Abstract
Introduction
Intracranial arteriovenous malformations (AVMs) display venous signals on arterial spin labeling (ASL) magnetic resonance (MR) imaging due to the presence of arteriovenous shunting. Our aim was to quantitatively correlate AVM signal intensity on ASL with the degree of arteriovenous shunting estimated on digital subtraction angiography (DSA) in AVMs.
Methods
MR imaging including pseudo-continuous ASL at 3 T and DSA were obtained on the same day in 40 patients with intracranial AVMs. Two reviewers assessed the nidus and venous signal intensities on ASL images to determine the presence of arteriovenous shunting. Interobserver agreement on ASL between the reviewers was determined. ASL signal intensity of the AVM lesion was correlated with AVM size and the time difference between normal and AVM venous transit times measured from the DSA images.
Results
Interobserver agreement between two reviewers for nidus and venous signal intensities was excellent (κ = 0.80 and 1.0, respectively). Interobserver agreement regarding the presence of arteriovenous shunting was perfect (κ = 1.0). AVM signal intensity showed a positive relationship with the time difference between normal and AVM venous transit times (r = 0.638, P < 0.001). AVM signal intensity also demonstrated a positive relationship with AVM size (r = 0.561, P < 0.001).
Conclusion
AVM signal intensity on ASL in patients with AVM correlates well with the degree of early vein opacification on DSA, which corresponds to the degree of arteriovenous shunting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial arteriovenous malformations (AVMs) are cerebral vascular malformations characterized by the presence of direct arteriovenous shunting with no intervening capillary beds. They form an abnormal tangle of blood vessels, the so-called nidus, which is extremely fragile and at high risk for bleeding; in fact, 40–70 % of patients with AVMs present with intracranial hemorrhages (ICHs), accounting for 2–4 % of overall hemorrhagic strokes [1–4]. Digital subtraction angiography (DSA) has long been the gold standard for the diagnosis of AVMs, because it clearly depicts angioarchitectural characteristics, such as the feeding artery, nidus, and venous drainage.
The treatment options for intracranial AVMs include microsurgery, stereotactic radiosurgery, and endovascular embolization [5]. As the treatment often comprises multimodality and/or multistep procedures [4] and as it may take up to 3 years or longer for AVMs to be obliterated after radiosurgery [6], many patients undergo frequent follow-up imaging studies, among which DSA is the mainstay. However, DSA is invasive and poses an inherent risk, although small [7]. Therefore, a non-invasive imaging modality would be desirable for AVM patients undergoing follow-up in particular.
Arterial spin labeling (ASL) is a relatively new magnetic resonance (MR) technique that utilizes water protons in the arterial water blood as endogenous tracers to assess cerebral blood flow (CBF) [8]. Arterial blood protons labeled at the proximal portion to the brain with radiofrequency pulses diffuse into the brain tissue once they reach the capillary bed. Obtaining a signal at a certain time delay, in which most labeled protons can be found in the capillaries, renders the tissue perfusion signal. Typically, the labeled protons lose their signal before entering the veins because the T1 decay occurs during the transit time and the tissue exchange. However, under the presence of arteriovenous shunting, arterial blood moves directly into the veins without passing the capillaries or brain tissue, and the labeled protons may retain their signals in the intracranial veins. Thus, this phenomenon contributes to the venous ASL signal intensity [9–12]. Recently, Le et al. [12] demonstrated that venous ASL signals improved the detection of small intracranial AVMs.
The detectability of AVMs using ASL may be influenced by the conspicuity of venous ASL signal intensity. Because the signal intensity in a voxel on ASL is theoretically determined by the numbers of labeled protons in the corresponding area, ASL signal intensity in the nidus or the draining vein of an AVM may reflect the degree of arteriovenous shunt. The degree of arteriovenous shunting, on the other hand, could be demonstrated by the degree of early visualization of AVM draining vein on DSA. Therefore, the purpose of this study was to correlate signal intensity of the AVM lesion measured on ASL with the degree of arteriovenous shunting estimated on DSA in patients with intracranial AVMs. To our knowledge, there have been no studies that have quantitatively correlated venous ASL signal intensity with DSA findings.
Materials and methods
This study was approved by the institutional review board at our institution, and informed consent was waived. Demographic and radiographic data prospectively recorded in the database were retrospectively reviewed.
