Abstract
We characterize the kinetic properties of a gill (Na+, K+)-ATPase from the pelagic marine seabob Xiphopenaeus kroyeri. Sucrose density gradient centrifugation revealed membrane fractions distributed mainly into a heavy fraction showing considerable (Na+, K+)-ATPase activity, but also containing mitochondrial F0F1- and Na+- and V-ATPases. Western blot analysis identified a single immunoreactive band against the (Na+, K+)-ATPase α-subunit with an Mr of ≈110 kDa. The α-subunit was immunolocalized to the intralamellar septum of the gill lamellae. The (Na+, K+)-ATPase hydrolyzed ATP obeying Michaelis–Menten kinetics with VM = 109.5 ± 3.2 nmol Pi min−1 mg−1 and KM = 0.03 ± 0.003 mmol L−1. Mg2+ (VM = 109.8 ± 2.1 nmol Pi min−1 mg−1, K0.5 = 0.60 ± 0.03 mmol L−1), Na+ (VM = 117.6 ± 3.5 nmol Pi min−1 mg−1, K0.5 = 5.36 ± 0.14 mmol L−1), K+ (VM = 112.9 ± 1.4 nmol Pi min−1 mg−1, K0.5 = 1.32 ± 0.08 mmol L−1), and NH4 + (VM = 200.8 ± 7.1 nmol Pi min−1 mg−1, K0.5 = 2.70 ± 0.04 mmol L−1) stimulated (Na+, K+)-ATPase activity following site–site interactions. K+ plus NH4 + does not synergistically stimulate (Na+, K+)-ATPase activity, although each ion modulates affinity of the other. The enzyme exhibits a single site for K+ binding that can be occupied by NH4 +, stimulating the enzyme. Ouabain (KI = 84.0 ± 2.1 µmol L−1) and orthovanadate (KI = 0.157 ± 0.001 µmol L−1) inhibited total ATPase activity by ≈50 and ≈44 %, respectively. Ouabain inhibition increases ≈80 % in the presence of NH4 + with a threefold lower KI, suggesting that NH4 + is likely transported as a K+ congener.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osmotic and ionic homeostasis in the Crustacea is accomplished by the multi-functional gills, together with excretory organs like the antennal glands (Péqueux 1995; Freire et al. 2008). The gill epithelial cells also play a central role in hemolymph acid–base regulation and in the excretion of nitrogenous metabolic end products (Péqueux 1995; Lucu and Towle 2003; Weihrauch et al. 2004). Most marine crustaceans are isosmotic with their surrounding medium, using Na+ and Cl− ions as their primary hemolymph osmolytes (Péqueux 1995; Lucu and Towle 2003; Kirschner 2004; Freire et al. 2008; McNamara and Faria 2012). However, the selection of a stable extracellular osmolyte concentration relative to that found in fluctuating salinities may have sustained the invasion of estuaries and low salinity biotopes by the Crustacea (Morris 2001; Lee et al. 2011). A species independence of fluctuations in environmental salinity relies on efficient mechanisms of salt uptake and excretion, finely regulated by the activities of specific ion transporters in its osmoregulatory organs, such as the gills (Lucu and Towle 2003; Freire et al. 2008).
Although gill-based osmoregulatory mechanisms have been intensively studied in decapods from distinct osmotic niches (Péqueux 1995; Morris 2001; Freire et al. 2008; McNamara and Faria 2012), the role of molecular components in such transport mechanisms is still under active discussion. One suggestion for coupled Na+ and Cl− transport across the gill epithelium of weakly hyper-osmoregulating crabs proposes that the gill epithelium uses the (Na+, K+)-ATPase located in the basal membrane invaginations to drive active Na+ uptake, maintaining hemolymph Na+ concentration fairly constant (Towle and Kays 1986; Freire et al. 2008; McNamara and Faria 2012). Na+ movement across the apical membrane can be provided by an apical Na+/K+/2Cl− symporter (Towle and Weihrauch 2001) and/or an apical Na+/H+ antiporter (Weihrauch and Towle 2000). This Na+ gradient is supplemented by apical Cs+-sensitive K+ channels (Onken et al. 2003) that hyperpolarize the apical membrane, driving Cl− efflux to the hemolymph through Cl− channels in the basal invaginations (Morris 2001; Freire et al. 2008). Sodium influx may also follow a paracellular route (Onken et al. 2003; Freire et al. 2008).
