Abstract
Liver mitochondria are involved in several important life processes; mitochondrial dysfunction and disorders are implicated in several human diseases. Alcohol permeates all tissues of the body and exerts some intrinsic hepatotoxicity. In this work, our results demonstrated that ethanol caused a series of mitochondria permeability transition pore (MPTP) opening factors such as mitochondrial swelling, increased permeability of H+ and K+, collapsed membrane potential, and increased membrane fluidity. Furthermore, mitochondrial ultrastructure alternation observed clearly by transmission electron microscopy and the release of Cytochrome c could explain the MPTP opening from another aspect. Moreover, ethanol damaged the mitochondrial respiration system and induced disturbance of mitochondrial energy metabolism which was monitored by polarographic and microcalorimetric methods, respectively. Considered together, these damages may promote both apoptotic and necrotic cell death and contribute to the onset or progression alcohol-induced liver diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol is widely used within medicine as an antiseptic, disinfectant, and antidote. Ethanol is the principal type of alcohol found in alcoholic beverages, produced by the fermentation of sugars by yeasts. It is a neurotoxic, psychoactive drug and causes alcohol intoxication when consumed in sufficient quantity (John 2010). Considerable evidence from both human and animal studies has indicated that ethanol consumption can enhance the oxidative stress, both by increasing the formation of reactive oxygen species (ROS) and decreasing the cellular defense mechanisms. The majority of ethanol metabolism occurs in the liver and liver injury is the principal clinical complication of alcohol abuse (Wu and Cederbanum 2009; Hoek et al. 2002). Besides known as the energy producers and providers, mitochondria play a crucial role in all cellular metabolic processes in liver and intimately involved in the generation of and defense against ROS. ROS generation leads to decrease in the activities of several key enzymes of energy metabolism, including those of the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation. A wide range of mitochondrial ROS-induced damages has been described, including protein carbonylation, lipid peroxidation, or mitochondrial DNA (mtDNA) damage (Marchi et al. 2012). Hence, ethanol administration disrupts the energy metabolism and inhibits oxidative phosphorylation (ATP synthesis) in mitochondria (Nohl et al. 2005). It is reasonable to consider mitochondria as an important intracellular target for ethanol.
Other previous studies reported the glutathione (GSH) depletion in ethanol-treated cells such as isolated hepatocyte; ethanol-added hepatocytes render to be more susceptible to the cytotoxic effects of inflammatory cytokines, e.g., tumor necrosis factor (THF)-α, by amplifying the generation of ROS within mitochondria (Kurose et al. 1997; Colell et al. 1998). Mitochondrial GSH level is required for the maintenance of the reduced form of thiol groups which protects against MPT. Thus, ethanol-induced GSH level decrease could damage the mitochondrial membrane integrity and open the MPTP (Hirano et al. 1992).
Moreover, excessive ethanol affects cellular homeostasis, increasing the Ca2+ levels in cytosol. To maintain homeostasis, Ca2+ uptake into the mitochondria is increased, which induces mitochondrial Ca2+ overload. With this, Ca2+ overload can lead to opening the MPTP which mechanistically follows by termination of oxidative phosphorylation, swelling, Cytochrome c release, eventually leading to apoptosis, and necrosis (Armstrong 2006; Santo-Domingo et al. 2010).
While it is clear that ethanol can induce mitochondrial impairment by several pathways mentioned above, the purpose of this study was to evaluate the biological effect of different concentrations of ethanol on the liver mitochondrial membrane function and ultrastructure, respiration function, and heat metabolic processes with spectroscopic, microscopic, polarographic (with an oxygen electrode), and microcalorimetric methods.
Materials and Methods
Chemicals
Rotenone, oligomycin, rhodamine 123 (Rh123), hematoporphyrin (HP), 1,6-diphenyl-1,3,5-hexatriene (DPH), ethylene glycol tetraacetic acid (EGTA), and ethylene diamine tetraacetie acid (EDTA) were purchased from Sigma (St. Louis, MO). Ethanol was chromatographic grade (purity >99.8%) and purchased from Aladdin (Shanghai, China). Other reagents were of analytical reagent grade. All solutions were prepared with aseptic double-distilled water.