Patient population
From March 2011 through February 2012, 40 patients with confirmed intracranial AVM on previous session of DSA who had been planning to undergo Gamma Knife surgery (GKS) were enrolled in the study. Of these, 25 patients had received no prior treatment for AVM, 14 patients had previously undergone one or more session of GKS, and one patient had been previously treated with embolization using Onyx (ev3 Neurovascular, Irvine, CA).
Imaging methods
All patients underwent catheter-based DSA and brain MR imaging, including ASL, to localize the AVMs for GKS on the same day. The biplane angiography unit (Integris Allura Systems; Philips Healthcare, Best, the Netherlands) was used for DSA examinations, which included anteroposterior and lateral projections with the selective injection of the appropriate internal carotid, external carotid, and/or vertebral arteries with nonionic monomeric iodine contrast medium (Iopamidol, Pamiray 250, Dongkook Pharmaceutical, Seoul, Korea), using a 5-Fr catheter. A power injector was used for contrast administration. The injection rate was determined after assessing the patient’s vascular conformation on test bolus injection, which mostly ranged from 5 to 9 mL/s.
MR imaging was performed on a 3-T MR scanner (Verio; Siemens Medical Solutions, Erlangen, Germany) with a pre- and post-enhanced T1-weighted three-dimensional spoiled gradient echo sequence of 1.5-mm slice thickness and fast spin echo T2-weighted sequence of 1.5-mm slice thickness. Contrast enhancement was achieved with 0.1 mmol/kg gadobutrol (Gadovist, Bayer Schering Pharmaceutical, Berlin, Germany).
The ASL perfusion imaging was performed using a pseudo-continuous ASL pulse sequence with a background-suppressed three-dimensional GRASE single-shot readout (labeling pulse duration = 1.5 s, post-labeling delay = 1.6 s, no flow crushing gradient, repetition time = 3660 ms, echo time = 14 ms, field of view = 24 × 24 cm2, matrix = 64 × 64, slice thickness = 5 mm, rate-2 GRAPPA, 60 pairs of tags and controls acquired in 4 min, whole brain coverage) [13]. The signal intensity change between the labeled image and the control image was fitted to a model, from which a quantitative perfusion map of CBF was obtained.
Image analysis
ASL images were independently reviewed by two neuroradiologists (C-H.S. and L.S.) blinded to the patient histories and DSA findings. The reviewers determined the presence of nidus or venous ASL signal intensity. Nidus signal intensity was defined as focal or serpiginous high signal intensity in the brain parenchyma. Venous signal intensity was defined as serpiginous high signal intensity along the location of the cortical veins or major venous structures (superior sagittal sinus, transverse sinus, sigmoid sinus, straight sinus, vein of Galen, and internal cerebral vein). The reviewers then stated whether they thought arteriovenous shunting was present. A region of interest (ROI) of approximately 10 mm2 was placed in the region of maximum ASL signal intensity within the nidus or veins of interest as determined visually from the ASL map. At least three separate ROI measurements were made, and the maximum ASL signal intensity value was recorded as the AVM signal intensity (SIAVM) in each subject. To assess the potential effect of partial volume averaging, the diameter of a draining vein of interest was also recorded.
The two reviewers also analyzed the DSA images by consensus, in terms of Spetzler-Martin grade [14], arterial transit time, nidus opacification time, draining vein opacification time, normal venous transit time, and AVM size. The time difference between AVM and normal venous transit time (ΔT normal-AVM, s) was calculated by subtracting the draining vein opacification time from the normal venous transit time in each patient. AVM size was indirectly measured using the ratio of maximum dimension on either anteroposterior or lateral projection DSA images to the interval between the radiopaque markers on a stereotactic frame. Because the actual distance between the markers was fixed as 5 cm, AVM size was determined by multiplying 5 to the measured ratio.
Statistical analysis
To assess interobserver agreement for the evaluation of ASL images, we calculated the κ statistic for the two reviewers. Agreement between the reviewers was expressed as a κ value that accounted for the chance agreement between the two reviewers. κ values of less than 0 indicated a negative agreement; those of 0–0.20 indicated a positive but poor agreement, those of 0.21–0.40 indicated a fair agreement, those of 0.41–0.60 indicated a moderate agreement, those of 0.61–0.80 indicated a good agreement, and those of greater than 0.81 indicated an excellent agreement.
