Abstract
Objectives
To evaluate the usefulness of 4D-MR angiography based on super-selective pseudo-continuous ASL combined with keyhole and view-sharing (4D-S-PACK) for vessel-selective visualization and to examine the ability of this technique to visualize brain arteriovenous malformations (AVMs).
Methods
In this retrospective study, 15 patients (ten men and five women, mean age 44.0 ± 16.9 years) with brain AVMs were enrolled. All patients were imaged with 4D-PACK (non-selective), 4D-S-PACK, and digital subtraction angiography (DSA). Observers evaluated vessel selectivity, identification of feeding arteries and venous drainage patterns, visualization scores, and contrast-to-noise ratio (CNR) for each AVM component. Measurements were compared between the MR methods.
Results
Vessel selectivity was graded 4 in 43/45 (95.6%, observer 1) and 42/45 (93.3%, observer 2) territories and graded 3 in two (observer 1) and three (observer 2) territories. The sensitivity and specificity for identification of feeding arteries for both observers was 88.9% and 100% on 4D-PACK, and 100% and 100% on 4D-S-PACK, respectively. For venous drainage, the sensitivity and specificity was 100% on both methods for observer 1. The sensitivity and specificity for observer 2 was 94.4% and 83.3% on 4D-PACK, and 94.4% and 91.7% on 4D-S-PACK, respectively. The CNRs at the timepoint of 1600 ms were slightly lower in 4D-S-PACK than in 4D-PACK for all AVM components (Feeding artery, p = .02; nidus, p = .001; and draining artery, p = .02). The visualization scores for both observers were not significantly different between 4D-PACK and 4D-S-PACK for all components.
Conclusions
4D-S-PACK could be a useful non-invasive clinical tool for assessing hemodynamics in brain AVMs.
Key Points
• The 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) enabled excellent vessel selectivity.
• The 4D-S-PACK enabled the perfect identification of feeding arteries of brain arteriovenous malformation (AVM).
• 4D-S-PACK could be a non-invasive clinical tool for assessing hemodynamics in brain AVMs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arteriovenous malformation (AVM) of the brain is a vascular disorder that can cause serious intracranial hemorrhage, with an annual rate of rupture of 2–3% [1, 2]. The resultant bleeding has a high morbidity and mortality rate; it was reported that the inpatient mortality rate among patients with hemorrhage due to brain AVM was 12.9% [3], and 23–33% of the hemorrhage survivors remained severely disabled [4,5,6]. The accurate evaluation of brain AVMs including grading is necessary to prevent future hemorrhages, reduce intraoperative complications, and ensure better clinical outcomes for patients. Thus, effective vascular imaging methods with high diagnostic performance are desirable for optimal medical care.
Digital subtraction angiography (DSA) is the current reference standard for the diagnosis, treatment planning, and post-treatment monitoring of brain AVMs. However, DSA is an invasive procedure that carries the risk of neurological complications and adverse reactions associated with contrast agents. Dynamic contrast-enhanced MR angiography is a method to visualize brain AVMs in a time-resolved fashion; however, both spatial and temporal resolutions are often limited [7]. Thus, it is desirable to establish non-contrast-enhanced examination methods [8].
The use of arterial spin labeling (ASL) for MR angiography has drawn increasing attention, especially for the intracranial region. As a non-invasive approach, ASL has shown a capacity for non-contrast-enhanced intracranial MR angiography with 3D spatial and 4D dynamic acquisition, in neurovascular diseases such as AVMs and dural arteriovenous fistula [9,10,11,12,13,14,15].
4D-MR angiography based on pseudo-continuous arterial spin labeling (pCASL) combined with contrast-enhanced timing-robust angiography (CENTRA)-keyhole and view-sharing, named 4D-PACK, has been proposed as a 4D-MR angiography technique [14, 16]. The keyhole and view-sharing are established acceleration techniques. In the keyhole technique, only the central portion of k-space is sampled for every timepoint, except at the reference point. The missing profiles in the peripheral portion of k-space are completed by data from reference scan. In the view-sharing technique, the central portion is subdivided into three regions. While the central region is acquired at every timepoint, the peripheral regions are acquired in an alternating fashion, and missing profiles are copied from the next data timepoint. By combining these techniques with ASL-based 4D MR angiography, good peripheral artery visualization, even with longer transit times as in Moyamoya disease, was demonstrated [14]. However, 4D-PACK, like many other techniques, lacks the ability to achieve vessel-specific visualization or vessel selectivity, targeting the vascular tree originating from the selected artery [17]. Vessel selectivity is necessary, especially for the accurate identification of feeding arteries, detailed preoperative evaluations or planning of DSA, interventional procedures, and surgical operations. Recently, there have been reports of 4D-MR angiography attempting vessel-selective flow visualization, another clinically important demand that DSA is capable of [18,19,20,21].