Isolation of Mitochondria
Isolation of mitochondria from female Wistar rat’s liver was according to a typical method, standard differential centrifugation procedures with some modifications, of which the details are described in Sect. 1, Supplementary material (Pon et al. 2007; Dong et al. 2013). Mitochondrial protein concentrations were determined by the Biuret method. BSA was used as a standard for protein measurement (Gornall et al. 1949).
Mitochondrial Swelling
Mitochondrial swelling was assessed through the changes of absorbance using a UV–Vis spectrophotometer (SQ-4802; UNICO, Dayton, NJ) set at 540 nm for 500 s at 25 °C (Lai et al. 2015). Mitochondria (0.5 mg protein/mL) were suspended in buffer MAB (2 mL) composed of 200 mM sucrose, 5 mM succinate, 1 mM Tris, 1 mM Na3PO4⋅10H2O, 1 mM MOPS, 1 μM EGTA, 2 μM rotenone, and 3 μg/ml oligomycin [pH 7.0–7.4] and treated with different concentrations of ethanol. The reduction of absorbance is related to the increase in mitochondrial swelling.
Mitochondrial Inner Membrane H+ and K+ Permeabilization
Mitochondrial inner membrane permeabilization of H+ or K+ was respectively detected in potassium acetate and potassium nitrate medium (Dong et al. 2016). Mitochondria (0.5 mg protein/mL) were suspended in a potassium acetate buffer, which contained 135 mM KAc, 0.1 mM EGTA, 5 mM HEPES, 0.2 mM EDTA, 2 μM Rotenone, and 1 μg/mL valinomycin [pH 7.0]. The presence of 1 μg/ml valinomycin was to permeabilize the mitochondrial inner membrane to K+. As well as, KNO3 was used alternatively in the potassium nitrate medium that contained 135 mM potassium nitrate, 5 mM HEPES, 0.2 mM EDTA, 0.1 mM EGTA, and 2 μM rotenone [pH 7.1]. The instrument and experiment parameter were the same as described above, in ‘‘Mitochondrial swelling.’’
Mitochondrial Membrane Potential
For mitochondrial membrane potential measurements, a cationic lipophilic fluorescent dye Rh123 which can permeate inner membrane and accumulate in the polarized mitochondrial matrix was used (Ricchelli et al. 2003). Isolated mitochondria (0.5 mg protein/mL) were suspended in buffer MAB (2 mL) as described above. Rh123 fluorescence intensity was continuously visualized and monitored with a fluorescence spectrometer (LS55; Perkin-Elmer, Downers Grove, IL) set at 488 nm excitation and 535 nm emission wavelengths at 25 °C.
Mitochondrial Membrane Fluidity
HP and DPH are two kinds of membrane lipid-bound probe molecules. Mitochondria (0.5 mg protein/mL) were suspended in buffer MAB (2 mL) with probes HP (5 μM) and DPH (10 μM) added, then incubated for 5 min and 30 min before measuring, respectively. Changes in anisotropy (r) of HP and DPH were used to evaluate the fluidity of the mitochondrial membranes. The values of steady-state anisotropy could be obtained at (λ ex = 520 nm / λ em = 626 nm, HP) and (λ ex = 362 nm /λ em = 454 nm, DPH) with a fluorescence spectrometer (LS55) at 25 °C. The anisotropy (r) is defined by the following equation:
where G = I ∏ / I ⊥ is the correction factor for instrumental artifacts. The emission polarizer is oriented parallel (∏) to the direction of the polarized excitation; the observed intensity is called I ∏. Likewise, when the polarizer is perpendicular (⊥) to the excitation the intensity is called I ⊥.
Mitochondrial Ultrastructure
Mitochondria were preincubated with ethanol at a concentration of 10% (v/v) for 30 min at 30 °C. After incubating, mitochondria were collected and fixed for another 30 min at 4 °C using glutaraldehyde at a final concentration of 2.5% (v/v) in 0.1 M cacodylate buffer, then post fixed with 1% osmium tetroxide and dehydrated (Petronilli et al. 2009). The ultrastructure of mitochondria observations was performed on a JEM-100CX transmission electron microscope (TEM).