Pearson’s regression model was used to describe the association between SIAVM and other variables such as ΔT normal-AVM, AVM size, and draining vein diameter. SIAVM was correlated with ΔT normal-AVM after logarithmic transformation, as we assumed that these parameters did not exhibit linear relationships. Student’s t tests were performed to compare the mean values of the variables. The Kolmogorov-Smirnov test was used to determine whether values were normally distributed.
All statistical analyses were performed with MedCalc Software (Version 14.8.1 for Microsoft Windows XP/Vista/7/8, MedCalc Software, Mariakerke, Belgium). The results with P values less than 0.05 were considered statistically significant.
Results
Patient demographics
The mean age of the patients was 37.4 ± 15.1 years (range, 14–72 years). There were 17 females and 23 males in the subjects. The presenting symptoms of untreated patients were as follows: headache in eight patients, ICH in six patients, seizure in six patients, visual field defect in two patients, asymptomatic in two patients, and hemiparesis in one patient. The follow-up period for the GKS-treated patients ranged from 25 to 108 months (mean, 53.0 ± 26.8 months). Among the previously treated patients, three subjects (3/15, 20 %) demonstrated complete obliteration of the nidus on DSA. Excluding these three cases, the mean size of AVMs was 1.92 ± 0.86 cm (range, 0.73 – 5.0 cm).
Image analysis
The κ values of interobserver agreement between the two reviewers for ASL findings are shown in Table 1. The agreements on the presence of nidus and venous signal intensities were excellent (κ = 0.80 and 1.0, respectively). The overall agreement for the presence of arteriovenous shunting was perfect (κ = 1.0).
The nidus signal intensity was interpreted as positive in 33 cases (82.5 %) and negative in five cases (12.5 %) by both reviewers. With respect to venous signal intensity, the reviewers concordantly responded as positive in the 33 cases (82.5 %). A representative case demonstrating nidus and venous signal intensities is described in Fig. 1.
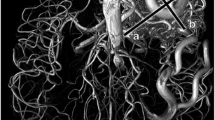
A 22-year-old male who presented with headache. a Axial arterial spin labeling (ASL) image shows a large mass with strong hyperintensity in the right occipitotemporal lobe (arrow). The image also shows intense signal intensity along the course of straight sinus (arrowheads). b Axial post-contrast T1-weighted image shows multiple tubular enhancing structures in the right occipitotemporal lobe (arrow). They represent a nidus with dilated veins. c Lateral digital subtraction angiography (DSA) confirms an arteriovenous malformation (AVM, arrow) fed by right posterior cerebral artery, with dilated venous structures draining into the straight sinus (white arrowheads) or superior sagittal sinus (SSS) via a cortical vein (black arrowheads). d Follow-up ASL image obtained 43 months after a Gamma Knife surgery (GKS) reveals a reduced nidus signal intensity (arrow) in the right occipitotemporal lobe. e Lateral DSA performed 2 months prior to (d) demonstrates a markedly diminished nidus (arrow)
Disagreement about the nidus signal was observed in two patients (5.0 %). The nidus signal was masked by a magnetic susceptibility artifact in one case (Fig. 2). In another case, the nidus was located in the vicinity of a draining cortical vein, so the nidus signal intensity was hard to differentiate from the venous signal intensity. There were four cases (10.0 %) that showed nidus ASL signal intensity but no discernible venous signal intensity (Fig. 3).
A 30-year-old male with left homonymous hemianopsia. a, b Axial ASL images. There is a focal asymmetric high signal intensity adjacent to the signal void area in the right temporal lobe (a, arrow). One of the reviewers overlooked this intensity, although he detected a serpiginous high signal intensity along the location of a cortical vein in the right temporal lobe (b, arrowheads), which he assessed as a venous signal intensity. After discussion, the two reviewers concluded that the high signal intensity in the right temporal lobe (a) represented a nidus signal. c Axial post-contrast T1-weighted image shows a small enhancing vascular lesion (arrow) in the right temporal lobe. d Lateral DSA confirms a temporal AVM (arrow) fed by multiple feeders originating from the right middle cerebral artery (MCA) and engorged veins (arrowheads) draining into the sphenoparietal or transverse sinus. e Lateral plain radiograph shows a radiopaque cast in the corresponding area (arrow). The patient had previously undergone endovascular embolization using Onyx (not shown)
A 54-year-old male who underwent GKS for a right frontal AVM 37 months ago. a Axial ASL image shows a high signal intensity in the right frontal lobe (arrowhead). Both reviewers interpreted this lesion as a nidus signal intensity. No venous signal intensity is clearly demonstrated. b Axial post-contrast T1-weighted image shows a small enhancing lesion (arrowhead) in the right frontal lobe. c Lateral DSA confirms a small nidus (arrow) with a cortical vein (arrowheads) draining into the SSS
In all three cases with complete obliteration of the nidus, the reviewers agreed that there was no nidus or venous signal on ASL. In one of these cases, the obliterated nidus showed focal contrast enhancement with surrounding T2 hyperintensity change, which was interpreted as a radiation-induced change (Fig. 4).