In 2010, the super-selective pCASL technique was introduced for single vessel-selective labeling for regional cerebral perfusion imaging [22, 23]. This technique was also applied to vessel-selective 3D angiography [24]. Recently, we proposed combining 4D-PACK with super-selective pCASL as a new vessel-selective 4D-MR angiography technique, referred to as 4D-S-PACK (4D-MRA based on super-selective pCASL with CENTRA-keyhole and view-sharing) hereafter [25]. The aim of the present study was to evaluate the vessel selectivity of 4D-S-PACK and its ability to visualize brain AVMs compared to that of non-selective 4D-PACK and DSA.
Materials and methods
This retrospective study was approved by the Institutional Review Board of Kyushu University Hospital University Hospital (No. 2019-367), and the requirement for informed consent was waived. Two authors (M.O., M.V.C.) were employees of Philips Healthcare and provided technical support for sequence development but were not involved in the study design or interpretation of the data. The institutional authors, who were not employees of Philips Healthcare, were responsible for all handling of data.
Patients
We analyzed the MRI data from 15 consecutive patients with brain AVMs identified between March 2016 and March 2019 who underwent both MR angiography and DSA examination within an interval of 3 months. The definitive diagnosis of brain AVM was made with DSA in all patients. The exclusion criteria for this study were as follows: (1) the DSA revealed no AVMs (N = 0); (2) new hemorrhages or other neurological events appeared in this period (N = 0); (3) severe motion artifacts were observed in the images (N = 0).
MR angiography
The MR angiography was performed using a 3-T scanner (Ingenia 3.0TX, Philips Healthcare). Figure 1 describes the principles of 4D-S-PACK. Labeling of the right and left internal carotid artery (ICA), and the bilateral vertebral arteries, was performed using this method. The labeling focus was placed in the upper cervical segment of the ICA or the second segment of the vertebral arteries (Fig. 2). For the labeling of ICAs, the gradient moment was set at 0.75 mT/m/ms in both the right-to-left and anterior-to-posterior directions, to create a circular labeling spot with a diameter of approximately 2 cm. For the labeling of the vertebral arteries, the gradient moment was set at 0.75 mT/m/ms only in the anterior-to-posterior direction to simultaneously label the bilateral vertebral arteries and not to label the ICAs.
Super-selective sequence and 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) scheme. a The gradient in the Gz direction of the blood flow and radiofrequency (RF) pulse train are applied as a pseudo-continuous arterial spin labeling (pCASL) sequence. In addition, the gradients perpendicular to the flow direction (Gx and Gy) are added as super-selective parts. This represents 32 ms of the total label duration, and this block is repeatedly applied according to the total label duration. b The inflow dynamic data were collected by multiple acquisitions, by changing the control or label duration. The data for 100 ms was used as a reference for the first four timepoints (100–800 ms), and 2000 ms was used as a reference for the last three timepoints (1200–2000 ms), with a keyhole percentage of 70%. Echo data were shared with adjacent timepoint data using the view-sharing technique to further accelerate the scan. The central disc in the K-space was subdivided into three regions, namely P+, C, and P−. The size of the central region C (central%) was set at 36.4% and, consequently, both peripheral regions, P+ and P−, to 16.8% of the total K-space samples. The scan time was 5 min 0 s
The locations of labeling spots. The size of the labeling focus can be adjusted by changing the zeroth moment of the transverse gradients to label individual vessels. The labeling focus was placed on the upper cervical segment of the internal carotid artery (ICA) or the second segment of the vertebral arteries. For the labeling of ICAs (top and middle rows), the gradient moment was set at 0.75 mT/m/ms in both right-to-left and anterior-to-posterior directions to create a circular labeling spot with a diameter of approximately 2 cm. For the labeling of the vertebral arteries (bottom row), the gradient moment was set at 0.75 mT/m ms in only the anterior-to-posterior direction to simultaneously label the bilateral vertebral arteries. The nidus (arrow) fed by the right posterior cerebral artery is depicted in the labeling of the vertebral arteries
Images were obtained by changing the label duration between 100, 200, 500, 800, 1200, 1600, and 2000 m. The imaging parameters were as follows: sequence, 3D T1 turbo field echo; repetition time/echo time, 5.0/1.8 ms; flip angle, 11°; echo train length, 60; slab thickness, 80 mm; voxel size, 1.0 × 1.4 × 1.6 mm; sensitivity encoding factor, 3.0. The keyhole (%) was set to 70%, the size of the central region C (central%) to 36.4%, and, consequently, both peripheral regions, P+ and P−, to 16.8% of the total k-space samples. Scans were further accelerated with view-sharing. The acquisition time for each artery was 5 min 0 s.
In 4D-PACK (non-selective), images were obtained with label durations of 100, 200, 400, 600, 800, 1200, 1600, and 2200 ms. The other parameters were the same as those used for 4D-S-PACK. The acquisition time was 6 min 5 s.