Mitochondrial Cytochrome c Release
Isolated mitochondrial Cytochrome c release was analyzed by Rat/Mouse Cyt c ELISA Kit (Shanghai Hua Yi Bio Technology Co. Ltd, China) according to the manufacturer’s protocol as described in Sect. 2, supplementary material.
Mitochondrial Respiration
Mitochondrial respiratory rate was evaluated by the consumption of oxygen using a Clark oxygen electrode and the Oxygraph software (Hatchtech, Dorchester, UK). Mitochondria (1 mg protein/mL) were added into a closed chamber equipped with magnetic stirring buffer MRB (1 mL), containing 250 mM sucrose, 20 mM KCl, 10 mM HEPES, 5 mM K2HPO4, 2 mM MgCl2, and 1 mM rotenone [pH 7.4] (Yang et al. 2016). Mitochondrial respiratory state 4 was initiated by adding 5 mM succinate. To induce respiration state 3, 5 mM succinate and 100 μM ADP were added into the medium. The slope of the oxygen consumption curve given in the oxygraph reflected the rate of oxygen consumption by mitochondria at each state. Respiratory Control Ratio (RCR) was calculated by dividing the rate of oxygen consumption of respiration state 3 by the rate of respiration state 4 (Xia et al. 2013).
Mitochondrial Succinate Dehydrogenase Activity
The activity of mitochondrial complex II (succinate dehydrogenase) was assayed through the measurement of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction. Briefly, 100 μL of mitochondrial MAB buffer suspensions (0.5 mg protein/mL) was incubated with different concentrations of ethanol at 30 °C for 30 min, and then 0.4% of MTT was added to the medium and incubated at 30 °C for 30 min. The product of purple formazan crystals was dissolved in 100 μL DMSO, and the absorbance at 490 nm was measured with a microplate reader (Victor™ X5, Perkin-Elmer, America).
Mitochondrial Cytochrome c Oxidase Activity
The activity of mitochondrial complex IV (Cytochrome c oxidase) was evaluated using Cytochrome c Oxidase Activity Assay Kit (GMS10014.2; Genmed Scientifics, Inc.) according to the manufacturer’s protocol as described in Sect. 2, supplementary material.
Mitochondrial Microcalorimetry Determination
The metabolic thermogenic curves of isolated mitochondria were performed using TAM air isothermal calorimeter with ampoule method. In the experiment, each sealed ampoule contained 2 mL of sample (5 mg protein/mL energized mitochondria suspension with different concentrations of ethanol) and 23 mL of air, which provided basically sufficient oxygen for the mitochondria metabolism. We chose pyruvate as the energy source and the buffer B was to supply the osmotic pressure (Zhao et al. 2016). The power–time curves about the heat output of mitochondria were continuously recorded at 30 °C.
Results
Mitochondrial Swelling
Mitochondrial swelling as an indicator of membrane permeability was evaluated by detecting the changes of absorbance at 540 nm. As shown in Fig. 1a, compared to the control, ethanol-induced mitochondrial swelling with a dose- and time-dependent effect. Ethanol concentrations used in this study, expressed as volume fractions, are within the concentration range used by other authors for pilot studies with tissues and cells (Syed et al. 2013; Lovett and Sweetman, 1983).
a Swelling of liver mitochondria induced by different concentrations of ethanol. b Effect of ethanol on mitochondrial inner membrane permeabilization to H+. c Effect of ethanol on mitochondrial inner membrane permeabilization to K+. d Ethanol-induced mitochondrial Δψ collapse. The concentrations used were 0, 1.25, 2.5, 5, 7.5, and 10% v/v (traces a–f)
Mitochondrial Inner Membrane Permeabilization to H+ and K+
The H+ gradient across the mitochondrial membrane was disrupted by the addition of ethanol. The effect of ethanol on inner membrane permeabilization to H+ displayed a dose- and time-dependent manner at the range of concentrations we chose (Fig. 1b). Likewise, the trend of K+ permeabilization of mitochondrial inner membrane exhibited the same pattern (Fig. 1c).