A 28-year-old male who underwent GKS for a right parietal AVM. Axial post-contrast T1-weighted image (a) and DSA (b) performed 38 months after initial GKS as a GKS-planning study. a A few enhancing tubular structures (arrow) indicate residual AVM at the right parietal lobe. b Lateral DSA depicts a residual AVM (arrows) supplied by the right MCA with a cortical vein (arrowheads) draining into the SSS. Axial post-contrast T1-weighted image (c), DSA (d), and axial ASL image (e) performed 88 months after initial GKS and 50 months after the second GKS. c There is remaining clustered enhancement at the right parietal lobe (arrow) that gives rise to the suspicion of a residual nidus. d However, the AVM is completely obliterated without demonstrable nidus on DSA. e On ASL, no abnormal venous signal intensity is noted in the corresponding area
There was a positive correlation between SIAVM and ΔT normal-AVM (Fig. 5, r = 0.638, P < 0.001). This relationship was still significant in both patients who received GKS treatment and those who did not (P = 0.015 and P = 0.042, respectively). SIAVM also exhibited a positive relationship with the size of the AVM (r = 0.561, P < 0.001). The mean diameter of the draining veins was 8.54 ± 1.85 mm (range, 3.56–12.04 mm). No significant correlation was noted between AVM signal intensity and the diameter of the draining vein (P = 0.093).
The ΔT normal-AVM in the treated group was significantly shorter than that in the untreated group (3.02 ± 1.00 s vs. 3.75 ± 0.70 s, P = 0.015). In the untreated group (n = 25), SIAVM was significantly lower in six patients who presented with hemorrhage than that in 19 patients who presented with symptoms other than hemorrhage or who were asymptomatic (562.4 ± 132.1 vs. 1137.4 ± 606.1, P < 0.001).
Spetzler-Martin grade of patients with residual AVMs (n = 37) ranged between grade 1 and grade 3 (Table 2). There was a trend towards larger AVM size with increasing Spetzler-Martin grade, but this was not statistically significant. There was also no significant difference in SIAVM or ΔT normal-AVM according to the Spetzler-Martin grade.
Discussion
In the present study, we have demonstrated that the maximum ASL signal intensity in an AVM nidus or the draining veins correlated well with the time difference between the normal vein opacification and AVM draining vein opacification and with the AVM size. In addition, we have shown that interobserver agreement on the presence of a nidus signal was excellent. Overall agreement for the presence of an arteriovenous shunting was perfect. Patients without hemorrhagic presentation showed a higher AVM ASL signal intensity than those who initially presented with hemorrhage.
Because there is no signal in the veins on ASL under normal conditions, nidus or venous ASL signal intensity is a robust sign of the presence of an arteriovenous shunt [10–12]. The intensity of venous signal on ASL in a patient with an AVM theoretically stands for the numbers of labeled protons in the veins, which is related to the degree of shunt. The higher the shunt rate is, the sooner the draining vein should be opacified. Therefore, we assumed that the degree of shunting on DSA could be expressed as the difference between the time of AVM draining vein opacification and the time at which the normal veins are opacified (ΔT normal-AVM). SIAVM showed a significant correlation with ΔT normal-AVM, in line with our hypothesis.
In this study, more than half of the AVMs were smaller than 2 cm (21/37, 56.8 %), but venous signal intensity proved useful in detecting arteriovenous shunt in patients with intracranial AVMs. The nidus of an AVM could also be identified in many cases by carefully tracing the venous signal to the upstream (Fig. 2). An AVM showing faint ASL signal intensity may not be easy to recognize (Fig. 3), but our results suggest that once it has been detected, it likely have a slow shunt flow. Therefore, we believe that ASL is applicable to the evaluation of small-sized AVMs with relatively slow shunts.
