intervention) and HC. In the graph of the LI, the points represent the means, error bars represent the SDs, and stars represent the significant differences between the two groups at a threshold of p < 0.05. L left, R right, HC healthy controls, PRE pre-intervention, POST post-intervention
Brain and behavior correlation
The correlation between the increments in the index of function connectivity (ΔFC: including the contralesional M1 and mSFG) and increments in the ΔFM-UL were not statistically significant (r = 0.16, p = 0.64; r = −0.03, p = 0.94; respectively). However, a significant positive correlation between the index of pre-intervention functional connectivity with the contralesional mSFG and ΔFM-UL was revealed (r = 0.68, p = 0.02) (Fig. 4).
Discussion
In the current study, we investigated the plasticity of a motor-related network associated with the 4-week rehabilitation intervention by resting-state fMRI. Our results demonstrated the characteristic of asymmetry in the resting-state functional connectivity of the ipsilesional M1 in subcortical stroke patients, and the dominant effect of a 4-week rehabilitation intervention was to restore inter-hemispheric symmetry; in addition, good recovery would involve improving motor preparation and motor execution circuits simultaneously. This study further examined whether changes in the motor-related cortex connectivity might correlate with motor improvement and found that the functional connectivity with the contralesional mSFG before intervention was positively correlated with increments in the FM-UL scores, which could be a prognostic value in predicting the efficiency of rehabilitation intervention.
Previous studies [17, 18, 27] have consistently reported reduced connectivity between the ipsilesional M1 and the contralesional SMC after a unilateral subcortical stroke. In agreement with previous studies, our findings have revealed a loss of functional connectivity between the ipsilesional M1 and contralesional SMC regions before intervention, which further suggests a breakdown in the normal interactions between the bilateral hemispheres, caused by the ipsilateral lesions. As expected, the functional connectivity of the ipsilesional M1 with the contralesional SMC regions was partially restored after intervention. The SMC, especially M1, is the primary origin of corticospinal neurons, and hence, the restored connectivity related to these areas in the unaffected side could indicate recruitment of the uncrossed corticospinal tract that originated from the contralesional hemisphere. This conduit could provide a route by which signals from the unaffected hemisphere could reach the muscles controlled by the affected hemisphere, to compensate for damage in the ipsilesional corticospinal tract. The increased connectivity of the ipsilesional M1 with contralesional SMC suggests that the motor execution circuits were enhanced. Otherwise, the role of M1 in the unaffected hemisphere remains controversial. Several tasks related to functional imaging studies have reported the activation of the contralesional M1; specifically, the posterior part of M1 activated was more evidently correlated with a poorer outcome in the long-term stroke patients [34]. Recent studies have also argued for a beneficial role of the SMC of the contralesional hemisphere in some aspects of effectively recovered motor behavior [35, 36]. In fact, we found no significant correlation between the increments in functional connectivity with the contralesional M1 and the increments in the FM-UL scores after intervention. The possible other reasons could be as follows: First, the motor recovery was associated with a redistribution of the motor function throughout the whole network rather than focused within a few regions, as is typically observed in the previous study [27]; second, it is well acknowledged that stroke lesions would induce motor behavior obstacles associated with functional impairments in the SMC, but the extent to which functional connectivity impairments of motor-related brain cortices accounting for the severity of motor performance dysfunction will be an important topic for future studies.