The TOF MR angiography was performed using the following parameters: sequence, 3D gradient-echo; repetition time/echo time, 20/3.5 ms; flip angle, 20°; slab thickness, 120 mm with four chunks: voxel size, 0.39 × 0.77 × 1.0 mm; sensitivity encoding acceleration factor, 2.0; and acquisition time, 6 min 9 s. For reference, several standard MR images, including T1-weighted, T2-weighted, and fluid attenuation inversion recovery, were acquired.
DSA
DSA was performed with a standard protocol on a biplane system (ARTIS zee, Siemens). Frontal and lateral views were obtained after the injection of a 4–10-mL bolus of iodinated contrast agent (Iopamiron, Bayer) at 2–5 mL/s in each ICA and (at least one) vertebral artery.
Image evaluation
Vessel selectivity
In the 4D-S-PACK series, the two observers evaluated vessel selectivity based on the grading system used in a previous study [19]: score 1, clearly depicting unlabeled vessels; score 2, partly depicting unlabeled vessels, influencing the imaging interpretation and diagnosis; score 3, slightly depicting unlabeled vessels with no influence on the diagnosis; score 4, complete selectivity with almost no visualization of unlabeled vessels.
Observer test
Two board-certified neuroradiologists (observer 1 with 11 years of experience and observer 2 with 5 years of experience), who were blinded to each patient’s clinical information to reduce bias, independently participated in the observer tests. Both observers took part in two reading sessions ≥ 1 month apart. Each session was comprised of half 4D-PACK and half 4D-S-PACK images in a random manner. One 4D-MR angiography (4D-PACK or 4D-S-PACK) image was displayed at a time, on a 20.8-in. liquid crystal display monitor of a picture archiving and communication system. Images other than those from the selected series were not displayed. The observers assessed only full maximum intensity projection (MIP) images of each 4D-MR angiography. The observers were asked to report the size of the nidus, the feeding arteries (anterior, middle, posterior cerebral arteries, and superior cerebellar artery), and the venous drainage pattern (superficial and/or deep). The size of nidus was defined as the longest diameter of the nidus measured on the full MIP images of each MR angiography (axial, coronal, or sagittal view). In addition, the Spetzler-Martin grade was assessed with the TOF MR angiography, 4D-PACK, or 4D-SPACK by both observers. The eloquence of the lesion location was assessed using both one of MR angiographies and T2-weiteged images since it was difficult to identify the anatomical location only with MR angiographies.
Visualization score for each AVM component
The visualization of each AVM component on the 4D-MR angiography was evaluated with a grading scale by the two neuroradiologists. The DSA image and an MR angiography (4D-PACK or 4D-S-PACK) image from the same patient were concurrently displayed on two monitors. The visualization of the feeding artery, nidus, and draining veins was assessed using the DSA image as the reference standard with a 5-point scale: 1, no visualization; 2, poor (1~25%); 3, moderate (26~50%); 4, good (51~75%, low signal); and 5, excellent (76~100%, high signal).
Quantitative assessment of visualization
The contrast-to-noise ratio (CNR) was measured in each AVM component by another board-certified neuroradiologist (19 years of experience). First, 3–5 circular or oval regions-of-interest (ROIs, typical size 50–70 mm2) were placed on each AVM component (feeding arteries, nidus, or draining veins), as well as on the background stationary tissues in the brain parenchyma. The ROI placement was performed with reference to the DSA, and best efforts were made to match locations of the ROIs on both types of MR angiographies.
The vessel-to-stationary tissue CNRs were calculated using the following equation: CNR = (Vesselmax − STave)/STSD, where Vesselmax is the maximum signal intensity in the ROIs [14]. The STave and STSD are the mean signal intensity and standard deviation in the stationary tissue ROI, respectively.
Statistical analysis
The nidus size measured with DSA and one of the MR angiographies was compared using a paired t test. The interobserver agreement between the two observers for the nidus size was determined using the intraclass correlation coefficient (ICC; ~ 0.4, poor agreement; 0.40~0.59, fair agreement; 0.60~0.74, good agreement; 0.75~1.0 excellent agreement) [26]. The identification of the feeding artery and the venous drainage pattern was compared between DSA, 4D-PACK, and 4D-S-PACK by the chi-square test. The Spetzler-Martin grade assessed with DSA and one of the MR angiographies (TOF MR angiography, 4D-PACK, or 4D-S-PACK) was compared using the Wilcoxon matched-pairs signed-rank test. The agreement between DSA and one of MR angiographies for the Spetzler-Martin grade was evaluated using the ICC. The scores for visualization of AVM components and the CNRs at the 1600-ms timepoint were compared between the 4D-PACK and 4D-S-PACK using the Wilcoxon matched-pairs signed-rank test. We selected this timepoint since this was the longest timepoint common to both methods. Statistical analyses were performed with Prism 5.0 (GraphPad Software), SPSS 21.0 (IBM), and JMP13 (SAS Institute). P values < 0.05 were considered significant.