Subgroup analysis revealed that the correlation between SIAVM and ΔT normal-AVM was also significant for both GKS-treated patients and non-GKS-treated patients. Pollock et al. [11] have exhibited that AVM nidal flow measured from ASL CBF map showed a trend of a serial decline over time after GKS. One subject in our study exhibited a nidus size reduction and a decreased venous ASL signal intensity on the follow-up study after GKS (Fig. 1). Another patient demonstrated disappearance of venous signal after 3 years’ interval, along with angiographical obliteration of the nidus. Taken together, after a validation study that includes pre- and post-treatment exams in each patient, venous signal intensity as a marker of the degree of shunt flow may have clinical implications in post-treatment monitoring and assessing the treatment efficacy.
According to Spetzler et al. [14], the major factors determining the difficulty of AVM operation include size, number of feeding arteries, amount of shunt flow, location, eloquence of neighboring brain, and venous drainage pattern. By simplifying these factors to three variables (size, eloquence of adjacent brain, and venous drainage), they proposed a grading system, namely the Spetzler-Martin grade, which is widely used in clinical practice to predict the surgical outcomes of patients with intracranial AVMs [15]. The amount of shunt flow is important in describing the steal phenomenon [16–20], which occurs as a result of blood flow deprivation in the adjacent brain tissue by a low-pressure system in an AVM. Certain previous reports [18, 20, 21] proposed that the presence of cerebral steal may be a protective factor for hemorrhage. In our study, we observed that patients who presented with hemorrhage showed significantly lower SIAVM than those who presented with symptoms other than hemorrhage or were asymptomatic. This might support the speculation that cerebral steal phenomenon, reflected as relatively higher SIAVM, affects the risk of hemorrhage. However, it is impossible to evaluate whether a lower SIAVM in a patient who presented with hemorrhage is a consequence of the hemorrhage or not in this study.
Radiation-induced changes following GKS include vascular damages with blood-brain barrier breakdown, ischemia, vasogenic edema, demyelination, and radiation necrosis [22, 23]. As a result, development of signal change on follow-up T2-weighted and/or FLAIR images was noted in 62 % of the patients who had undergone GKS in one report [6]. Likewise, abnormal enhancement was observed in 60 % of the patients with obliterated AVMs in another report [22]. In this study, there were two such cases which showed mild residual enhancement around the areas of angiographically confirmed obliterated AVMs but did not reveal any nidus or venous signal intensity on ASL (Fig. 4). This finding suggests a clue that venous ASL signal intensity might help differentiating radiation-induced change from residual arteriovenous shunting.
ASL has been recently drawing increased interest from clinicians and radiologists due to its capacity to quantify absolute CBF data without using a contrast medium. As such, ASL can be conveniently performed and reliably evaluated in a patient who requires repeated follow-up imaging studies. One limitation of ASL is its susceptibility to the magnetic field distortion caused by neurosurgical hardware, calcification, blood products, and air, any of which can lead to a decreased signal intensity [24]. A high shunt flow also produces signal loss and may hinder the interpretation of nidus signal intensity. Under these circumstances, recognizing abnormal signals in the sinus becomes particularly helpful for detecting abnormal arteriovenous shunting (Fig. 2). ASL also suffers from the intrinsic low spatial resolution. To minimize the potential influence of partial volume averaging effect, we intended to draw as small ROIs as possible (approximately 3.5 mm in diameter). As a result, no significant correlation was noted between AVM signal intensity and the diameter of the draining vein (P = 0.093). Hence, we believe the partial volume averaging effect did not play a significant role in our results.
Aside from the retrospective design, there are a few limitations in this study. First, the sample size was relatively small, and there was no follow-up study in each patient. In addition, the case number of negative control group is too small compared with that of the study group. Second, we did not perform comparison studies with conventional imaging findings to seek for the added values of venous signal intensity on ASL in the diagnosis of AVM. Third, we used a fixed post-labeling delay time without changing this value from the routine imaging studies. Because the rate of shunt differs among the patients, there could be unknown bias in our results, although we believe such a value would be small. Considering that ASL is free from issues pertaining to radiation and contrast injection, the performance of a prospective study design that includes negative controls with follow-up imaging studies would be desirable.