In terms of the LI-SMC, it was much higher than the healthy controls before intervention, which means that the functional connectivity between the ipsilesional M1 and bilateral SMC was more highly lateralized to the ipsilesional M1 with the greatest asymmetry; however, it reduced quickly and was close to that of HC after a 4-week intervention, which implies a trend to restore relatively symmetrical connectivity. The restored pattern related with the SMC suggested that the sensorimotor cortex regions of bilateral hemispheres have established a balance to accelerate motor improvement by intervention, which reflects that good inter-hemispheric coherence is quite important for normal motor function in the sensorimotor system [37, 38]. Therefore, first, our present study verified the notion that the bilateral brain hemisphere will be restored toward the patterns of HC with motor improvement; second, the results of this study are distinctive to reveal functional connectivity changes induced by the intervention; however, they cannot be considered to correspond with the rearrangements of activation over the bi-hemispheric sensorimotor system in task-based fMRI. The basic reason is that motor task-related changes measured from functional activities might simply reflect changes in the motor behavior when performing the tasks, but the functionally related resting-state motor network is engaged in the absence of an overt behavior. In other words, motor task-related activities can be regarded as the resting-state motor network’s response to an overt motor behavior stimulation [39]. Of course, they are comparable with one another by a plausible association between resting-state connectivity and motor task activation. The functional connectivity changes in the current study could also help us to better understand the cortical activation patterns (increases in or focusing of recruitment to the contralesional SMC region) that presented in previous task-based fMRI study [9]. It becomes possible to understand the inconsistency between good hand motor recovery and the persistent abnormal brain activation that occurs in some stroke patients [6]. Here, we should emphasize the importance of observing the reestablished symmetry between the bilateral cerebral hemisphere, not measuring quantitative changes of functional activations that were evoked by motor tasks or functional connectivity purely in one side of the brain, to evaluate the efficiency of rehabilitation intervention.
We also found a decreased connectivity of the ipsilesional M1 with bilateral SMA and IPL before intervention in comparison with the HC. Functionally, SMA is involved in the performance of difficult tasks and of tasks that demand high levels of accuracy [40], and IPL is related not only to higher order processing of sensory information but also to sensorimotor integration [41]. As important parts of secondary sensorimotor areas, both of these areas returned to be normal after intervention, which implies that the intervention makes these secondary sensorimotor areas superior to the primary motor area to recover. The enhancement connectivity with the two areas could of course positively influence the motor recovery of the affected hand. Furthermore, changes in the functional connectivity during stroke recovery did not involve all of the brain regions to the same extent, which suggests that there is a heterogeneous plasticity of the overall network structure.
The prefrontal lobe is not regarded as a primary or secondary sensorimotor region but as a region that is associated with high-level executive functions and decision-related processes. Before the intervention, the enhanced connectivity with the bilateral middle frontal gyrus possibly implied that motor cognitive manipulation had become stronger because of the shortage of motor output affected by the stroke lesion. However, the increased connectivity with the ipsilesional superior frontal gyrus after intervention could be related to motor improvement of the affected hand. More specifically, the connectivity magnitude of the ipsilesional M1 with the contralesional mSFG before the intervention was proportional to the increments in the FM-UL scores after intervention across all of the stroke patients, which suggests that the recruitment of mSFG could be helpful in reinforcement of the management of the cognitive load that is required for motor performance (motor preparation or motor planning) [42]. Notably, the ipsilesional inferior frontal gyrus appeared to have increased connectivity after intervention, which is possibly related to the direct effect of the motor imagery therapy, as six patients in our study adopted conventional therapy plus motor imagery training. The internal simulation of an action during motor imagery has been considered the core element of motor planning [43]. Moreover, the decreased connection with the contralesional cerebellum before intervention was consistent with the previous study, which reported decreased cerebellar activation in clinically stable stroke patients [8]. In contrast, the significantly increased connectivity with the contralesional cerebellum after intervention could be related to the ongoing relearning of motor skills induced by a 4-week intervention [44]. Otherwise, the subcortical structures (e.g., the basal ganglia and thalamus) sustained increased connectivity after intervention, which possibly reflects functional compensation around the ipsilateral lesions. In summary, we provide here the first description of the modulation of specific resting-state networks by rehabilitation intervention, which reveals a novel association between the neural plasticity mechanisms of the rehabilitation and the sensorimotor system resting-state activity. Our approach could provide a powerful tool for exploration of the systems that are involved in functional reorganization following a stroke.
There are some limitations of this study. First, because the rehabilitation intervention included physical and occupational therapy of the upper limbs, electrical stimulation, Chinese acupuncture, massage, and MIT, we could not detect the specific effect of any one treatment according to our current results. Thus, it is necessary to further subdivide the patients by rehabilitation therapies to make certain the specific relationships between the changes of brain functional connectivity and rehabilitation intervention. Second, consistent results were a dynamic reorganization that went along with recovery; it is impossible to fully understand the dynamic changes in the patterns of functional connectivity following a stroke due to our shorter intervention time. Finally, in this study, we focused on identifying the functional connectivity changes of the motor-related network, especially of the motor executive and motor cognitive manipulative cortices that were affected by intervention. However, recent studies have indicated that the disrupted functional connectivity was also related to injuries in white matter tracts that were measured by diffusion tensor imaging [45, 46]. Further study combining functional connectivity with anatomical research is required to better understand the neural mechanisms of rehabilitative intervention.