Results
A total of 15 patients, ten men and five women, mean age 44.0 ± 16.7 years, median age 47.0 years, and age range 10–66 years (Table 1), were identified based on the above criteria. Nine out of 15 patients experienced cerebral hemorrhages due to ruptures of the AVMs prior to MR imaging. The mean interval between the DSA and 4D-MR examination was 7.3 ± 15.3 days.
Vessel selectivity
The vessel selectivity was graded 4 in 43/45 (95.6%, observer 1) and 42/45 (93.3%, observer 2) territories and graded 3 in two (4.4%, observer 1) and three (6.7%, observer 2) territories. No territories were graded 1 or 2 for both observers. No external carotid artery (ECA) system was visualized in any patients.
Observer test
The nidus size measured on DSA (31.3 ± 14.6 mm) was not significantly different from those measured on 4D-PACK (observer 1, 30.3 ± 15.0 mm, p = .54; observer 2, 28.7 ± 16.4 mm, p = 0.11), or 4D-S-PACK (observer 1, 30.4 ± 14.1 mm, p = 0.53; observer 2, 27.7 ± 11.7 mm, p = 0.07). The interobserver agreement between the two observers for the nidus size was excellent on both 4D-PACK (ICC = 0.900) and 4D-S-PACK (ICC = 0.843). Figure 3, Supplementary Material 1, and Supplementary Material 2 show representative cases in which 4D-S-PACK visualized the AVM as seen on DSA.
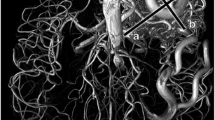
A 10-year-old man with a brain arteriovenous malformation (AVM) supplied by the left middle cerebral artery. The 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) labeling of the left internal carotid artery (ICA) clearly depicts the feeding artery (white arrows), nidus (black arrows), and draining veins (arrowheads) as seen on digital subtraction angiography (DSA). Note that complete vessel selectivity was achieved, and the external carotid artery (ECA) and the vertebrobasilar system are not visualized
The identification of the feeding artery on both MR angiographies is summarized in Table 2. The sensitivity, specificity, and accuracy for both observers was 24/27 (88.9%), 93/93 (100%), and 117/120 (97.5%) on 4D-PACK and 27/27 (100%), 93/93 (100%), and 120/120 (100%) on 4D-S-PACK (p = 0.86 for both observers), respectively. Figure 4 shows a representative case in which 4D-S-PACK demonstrated accurate identification of feeding arteries.
A 47-year-old woman with a brain arteriovenous malformation (AVM) supplied by the left anterior, middle, and posterior cerebral arteries. The 4D-MR angiography based on pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-PACK) (top row) shows a large AVM in the left temporoparietal lobes. Both observers identified the feeding arteries from the left middle and posterior cerebral arteries; however, both of them failed to detect the feeding artery from the left anterior cerebral artery on 4D-PACK. The 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) labeled the left internal carotid artery (ICA) (second row), the right ICA (third row), and the vertebral arteries (bottom row), revealing the AVM was supplied by all three main cerebral arteries, the left middle cerebral artery (white arrow), the left anterior cerebral artery (arrowhead), and the left posterior cerebral artery (black arrow). Note that the A1 segment of the left anterior cerebral artery is hypoplastic, and the left anterior cerebral artery is supplied by the right ICA system through the anterior communicating artery. Both observers identified all feeding arteries on 4D-S-PACK. The complete vessel selectivity was achieved, and no ECA system is observed
The identification of the venous drainage pattern on both MR angiographies is summarized in Table 3. The sensitivity (18/18), specificity (12/12), and accuracy (30/30) were all 100% on both MR angiographies for observer 1 (p > 0.99). The sensitivity, specificity, and accuracy for observer 2 was 17/18 (94.4%), 10/12(83.3%), and 27/30 (90.0%) on 4D-PACK and 17/18 (94.4%), 11/12 (91.7%), and 28/30 (93.3%) on 4D-S-PACK, respectively (p = 0.95).