Mitochondrial Membrane Potential (Δψ) Collapse
Due to its cationic property, Rh123 could accumulate in the mitochondrial inner membrane, thus trapping inside the sub-cellular organelle which led to fluorescence quenching. When Δѱ decreased, Rh123 was released into the medium and its fluorescence intensity would increase subsequently. As shown in Fig. 1d, mitochondrial membrane potential significantly decreased with different concentrations of ethanol.
Mitochondrial Membrane Fluidity
In biological membranes, HP mostly accumulated in polar regions or interacted with the protein sites in the mitochondrial membrane, while DPH was typically used to probe highly hydrophobic lipid regions of the mitochondrial membrane. The change of fluorescence anisotropy of mitochondria-bound dyes reflected modification of membrane fluidity and conformational changes of the MPTP (Ricchelli et al. 1999). As shown in Fig. 2a, addition of ethanol caused a significant decrease of the HP anisotropy which corresponded to the increase of membrane fluidity. On the contrary, the effect of ethanol on the DPH-labeled membrane fluidity was not obvious (Fig. 2b).
Mitochondria Ultrastructure
The ultrastructure of mitochondria was detected by TEM for better show of mitochondrial changes treated with or without ethanol. As shown in Fig. 3a, mitochondria maintained their integrity with the classical ultrastructure, which contained a well-defined outer membrane, a confined intermembrane space, and compact cristae. With the addition of 10% (v/v) ethanol, the number of completed mitochondria decreased and mitochondrial structure was broken down, appearing a typical pattern of inner membrane unfolding and a larger inner membrane space (Fig. 3b).
Mitochondrial Cyt c Release
The opening of MPTP initiated the mitochondrial inner membrane permeabilization, followed by matrix osmotic swelling, rupture of the mitochondrial outer membrane, and release of Cyt c, which ultimately activates the caspase family of proteases, the primary trigger leading to the onset of apoptosis (Kobayashi et al. 2003; Liu et al. 2015). Cyt c was associated with the inner membrane through electrostatic interaction with acidic phospholipids, complex III and IV, as well as the hinge protein (Garrido et al. 2006). Table 1 showed that Cyt c was released from isolated mitochondria incubated with ethanol during the incubation period.
Mitochondrial Respiratory Activities
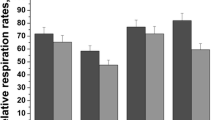
Mitochondrial oxygen consumption was monitored in the presence of different concentrations of ethanol. The high respiratory rate at state 3 indicated an intact respiratory chain and ATP synthesis, while a low rate at state 4 manifested an intact mitochondrial inner membrane (Adlam et al. 2005, Li et al. 2011). At the tested concentration, ethanol stimulated mitochondrial respiratory state 3 and state 4 explicitly at the concentrations from 0 to 10% (v/v), where the degree of inhibition increased in a concentration-dependent manner. The decrease of RCR ratio demonstrated that the addition of ethanol inhibited the mitochondrial respiration function (Fig. 4).
Mitochondrial Complex II and IV Activities
Succinate dehydrogenase (complex II) activity was assessed using the MTT test after a 30 min incubation of mitochondria with different concentrations of ethanol (0, 2.5, 5, 7.5, and 10% v/v). Figure 5a shows a decrease in the mitochondrial metabolism of MTT to formazan (P < 0.05) following the addition of various concentrations of ethanol.
Cytochrome c oxidase (complex IV), the last enzyme in the mitochondrial respiratory electron transfer chain, which catalyzed the last step of the respiration, the transfer of electrons from cytochrome c to molecular oxygen (Flavia et al. 2008). As shown in Fig. 5b, significant collapses in the activity of this enzyme were observed following the addition of ethanol (0, 2.5, 5, 7.5, and 10% v/v) during the incubation time (P < 0.05).