In conclusion, venous ASL signal intensity can help determining the presence of arteriovenous shunting reproducibly. AVM signal intensity on ASL correlates well with the degree of early vein opacification on DSA, which in turn corresponds to the degree of arteriovenous shunting.
References
Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A (2008) Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 63:823–829
Crawford PM, West CR, Chadwick DW, Shaw MD (1986) Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry 49:1–10
Ondra SL, Troupp H, George ED, Schwab K (1990) The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg 73:387–391
Choi JH, Mohr JP (2005) Brain arteriovenous malformations in adults. Lancet Neurol 4:299–308
van Beijnum J, van der Worp HB, Buis DR et al (2011) Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 306:2011–2019
Han JH, Kim DG, Chung HT et al (2008) Clinical and neuroimaging outcome of cerebral arteriovenous malformations after Gamma Knife surgery: analysis of the radiation injury rate depending on the arteriovenous malformation volume. J Neurosurg 109:191–198
Kaufmann TJ, Huston J 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF (2007) Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 243:812–819
Detre JA, Zhang W, Roberts DA et al (1994) Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed 7:75–82
Kukuk GM, Hadizadeh DR, Bostrom A et al (2010) Cerebral arteriovenous malformations at 3.0 T: intraindividual comparative study of 4D-MRA in combination with selective arterial spin labeling and digital subtraction angiography. Investig Radiol 45:126–132
Wolf RL, Wang J, Detre JA, Zager EL, Hurst RW (2008) Arteriovenous shunt visualization in arteriovenous malformations with arterial spin-labeling MR imaging. AJNR Am J Neuroradiol 29:681–687
Pollock JM, Whitlow CT, Simonds J et al (2011) Response of arteriovenous malformations to gamma knife therapy evaluated with pulsed arterial spin-labeling MRI perfusion. AJR Am J Roentgenol 196:15–22
Le TT, Fischbein NJ, Andre JB, Wijman C, Rosenberg J, Zaharchuk G (2012) Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations. AJNR Am J Neuroradiol 33:61–68
Fernandez-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA (2008) Minimizing acquisition time of arterial spin labeling at 3 T. Magn Reson Med 59:1467–1471
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
Hamilton MG, Spetzler RF (1994) The prospective application of a grading system for arteriovenous malformations. Neurosurgery 34:2–6
Nornes H, Grip A, Wikeby P (1979) Intraoperative evaluation of cerebral hemodynamics using directional Doppler technique. Part 1: arteriovenous malformations. J Neurosurg 50:145–151
Homan RW, Devous MD Sr, Stokely EM, Bonte FJ (1986) Quantification of intracerebral steal in patients with arteriovenous malformation. Arch Neurol 43:779–785
Nataf F, Meder JF, Roux FX et al (1997) Angioarchitecture associated with haemorrhage in cerebral arteriovenous malformations: a prognostic statistical model. Neuroradiology 39:52–58
Nornes H, Grip A (1980) Hemodynamic aspects of cerebral arteriovenous malformations. J Neurosurg 53:456–464
Norbash AM, Marks MP, Lane B (1994) Correlation of pressure measurements with angiographic characteristics predisposing to hemorrhage and steal in cerebral arteriovenous malformations. AJNR Am J Neuroradiol 15:809–813
Marks MP, Lane B, Steinberg GK, Chang PJ (1990) Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology 176:807–813
Kihlstrom L, Guo WY, Karlsson B, Lindquist C, Lindqvist M (1997) Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery. J Neurosurg 86:589–593
Izawa M, Hayashi M, Chernov M et al (2005) Long-term complications after gamma knife surgery for arteriovenous malformations. J Neurosurg 102(Suppl):34–37
Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA (2008) Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol 29:1228–1234
Ethical standards and patient consent
We declare that all human and animal studies have been approved by our Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that due to the retrospective nature of this study, informed consent was waived.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunwoo, L., Sohn, CH., Lee, J.Y. et al. Evaluation of the degree of arteriovenous shunting in intracranial arteriovenous malformations using pseudo-continuous arterial spin labeling magnetic resonance imaging. Neuroradiology 57, 775–782 (2015). https://doi.org/10.1007/s00234-015-1533-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1533-5