The Spetzler-Martin grades assessed on DSA (2.2 ± 0.9) were not significantly different from those assessed on TOF MR angiography (observer 1, 1.9 ± 0.9, p = 0.10; observer 2, 2.3 ± 0.8, p = 0.43), 4D-PACK (observer 1, 2.3 ± 1.0, p = 0.33; observer 2, 2.3 ± 1.9, p = 0.50), and 4D-S-PACK (observer 1, 2.3 ± 1.0, p = 0.33; observer 2, 2.2 ± 0.7, p = 0.43). The nidus size measured on TOF MR angiography (observer 1, 23.6 ± 11.2 mm, p = 0.0004; observer 2, 29.8 ± 13.1 mm, p = 0.50) was significantly smaller than that on DSA (31.3 ± 14.6 mm) for observer 1 while no difference was found for observer 2. Observer 1 failed to detect deep venous drainage in two patients with TOF MR angiography but successfully detected all deep venous drainage with 4D-PACK or 4D-S-PACK. Observer 2 correctly detected deep venous drainage with TOF in all patients. The interobserver agreement between DSA and MR angiography for the Spetzler-Martin grade evaluated by observer 1 was excellent on TOF MR angiography (ICC = 0.789), 4D-PACK (ICC = 0.966), or 4D-S-PACK (ICC = 0.966). This agreement as evaluated by observer 2 was good on TOF MR angiography (ICC = 0.736), 4D-PACK (ICC = 0.699), or 4D-S-PACK (ICC = 0.681).
Visualization score for each AVM component
The visualization scores are summarized in Table 4. The scores for both observers were not significantly different between 4D-PACK and 4D-S-PACK in the visualization of the feeding arteries, nidus, or draining veins.
Quantitative assessment of visualization
The temporal changes of the CNR are shown in Fig. 5. The CNRs at the timepoint of 1600 ms were significantly lower in 4D-S-PACK than in 4D-PACK for the feeding artery (4D-PACK, 71.5 ± 20.6; 4D-S-PACK, 58.3 ± 19.3; p = 0.02), nidus (4D-PACK, 75.8 ± 22.2; 4D-S-PACK, 55.6 ± 21.6; p = 0.001), and draining vein (4D-PACK, 43.8 ± 18.9; 4D-S-PACK, 32.9 ± 14.2; p = 0.02).
The temporal changes in the contrast-to-noise ratio (CNR). The CNRs were lower in 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) than in 4D-MR angiography based on pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-PACK) for (a) the feeding artery, (b) the nidus, and (c) the draining vein
Discussion
Our results indicate that the 4D-S-PACK showed good vessel selectivity. The sensitivity and specificity for feeding arteries was 100% for both observer 1 and observer 2. The venous drainage was 100% for both sensitivity and specificity for observer 1 and 94.4% and 91.7%, respectively, for observer 2. Although the CNR at 1600 ms was lower in 4D-S-PACK than in 4D-PACK for all AVM components, the visualization scores were not significantly different between the methods for all AVM components.
Very few studies have used non-selective ASL-based 4D-MR angiography for visualizing brain AVMs [27,28,29]. In all previous studies, pulsed ASL was used for labeling. Xu et al reported that the inter-modality agreement between DSA and 4D-MR angiography was worse for venous drainage, when compared to the feeding artery and nidus size [27]. Similarly, Yu et al reported that diagnostic confidence scores were poor for draining veins [28]. Their results indicated that the signal of the labeled blood would be lost over time during the acquisition of images at multi-timepoints after the pulsed ASL, which could degrade the visualization of draining veins. The 4D-PACK would improve the visualization of blood flow with a long transit time using pCASL [14, 16]. It was reported that 4D-PACK successfully visualized the leptomeningeal collateral flow with a very long transit time in Moyamoya disease [14]. Thus, the depiction of draining veins in brain AVMs would be enhanced by this method. In fact, the venous drainage pattern was correctly identified in both 4D-PACK and 4D-S-PACK in most of the patients in the present study.
To the best of our knowledge, there has been only one study that performed vessel-selective 4D-MR angiography using ASL in patients with brain AVMs. Fujima et al employed the pulsed ASL-based technique named contrast-inherent inflow-enhanced multi-phase angiography (CINEMA) combined with vessel-selective ASL for 4D-MR angiography [19]. In CINEMA, data from multiple timepoints can be acquired with Look-Locker scheme in which trains of low-flip angle readout RF pulses are applied after a pulsed ASL. The 4D-S-PACK has theoretical advantages over vessel-selective CINEMA in terms of the signal-to-noise ratio and vessel selectivity. The 4D-S-PACK employs the pCASL labeling scheme, whereas CINEMA uses pulsed ASL. In 4D-S-PACK, the images are acquired immediately after labeling, to minimize the signal saturation and relaxation of labeled blood. Since Look-Locker sampling is used in CINEMA to acquire multi-timepoint data, signal saturation by consecutive RF pulses and T1 relaxation can lead to insufficient vessel visualization, especially at later timepoints. It is difficult for vessel-selective CINEMA to separately visualize territorial flow of the ICA and the ECA, as spatially non-selective label pulse is used in a foot-to-head direction and the label slab typically includes the common carotid artery [19]. On the other hand, super-selective pCASL can selectively label the ICAs. The ECA was not visualized in any patients when labeling of the ICAs on 4D-S-PACK. Clear visualization of ICA territorial flow with no visualization of the ECA territorial flow would be clinically significant, since this would improve identification of related vessels.