Mitochondrial Metabolic Thermogenic Curves
The heat flux curve of isolated mitochondria energized by pyruvate revealed mitochondrial metabolism (Zhao et al. 2014). As we see from Fig. 6a, the thermogenic curve could be divided into four phases: lag phase, activity recovery phase, stationary increase phase, and decline phase, suggesting that the mitochondria had a good degree of purification and metabolic activity. In the first 500 min, mitochondria had a slow process of recovery to adapt a new physiological environment. An obvious activity recovery phase suggested that the isolated mitochondria had a well degree of metabolic activity. After the short activity recovery, mitochondria had a stationary increase in phase and lasted about 1000 min. In the end, when the environment nutrition exhausted, mitochondria had a decline phase. Figure 6b displayed the corresponding metabolic curves of isolated mitochondria with different concentrations of ethanol. As shown in Fig. 6b, lag phase was little affected with the addition of ethanol. Moreover, activity recovery phase was obviously inhibited and it was difficult to separate activity recovery phase and stationary increase phase with the concentration of ethanol increasing. Low levels of ethanol stimulated the metabolic activity of energized mitochondria and the inhibition was discovered with high concentrations of ethanol. The metabolism of energized mitochondria was inhibited absolutely with the treatment of ethanol at 5% (v/v).
Discussion
Mitochondrial dysfunction is a fundamental pathogenic mechanism that leads to various human disorders, such as Alzheimer’s diseases, Parkinson diseases, diabetes diseases, Huntington diseases, and even the cancers especially those associated with liver (Castanha Zanoli et al. 2012). To begin with, ethanol is able to elicit mitochondrial swelling. As a sign of mitochondrial dysfunction, swelling is one of the most important indicators of the MPTP opening. Mitochondrial swelling occurs as a fast decrease in the absorbance of the mitochondrial suspension (Passarella et al. 2003). As shown in Fig. 1a, different concentrations of ethanol could enhance permeabilization of mitochondrial membrane to sucrose with a two-phase process, thus leading to the occurrence of swelling. At the first phase of the process, ethanol addition caused an instantaneous decrease of absorbance. On the one hand, this phenomenon was caused by dilution of the whole medium volume. On the other hand, the MPT allowed ethanol and water to redistribute freely across the mitochondrial inner membrane resulting, in a concentration-dependent manner, matrix swelling, and mechanical rupture of the outer membrane. During the second phase, high concentration of ethanol caused mitochondrial swelling in a time-dependent manner. Furthermore, the mitochondria suspension incubated with a high concentration of ethanol (5% v/v) was centrifuged and the mitochondrial pellet was resuspended with MAB and then the swelling of which was monitored. Its swelling extents were only a little more than the mitochondria without recentrifugation in 10 min (Table S1). This indicated that the ethanol-induced changes were irreversible. The same result was obtained in the experiment of mitochondrial fluidity, as shown in Fig. S1.
Under physiological conditions, H+ and K+ gradient across the mitochondria inner membrane must be in dynamic balance, in order to maintain a high efficiency of energy conversion. Influx of H+ into the mitochondrial matrix, producing a proton gradient which enables the ATP Synthase to synthesize ATP (Liu et al. 2011). Meanwhile, most of the oxygen consumed is derived from H+ leakage across the mitochondrial membrane during mitochondrial respiration state 4 (Murphy, 2001). As shown in Fig. 4, the ethanol-induced stimulation of respiration state 4 was consistent with the change of mitochondrial inner membrane permeabilization to H+ (Fig. 1b). Correspondingly, our present results had shown that the K+ gradient across the mitochondrial membrane got disrupted with the addition of ethanol (Fig. 1c). Intracellular K+ is a predominant ion that acts as a repressor of apoptotic effectors, and the depletion of K+ may serve as a key step in apoptosis (Eliseev et al. 2003). The K+ influx induced the decrease of Δѱ, which explains the MPT and, thus, mitochondrial swelling, disruption of the outer membrane, and cyt c release (Bernardi et al. 1992). The increase in K+ permeability may accompany an increased permeability to protons. In light of this, our data on H+ (Fig. 1b) and K+ permeabilization (Fig. 1c) were consistent with the data on mitochondrial swelling (Fig. 1a), Δѱ (Fig. 1d), and Cyt c (Table 1) in this article.