Conclusions
Our findings revealed that the rehabilitation intervention contributed to restoring symmetry of the functional connectivity pattern between the left and right SMC and simultaneously increasing the connectivity with the motor cognitive cortices. We also found that systematic assessment of resting-state functional connectivity may provide prognostic insight for later motor improvement. This study not only shed light on the neural mechanisms of rehabilitative intervention after stroke but also provided a useful approach for evaluating therapeutic strategies of motor rehabilitation.
Reference
Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J (1992) Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke; a J Cerebral Circ 23(8):1084–1089
Calautti C, Baron JC (2003) Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke; A J Cereb Circ 34(6):1553–1566. doi:10.1161/01.str.0000071761.36075.a6
Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, Manelfe C, Chollet F (2003) Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage 20(4):2166–2180
Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F (2007) Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex (New York, NY: 1991) 17(12):2980–2987. doi:10.1093/cercor/bhm023
Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C (2011) Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage 55(3):1147–1158. doi:10.1016/j.neuroimage.2011.01.014
Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F (2004) A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage 23(3):827–839. doi:10.1016/j.neuroimage.2004.07.058
Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR (1997) A functional MRI study of subjects recovered from hemiparetic stroke. Stroke; A J Cereb Circ 28(12):2518–2527
Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM (2002) Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke; A J Cereb Circ 33(1):103–109
Sun L, Yin D, Zhu Y, Fan M, Zang L, Wu Y, Jia J, Bai Y, Zhu B, Hu Y (2013) Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: a longitudinal fMRI study. Neuroradiology 55(7):913–925. doi:10.1007/s00234-013-1188-z
Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain : J Neurol 126(Pt 11):2476–2496. doi:10.1093/brain/awg245
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103(37):13848–13853. doi:10.1073/pnas.0601417103
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100(1):253–258. doi:10.1073/pnas.0135058100
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356. doi:10.1523/jneurosci.5587-06.2007
Park JY, Kim YH, Chang WH, Park CH, Shin YI, Kim ST, Pascual-Leone A (2014) Significance of longitudinal changes in the default-mode network for cognitive recovery after stroke. European J Neurosci 40(4):2715–2722. doi:10.1111/ejn.12640
van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA (2014) A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Hum Brain Mapp 35(8):3919–3931. doi:10.1002/hbm.22448
Yin D, Song F, Xu D, Peterson BS, Sun L, Men W, Yan X, Fan M (2012) Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS One 7(12):e52727. doi:10.1371/journal.pone.0052727
Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH (2011) Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke; J Cereb Circ 42(5):1357–1362. doi:10.1161/strokeaha.110.596155
Golestani AM, Tymchuk S, Demchuk A, Goodyear BG (2013) Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair 27(2):153–163. doi:10.1177/1545968312457827
Barzel A, Ketels G, Tetzlaff B, Kruger H, Haevernick K, Daubmann A, Wegscheider K, Scherer M (2013) Enhancing activities of daily living of chronic stroke patients in primary health care by modified constraint-induced movement therapy (HOMECIMT): study protocol for a cluster randomized controlled trial. Trials 14:334. doi:10.1186/1745-6215-14-334
Braun SM, Beurskens AJ, Borm PJ, Schack T, Wade DT (2006) The effects of mental practice in stroke rehabilitation: a systematic review. Arch Phys Med Rehabil 87(6):842–852. doi:10.1016/j.apmr.2006.02.034
Brunnstrom S (1966) Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46(4):357–375
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S (1975) The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7(1):13–31
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6(9):e25031. doi:10.1371/journal.pone.0025031
Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2001) Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol 22(7):1326–1333
Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C (2010) Dynamic functional reorganization of the motor execution network after stroke. Brain : J Neurol 133(Pt 4):1224–1238. doi:10.1093/brain/awq043
Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC (2002) Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15(4):247–262
Tang Q, Li G, Liu T, Wang A, Feng S, Liao X, Jin Y, Guo Z, He B, McClure MA, Xing G, Mu Q (2015) Modulation of interhemispheric activation balance in motor-related areas of stroke patients with motor recovery: systematic review and meta-analysis of fMRI studies. Neurosci Biobehav Rev 57:392–400. doi:10.1016/j.neubiorev.2015.09.003
Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC (2007) The relationship between motor deficit and hemisphere activation balance after stroke: a 3T fMRI study. NeuroImage 34(1):322–331. doi:10.1016/j.neuroimage.2006.08.026
Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL (2000) Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke; J Cereb Circ 31(3):656–661
Woo CW, Krishnan A, Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage 91:412–419. doi:10.1016/j.neuroimage.2013.12.058
Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M (2010) Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67(3):365–375. doi:10.1002/ana.21905
Calautti C, Leroy F, Guincestre JY, Baron JC (2001) Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a fixed-performance paradigm. Stroke; J Cereb Circ 32(11):2534–2542
Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M (2006) Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain : J Neurol 129(Pt 3):791–808. doi:10.1093/brain/awh713
Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C (2006) The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26(22):6096–6102. doi:10.1523/jneurosci.4564-05.2006
Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55(3):400–409. doi:10.1002/ana.10848
Ward NS (2005) Mechanisms underlying recovery of motor function after stroke. Postgrad Med J 81(958):510–514. doi:10.1136/pgmj.2004.030809
Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007) Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56(1):171–184. doi:10.1016/j.neuron.2007.08.023
Azari NP, Binkofski F, Pettigrew KD, Freund HJ, Seitz RJ (1996) Enhanced regional cerebral metabolic interactions in thalamic circuitry predicts motor recovery in hemiparetic stroke. Hum Brain Mapp 4(4):240–253. doi:10.1002/(SICI)1097-0193(1996)4:4<240::AID-HBM2>3.0.CO;2-3 10.1002/(sici)1097-0193(1996)4:4<240::aid-hbm2>3.0.co;2-3
Leiguarda RC, Marsden CD (2000) Limb apraxias: higher-order disorders of sensorimotor integration. Brain : J Neurol 123(Pt 5):860–879
Talati A, Hirsch J (2005) Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on "what," "when," and "where" related information: an fMRI study. J Cogn Neurosci 17(7):981–993. doi:10.1162/0898929054475226
Jeannerod M, Frak V (1999) Mental imaging of motor activity in humans. Curr Opin Neurobiol 9(6):735–739
Perrett SP, Ruiz BP, Mauk MD (1993) Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci 13(4):1708–1718
He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M (2007) Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53(6):905–918. doi:10.1016/j.neuron.2007.02.013
Liu J, Qin W, Zhang J, Zhang X, Yu C (2015) Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke; J Cereb Circ 46(4):1045–1051. doi:10.1161/strokeaha.114.007044
Acknowledgments
This research was supported by the China National Nature Science Foundation (grant no. 81471651), the China National Nature Science Young Foundation (grant no. 81401859), the 12th Five-Year Plan supporting project of Ministry of Science and Technology of the People’s Republic of China (grant no. 2013BAI10B03), the Shanghai Zhabei District Health Bureau (grant no. 2014MS06) and the Shanghai Commission of Healthy and Family Planning (grant no. 201440634). We would like to thank all of the volunteers and stroke patients for their participation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the Institutional Ethics Committee of East China Normal University, Shanghai, China, and have therefore been performed in accordance with the ethical laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
XZ and LS are joint first authors and contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Comparison of the functional connectivity of the ipsilesional M1 between stroke patients (at both pre- and post-intervention) and HC. (DOCX 2500 kb)
Rights and permissions
About this article
Cite this article
Zheng, X., Sun, L., Yin, D. et al. The plasticity of intrinsic functional connectivity patterns associated with rehabilitation intervention in chronic stroke patients. Neuroradiology 58, 417–427 (2016). https://doi.org/10.1007/s00234-016-1647-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1647-4