The identification of feeding arteries on 4D-S-PACK was perfect for both observers. Although we expected an improvement in the identification of feeding arteries using 4D-S-PACK, there were no statistically significant differences with 4D-PACK, which also provided good identification. This could be because we used a simplified classification method based just on the main cerebral arteries, and did not take their branches into account. Therefore, it was not difficult to identify feeding arteries using 4D-PACK. Nevertheless, 4D-S-PACK could detect feeding arteries that 4D-PACK could not. The feeding arteries from the anterior cerebral artery were not detected on 4D-PACK in three patients for both observers, while they were successfully detected on 4D-S-PACK. Multiple arteries overlapped in the maximum intensity projection image, making it difficult to identify these arteries on 4D-PACK. The 4D-S-PACK was able to distinguish these feeding arteries from other unrelated arteries. There were no differences in the identification of feeding arteries between observer 1 (11 years of experience) and observer 2 (5 years of experience) with 4D-PACK or 4D-S-PACK. They failed to detect the same three feeding arteries with 4D-PACK. Similarly, the results for identification of venous drainage pattern were almost identical for both observers with both MR methods. These results suggest that the evaluation of both feeding arteries and venous drainage pattern with 4D-ASL-based MR angiographies was reproducible regardless of the observer’s experience of neuroradiology.
In the assessment of the ability to achieve vessel-specific visualization or vessel selectivity, targeting the vascular tree originating from either the ICA or the vertebral artery, the targeted circulation was visualized in all patients. The vessel selectivity was complete (almost no visualization of unlabeled vessels) in the majority of cases (95.6% for observer 1, and 93.3% for observer 2), which suggests that the gradient moment of 0.75 mT/m in super-selective pCASL is effective in selectively labeling ICAs and vertebral arteries. In a few patients, an unlabeled artery was slightly visualized, although there was no influence on the diagnosis. In such patients, the opposite ICA was slightly visible when labeling occurred at some timepoints. This could be due to the sidebands of the selective labeling, as a result of the repetitive pattern of transversal gradients, giving rise to a sinusoidal pattern of the labeling efficiency outside of the labeling spot. In other patients, one ICA was slightly visualized when labeling the vertebral arteries. When labeling vertebral arteries, the selective gradient was applied only in the anterior-to-posterior direction to simultaneously label bilateral vertebral arteries. Therefore, it was possible that the ICA was partially included in the labeling area and could not be completely separated from the vertebral arteries. The super-selective pCASL requires careful planning of the label spot, which leads to prolongation of imaging time; otherwise, insufficient or incorrect label selectivity could occur. Effective operator training or, as already reported, advanced automatic planning would be crucial for clinical use [30].
The CNRs were lower at all timepoints for the all the components in 4D-S-PACK than in 4D-PACK. This was most likely due to the reduced amount of labeled blood due to the super-selective labeling. However, this signal loss on 4D-S-PACK would be clinically acceptable since the observer study demonstrated that the visualization scores for all components of the AVMs were well preserved.
We did not find any significant differences in the Spetzler-Martin grade between DSA and any of MR angiographies. However, the nidus size measured on TOF MR angiography was significantly smaller than that on DSA for observer 1, and observer 1 failed to detect deep venous drainage in two patients with TOF MR angiography while successfully detecting all deep venous drainage with 4D-PACK or 4D-S-PACK. This might indicate a potential advantage of 4D-ASL-based MR angiography for assessing Spetzler-Martin grade, despite the results not showing a significant difference.
This study had limitations. First, the number of subjects was small. Although 4D-S-PACK demonstrated good visualization of brain AVMs and excellent vessel selectivity, the efficacy should be tested in a larger patient population across multiple institutions. Second, super-selective labeling is sensitive to patient movement, since the labeling spots are small to allow only a single artery to be targeted. Movement between the positioning of the labeling spot and imaging would hamper vessel selectivity. Although no severe image degradation due to motion was observed in this study, subtle motion could have reduced the vessel selectivity. Finally, the usefulness of 4D-S-PACK for evaluating smaller and/or lower flow brain AVMs has not been proven yet. Further studies are necessary to evaluate the efficacy of the method in such lesions.
In conclusion, the 4D-S-PACK enabled excellent vessel selectivity and facilitated the identification of feeding arteries, and this method was also useful in the accurate measurement of nidus size, and the correct determination of venous drainage patterns in brain AVMs. Although the CNRs were slightly lower in 4D-S-PACK than in 4D-PACK because of the label loss in the super-selective labeling, this was acceptable since visualization was well preserved. 4D-S-PACK could be a non-invasive clinical tool for assessing brain AVMs.