The MPTP opening is always accompanied with the changes of membrane fluidity, and this can reflect the dynamic properties of mitochondrial membranes. We chose probe HP which could preferentially accumulate in the protein regions surrounding some inner mitochondrial carriers specifically. The HP-binding sites are involved in MPTP structure or regulation (Ricchelli et al. 1999, 2005). Figure 2a indicates that ethanol induced significant increase of the membrane fluidity properties. Instead, influence of ethanol on DPH-labeled highly hydrophobic lipid phases of mitochondrial membrane was unobvious (Fig. 2b), thus suggesting that ethanol could induce alterations of mitochondrial inner membrane pore-forming protein conformation involved in MPTP formation, which confirmed the conclusion that ethanol stimulated the opening of MPTP.
Moreover, our data indicated that the ultrastructure of mitochondria was changed by 10% v/v ethanol. Compared with the control group, addition of 10% v/v ethanol caused mitochondrial swelling and a rupture in the membrane, as the most direct evidence of MPT occurrence (Fig. 3).
As shown in Fig. 4, ethanol stimulated mitochondrial respiration state and caused a net decrease of RCR ratio which was reasonable to suggest that mitochondrial inner membrane function about respiration would be influenced when ethanol induced the mitochondrial dysfunction. Mitochondria play the most crucial role in cellular energy generation (adenosine triphosphate, ATP), as reflected by respiration electron chain located within the inner mitochondrial membrane (Newmeyer and Ferguson-Miller 2003). Observing the activity of respiratory enzymes is a straightforward approach for investigating mitochondrial dysfunction. Previous studies showed that Complex I and III are the main sources of ROS production in the respiration chain (Barja 2002), and ethanol treatment promotes ROS formation by mitochondria. Although other respiratory complexes could be investigated, Complexes II and IV were the most amenable to histochemical examination. Our results (Fig. 5) showed a decrease in the activity of two enzymes with ethanol addition.
Microcalorimetry is a very suitable and highly sensitive tool in the assessment of the action of environmental chemicals on living biological systems by providing a variety of kinetic and thermodynamic information. In this paper, we found some complicated alterations of mitochondrial metabolic thermogenic curves with the addition of ethanol. To be more specific, we analyzed the relationship between the thermogenic parameters and concentrations of ethanol respectively. As shown in Fig. 6b, if the heat output power was P 0 at time = 0, and P t at time t, then thermokinetic equation was
The growth rate constant (k) can be obtained from the activity recovery phase as described above Eq. (1), and other parameters were obtained from Fig. 6b directly, which included the maximum power output (P m), the maximum power output time (t m), and total heat output (Q) as shown in Table 2. The growth rate constant (k) and the maximum power output (P m) increased with the addition of ethanol (0.5%–1.5%, v/v) and decreased with high concentration (1.5–5%, v/v). The changes of the maximum power output time (t m) were contrary to the maximum power output (P m) at the test concentrations. The addition of ethanol had a regular effect on the total heat output (Q) which indicated that high concentration of ethanol may decelerate ATP consumption. A possible explanation for the differences is that there may be two kinds of action for different concentrations of ethanol to mitochondrial energy metabolism. At the activity recovery phase, the addition of ethanol caused an inhibition of normal mitochondrial recovery at the tested concentration. But as experiment time went on, mitochondria could regulate its adaptation to the additional physiological stresses (0.5%–1.5%, v/v, ethanol); moreover it promoted mitochondrial respiration state (Fig. 4) which reported that the increase of respiratory rate was conjugated by increased heat production (Levine and Cheney 2000). Nevertheless, when the concentration of ethanol (1.5%–5%, v/v) increased, the restrained metabolism phases and decreased total heat output indicated that mitochondria had been destroyed or killed which was consistent with the results of MPTP opening and respiration damage induced by ethanol.