Abbreviations
- 4D-PACK:
-
4D-MR angiography based on pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing
- 4D-S-PACK:
-
4D-MR angiography based on super-selective pCASL with CENTRA-keyhole and view-sharing
- AVM:
-
Arteriovenous malformation
- CINEMA:
-
Contrast-inherent inflow-enhanced multi-phase angiography
- CNR:
-
Contrast-to-noise ratio
- DSA:
-
Digital subtraction angiography
- ECA:
-
External carotid artery
- ICA:
-
Internal carotid artery
- MIP:
-
Maximum intensity projection
- pCASL:
-
Pseudo-continuous arterial spin labeling
References
Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A (2008) Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 63:823–829 discussion 829-831
Laakso A, Dashti R, Juvela S, Isarakul P, Niemela M, Hernesniemi J (2011) Risk of hemorrhage in patients with untreated Spetzler-Martin grade IV and V arteriovenous malformations: a long-term follow-up study in 63 patients. Neurosurgery 68:372–377 discussion 378
Murthy SB, Merkler AE, Omran SS et al (2017) Outcomes after intracerebral hemorrhage from arteriovenous malformations. Neurology 88:1882–1888
ApSimon HT, Reef H, Phadke RV, Popovic EA (2002) A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke 33:2794–2800
Brown RD Jr, Wiebers DO, Forbes G et al (1988) The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg 68:352–357
Halim AX, Johnston SC, Singh V et al (2004) Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 35:1697–1702
Griffiths PD, Hoggard N, Warren DJ, Wilkinson ID, Anderson B, Romanowski CA (2000) Brain arteriovenous malformations: assessment with dynamic MR digital subtraction angiography. AJNR Am J Neuroradiol 21:1892–1899
Falk Delgado A, Van Westen D, Nilsson M et al (2019) Diagnostic value of alternative techniques to gadolinium-based contrast agents in MR neuroimaging-a comprehensive overview. Insights Imaging 10:84
Uchino H, Ito M, Fujima N et al (2015) A novel application of four-dimensional magnetic resonance angiography using an arterial spin labeling technique for noninvasive diagnosis of Moyamoya disease. Clin Neurol Neurosurg 137:105–111
Iryo Y, Hirai T, Kai Y et al (2014) Intracranial dural arteriovenous fistulas: evaluation with 3-T four-dimensional MR angiography using arterial spin labeling. Radiology 271:193–199
Iryo Y, Hirai T, Nakamura M et al (2015) Collateral circulation via the circle of Willis in patients with carotid artery steno-occlusive disease: evaluation on 3-T 4D MRA using arterial spin labelling. Clin Radiol 70:960–965
Iryo Y, Hirai T, Nakamura M et al (2016) Evaluation of intracranial arteriovenous malformations with four-dimensional arterial-spin labeling-based 3-T magnetic resonance angiography. J Comput Assist Tomogr 40:290–296
Togao O, Hiwatashi A, Obara M et al (2018) Acceleration-selective arterial spin-labeling MR angiography used to visualize distal cerebral arteries and collateral vessels in Moyamoya disease. Radiology 286:611–621
Togao O, Hiwatashi A, Obara M et al (2018) 4D ASL-based MR angiography for visualization of distal arteries and leptomeningeal collateral vessels in moyamoya disease: a comparison of techniques. Eur Radiol 28:4871–4881
Togao O, Hiwatashi A, Yamashita K et al (2019) Acceleration-selective arterial spin labeling MR angiography for visualization of brain arteriovenous malformations. Neuroradiology 61:979–989
Obara M, Togao O, Beck GM et al (2018) Non- non-cofvced 4D intracranial MR angiography based on pseudo-continuous arterial spin labeling with the keyhole and view-sharing technique. Magn Reson Med 80:719–725
Suzuki Y, Fujima N, van Osch MJP (2019) Intracranial 3D and 4D MR angiography using arterial spin labeling: technical considerations. Magn Reson Med Sci. https://doi.org/10.2463/mrms.rev.2019-0096
Lindner T, Jensen-Kondering U, van Osch MJ, Jansen O, Helle M (2015) 3D time-resolved vessel-selective angiography based on pseudo-continuous arterial spin labeling. Magn Reson Imaging 33:840–846
Fujima N, Osanai T, Shimizu Y et al (2016) Utility of noncontrast-enhanced time-resolved four-dimensional MR angiography with a vessel-selective technique for intracranial arteriovenous malformations. J Magn Reson Imaging 44:834–845
Okell TW, Schmitt P, Bi X et al (2016) Optimization of 4D vessel-selective arterial spin labeling angiography using balanced steady-state free precession and vessel-encoding. NMR Biomed 29:776–786
Suzuki Y, Okell TW, Fujima N, van Osch MJP (2019) Acceleration of vessel-selective dynamic MR angiography by pseudocontinuous arterial spin labeling in combination with acquisition of ConTRol and labEled images in the same shot (ACTRESS). Magn Reson Med 81:2995–3006