According to the above discussion, several possible pathway mechanisms of mitochondrial dysfunction induced by ethanol can be proposed and elucidated briefly in Fig. 7. In summary, we investigated the Wistar rat liver mitochondrial dysfunction induced by ethanol by spectroscopic, microscopic, polarographic, and microcalorimetric methods. Overdose ethanol could trigger mitochondrial swelling, permeate to H+/K+, collapse membrane potential, increase membrane fluidity, cause rupture of mitochondrial ultrastructure, and increase Cyt c release which indicated that ethanol caused MPTP opening. Additionally, ethanol had stimulated mitochondrial respiration in rapid oxygen consumption phase and inhibited mitochondrial respiratory chain enzyme activities and heat production. In short, ethanol was supposed to influence not only mitochondrial MPT but also respiratory dysfunction and energy metabolism disturbance, thus leading to mitochondrial dysfunction and even apoptosis.
References
Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, Sammut IA (2005) Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19(9):1088–1095
Armstrong JS (2006) The role of the mitochondrial permeability transition in cell death. Mitochondrion 6(5):225–234
Baracca A, Sgarbi G, Solaini G, Lenaz G (2003) Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta 1606(1–3):137–146
Barja G (2002) The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenergy Biomembr 34(3):227–233
Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M (1992) Modulation of the mitochondrial permeability transition pore. effect of protons and divalent cations. J Biol Chem 267(5):2934–2939
Castanha Zanoli JC, Maioli MA, Medeiros HCD, Mingatto FE (2012) Abamectin affects the bioenergetics of liver mitochondria: a potential mechanism of hepatotoxicity. Toxicol In Vitro 26(1):51–56
Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Frenandez-Checa C (1998) Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology 115(6):1541–1551
Dong JX, Zhao GY, Yu QLY, Li R, Yuan L, Chen J, Liu Y (2013) Mitochondrial dysfunction induced by Honokiol. J Membr Biol 246(5):375–381
Dong P, Li JH, Xu SP, Wu XJ, Xiang X, Yang QQ, Jin JC, Liu Y, Jiang FL (2016) Mitochondrial dysfunction induced by ultra-small silver nanoclusters with a distinct toxic mechanism. J Hazard Mater 308(5):139–148
Eliseev RA, Salter JD, Gunter KK, Gunter TE (2003) Bcl-2 and tBid proteins counter-regulate mitochondrial potassium transport. Biochim Biophys Acta 1604(1):1–5
Flavia F, Ileana CS, Antoni B (2008) Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life 60(9):557–568
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13(9):1423–1433
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Hirano T, Neil K, Tsukamoto H, Kamimura S, Fernández-Checa JC (1992) Hepatic mitochondrial GSH depletion and progression of experimental alcoholic liver disease in rats. Hepatology 16(6):1423–1428
Hoek JB, Cahill A, Pastorino JG (2002) Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122(7):2049–2063
John CMB (2010) Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int J Environ Res Public Health 7(4):1540–1557
Kobayashi T, Kuroda S, Tada M, Houkin K, Iwasaki Y, Abe H (2003) Calcium-induced mitochondrial swelling and cytochrome c release in the brain: its biochemical characteristics and implication in ischemic neuronal injury. Brain Res 960(1–2):62–70
Kurose I, Higuchi H, Kato S, Miura S, Watanabe N, Kamegaya Y, Tomita K, Takaishi K, Horie Y, Fukuda M, Mizukami K, Ishii H (1997) Oxidative stress on mitochondria and cell membrane of cultured rat hepatocytes and perfused liver exposed to ethanol. Gastroenterology 112(4):1331–1343
Lai L, Jin JC, Xu ZQ, Ge YS, Jiang FL, Liu Y (2015) Spectroscopic and microscopic studies on the mechanism of mitochondrial toxicity Induced by CdTe QDs modified with different ligands. J Membrane Biol 248(4):727–740
Levine C, Cheney MA (2000) Metabolic responses to acute toxicity of alkylphenols and alkylphenol polyethoxylates in Eliptio Complanata measured by calorespirometry. Environ Toxicol Chem 19(19):1906–1910
Li JH, Zhang Y, Xiao Q, Tian FF, Liu XR, Li R, Zhao GY, Jiang FL, Liu Y (2011) Mitochondria as target of quantum dots toxicity. J Hazard Mater 194(30):440–444
Liu XR, Alex B, Chen JH (2015) Dynamics of the BH3-only protein binding interface of Bcl-xL. Biophys J 109(5):1049–1057
Liu XR, Li JH, Zhang Y, Ge YS, Tian FF, Dai J, Jiang FL, Liu Y (2011) Mitochondrial permeability transition induced by different concentrations of Zinc. J Membr Biol 244(3):105–112
Lovett GS, Sweetman AJ (1983) The analysis of drug action on mitochondrial oxidative phosphorylation. The choice of organic solvent for water-insoluble drugs. Methods Find Exp Clin Pharmacol 5(10):695–699
Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P (2012) Mitochondria-Ros crosstalk in the control of cell death and aging. J Signal Transduct, Article ID 329635. doi:10.1155/2012/329635
Murphy MP (2001) How understanding the control of energy metabolism can help investigation of mitochondrial dysfunction, regulation and pharmacology. Biochim Biophys Acta 1504(1):1–11
Newmeyer DD, Ferguson-Miller S (2003) Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112(4):481–490
Nohl H, Gille L, Stainek K (2005) Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol 69(5):719–723
Passarella S, Atlante A, Valenti D, de BL (2003) The role of mitochondrial transport in energy metabolism. Mitochondrion 2(5):319–343
Petronilli V, Sileikyte J, Zulian A, Dabbeni-Sala F, Jori G, Gobbo S, Tognon G, Nikolov P, Bernardi P, Ricchelli F (2009) Switch from inhibition to activation of the mitochondrial permeability transition during hematoporphyrin-mediated photooxidative stress: unmasking pore-regulating external thiols. Biochim Et Biophys Acta 1787(7):897–904
Pon LA, Schon EA (2007) Methods in cell biology, vol 80: mitochondria, 2nd edn. Academic Press, New York
Ricchelli F, Gobbo S, Moreno G, Salet C (1999) Changes of the fluidity of mitochondrial membranes induced by the permeability transition. BioChemistry 38(29):9295–9300
Ricchelli F, Beghetto C, Gobbo S, Tognon G, Moretto V, Crisma M (2003) Structural modifications of the permeability transition pore complex in resealed mitochondria induced by matrix-entrapped disaccharides. Arch Biochem Biophys 410(1):155–160
Ricchelli F, Jori G, Gobbo S, Nikolov P, Petronilli V (2005) Discrimination between two steps in the mitochondrial permeability transition process. Int J Biochem Cell Biol 37(9):1858–1868
Santo-Domingo J, Demaurex N (2010) Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 1797(6–7):907–912
Syed M, Skonberg C, Hansen SH (2013) Effect of organic solvents on oxidative phosphorylation in rat liver mitochondria: choice of organic solvents. Toxicol In Vitro 27(8):2135–2141
Wu DF, Cederbaum AI (2009) Oxidative stress and alcoholic liver disease. Semin Liver Dis 29(2):141–154
Xia CF, Jin JC, Yuan L, Zhao J, Chen XY, Jiang FL, Qin CQ, Dai J, Liu Y (2013) Microcalorimetric studies of the effect of cerium(III) on isolated rice mitochondria fed by pyruvate. Chemosphere 91(11):1577–1582
Yang LY, Gao JL, Gao T, Dong P, Ma L, Jiang FL, Liu Y (2016) Toxicity of polyhydrolated fullerene to mitochondria. J Hazard Mater 301(15):119–126
Zhao J, Zhou ZQ, Jin JC, Yuan L, He H, Jiang FL, Yang XG, Dai J, Liu Y (2014) Mitochondrial dysfunction induced by different concentrations of gadolinium ion. Chemosphere 100:194–199
Zhao J, Ma L, Xiang X, Guo QL, Jiang FL, Liu Y (2016) Microcalorimetric studies on the energy release of isolated rat mitochondria under different concentrations of gadolinium (III). Chemosphere 153:414–418
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21463008); Guangxi Natural Science Foundation Program (No. 2012GXNSFBA053119); and the Foundation of State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2012 -A10).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, L., Dong, JX., Wu, C. et al. Spectroscopic, Polarographic, and Microcalorimetric Studies on Mitochondrial Dysfunction Induced by Ethanol. J Membrane Biol 250, 195–204 (2017). https://doi.org/10.1007/s00232-017-9947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-017-9947-0