Helle M, Norris DG, Rufer S, Alfke K, Jansen O, van Osch MJ (2010) Superselective pseudocontinuous arterial spin labeling. Magn Reson Med 64:777–786
Lindner T, Austein F, Jansen O, Helle M (2018) Self-controlled super-selective arterial spin labelling. Eur Radiol 28:1227–1233
Jensen-Kondering U, Lindner T, van Osch MJ, Rohr A, Jansen O, Helle M (2015) Superselective pseudo-continuous arterial spin labeling angiography. Eur J Radiol 84:1758–1767
Obara M, Togao O, Helle M et al (2018) Investigation of intracranial artery selective visualization in superselective 4D-MR angiography with pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (SS-4D-PACK). Proc Int Soc Magn Reson Med 27:185
Cicchetti DV, Sparrow SA (1981) Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86:127–137
Xu J, Shi D, Chen C et al (2011) Noncontrast-enhanced four-dimensional MR angiography for the evaluation of cerebral arteriovenous malformation: a preliminary trial. J Magn Reson Imaging 34:1199–1205
Yu S, Yan L, Yao Y et al (2012) Noncontrast dynamic MRA in intracranial arteriovenous malformation (AVM), comparison with time of flight (TOF) and digital subtraction angiography (DSA). Magn Reson Imaging 30:869–877
Raoult H, Bannier E, Robert B, Barillot C, Schmitt P, Gauvrit JY (2014) Time-resolved spin-labeled MR angiography for the depiction of cerebral arteriovenous malformations: a comparison of techniques. Radiology 271:524–533
Helle M, Wenzel F, van de Ven K, Boernert P (2018) Advanced automatic planning for super-selective arterial spin labeling flow territory mapping. Proc Int Soc Magn Reson Med 26:302
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP17K10410 and JP20K08111.
Funding
This work was supported by JSPS KAKENHI Grant Number JP17K10410 and JP20K08111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Akio Hiwatashi.
Conflict of interest
Two authors (M.O., M.V.C.) were employees of Philips Healthcare and provided technical support for sequence development but were not involved in the study design or interpretation of the data. The institutional authors, who were not employees of Philips Healthcare, were in control of all the data.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional review board approval was obtained
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Material 1
(Movies). A 60-year-old woman with brain AVM. Left internal carotid arteriograms (A. lateral view) shows that the AVM is supplied by multiple branches from the left middle cerebral artery. Vertebral arteriograms (AVI 526 kb)
330_2020_7057_MOESM2_ESM.avi
B. lateral view) show that this AVM is also supplied by the left posterior cerebral artery. The 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) labelling the left internal carotid artery (AVI 550 kb)
330_2020_7057_MOESM3_ESM.avi
C. sagittal view) shows the AVM is supplied by the left middle cerebral artery as seen on the digital subtraction angiography. The 4D-S-PACK labelling the bilateral vertebral arteries (AVI 54 kb)
330_2020_7057_MOESM4_ESM.avi
D. sagittal view) shows the AVM is also supplied by the left posterior cerebral artery as seen on the digital subtraction angiography. On the nonselective 4D-MR angiography based on pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-PACK (AVI 48 kb)
330_2020_7057_MOESM5_ESM.avi
E. multiple arteries overlapped in the maximum intensity projection image, making it difficult to identify the feeding arteries. (AVI 83 kb)
Supplementary Material 2
(Movies). A 47-year-old woman with brain AVM. Left internal carotid arteriograms (A. anterior-posterior view (AVI 741 kb)
330_2020_7057_MOESM7_ESM.avi
B. lateral view) show that the AVM in the left cerebrum is supplied by the left middle cerebral artery. Contrast-enhanced 4D-CT angiography (AVI 649 kb)
330_2020_7057_MOESM9_ESM.avi
D. sagittal view) clearly depicts all compartments of the AVM; however temporal resolution (1 sec) is limited, and it is difficult to distinguish feeding arteries because of the lack of vessel selectivity. The 4D-MR angiography based on super-selective pseudo-continuous arterial spin labeling combined with CENTRA-keyhole and view-sharing (4D-S-PACK) labelling the left internal carotid artery (AVI 195 kb)
330_2020_7057_MOESM12_ESM.avi
G. axial view) shows the AVM is supplied by the left middle cerebral artery as seen on the digital subtraction angiography. (AVI 107 kb)
Rights and permissions
About this article
Cite this article
Togao, O., Obara, M., Helle, M. et al. Vessel-selective 4D-MR angiography using super-selective pseudo-continuous arterial spin labeling may be a useful tool for assessing brain AVM hemodynamics. Eur Radiol 30, 6452–6463 (2020). https://doi.org/10.1007/s00330-020-07057-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07057-4