Most investigations of crustacean osmoregulation have focused on reduced salinity and its effects on gill and excretory organ functions, evaluating compensatory ion uptake mechanisms (Freire et al. 2008) and alterations in urine volume and concentration (Robinson 1982; Péqueux 1995; Sáez et al. 2009). The gill (Na+, K+)-ATPase also appears to play a major role in ammonia excretion (Weihrauch et al. 2004). NH4 + can replace K+ in sustaining ATP hydrolysis (Furriel et al. 2000; Masui et al. 2002, 2005; Garçon et al. 2009; Lucena et al. 2012; Leone et al. 2014) as also seen in the vertebrate enzyme (Skou and Esmann 1992). The synergistic stimulation by K+ and NH4 + of gill (Na+, K+)-ATPase activity in various crustaceans suggests a significant physiological role for the (Na+, K+)-ATPase in active nitrogen excretion by the gill epithelium (Masui et al. 2002; Gonçalves et al. 2006; Garçon et al. 2007; Santos et al. 2007; Lucena et al. 2012; França et al. 2013; Leone et al. 2014).
The (Na+, K+)-ATPase (E.C.3.6.1.37) or sodium pump belongs to the P2-type ATPase enzyme family, and is phosphorylated by an ATP-derived γ-phosphate group at an aspartate residue in the highly conserved DKTGS/T sequence during the ion transport cycle (Palmgren and Nissen 2011). The (Na+, K+)-ATPase is an oligomeric enzyme consisting of a catalytic α-subunit and a β-subunit required for the correct folding, stabilization and expression of the active α-protomer in the plasma membrane (Kaplan 2002; Morth et al. 2007; Poulsen et al. 2010). Recently, a short, single-span membrane protein belonging to the FXYD2 peptide family has been shown to interact with transmembrane helix αM9, fine-tuning the kinetic behavior of the (Na+, K+)-ATPase to the specific needs of a given cell type, tissue or physiological state (Garty and Karlish 2006; Geering 2008; Shindo et al. 2011). This FXYD peptide is also present in the crustacean (Na+, K+)-ATPase (Silva et al. 2012).
All P-type ATPases function similarly, hydrolyzing ATP and occluding ions during the translocation process within the membrane-embedded protein moiety. As a consequence, the specific ion binding sites on the enzyme are accessible from one side of the membrane only. The overall reaction mechanism of the (Na+, K+)-ATPase involves at least two different conformations: E1, exhibiting high affinity for intracellular Na+, and E2, showing high affinity for extracellular K+, each existing in a phosphorylated or dephosphorylated form (Horisberger 2004). Cycling between the E1 and E2 forms results in the counter transport of three Na+ ions from the cytosol and two K+ ions into the cytosol across the cell membrane, at the expense of ATP hydrolysis (Kaplan 2002; Jorgensen et al. 2003).
The pelagic marine shrimp Xiphopenaeus kroyeri (Heller 1862) is widely distributed along the coast of the Western Atlantic ocean from Virginia (USA), throughout the Gulf of Mexico and the Caribbean Sea to Southern Brazil (Rio Grande do Sul) (Williams 1984; Costa et al. 2003). This species, popularly known as the “camarão sete-barbas” in Brazilian waters, or the “seabob” around the world, is one of the most important commercial shallow water crustaceans exploited along the northern coast of São Paulo State. It is subject to intensive trawling and accounts for approximately 80 % of the penaeid shrimp catch (Mantelatto et al. 1999). The species’ plays an important ecological role through its trophic relationships, maintaining the stability of benthic communities (Pires 1992).
Adult X. kroyeri migrates to deep offshore areas during their reproductive period, and various physiological and biological processes establish the recruitment pattern of the juveniles (Dall et al. 1990). While juvenile Xiphopenaeus spp. inhabit estuarine areas, their migration patterns between the estuarine and fully marine biotopes are poorly known, and it is uncertain to what extent they depend on estuaries as recruitment sites. The penaeid shrimps possess bi-lobate, dendrobranchiate gills (Taylor and Taylor 1992). However, there is no information available as to gill morphology and epithelial ultrastructure in X. kroyeri. While knowledge of the biology and ecology of X. kroyeri has slowly accumulated (for review see Heckler et al. 2014), there is a dearth of information on the species’ physiology and biochemistry.
One of the novelties of this work is the species examined (X. kroyeri), a penaeid shrimp with a complex life cycle that unfolds in different habitats. Such a life history is underpinned by a complex physiology and biochemistry, as demonstrated by our findings for the gill (Na+, K+)-ATPase. This is the first study to evaluate these aspects in a very intriguing species. Our findings lay useful groundwork for future studies and aid in comprehending the evolution of biochemical processes related to osmoregulation in decapod crustaceans.
We provide a full kinetic characterization of the (Na+, K+)-ATPase present in a microsomal fraction from the gill epithelium of X. kroyeri held in seawater in an effort to better understand the role of this enzyme in the active excretion of NH4 + by the gills in different decapod crustacean groups. We also examine the distribution of (Na+, K+)-ATPase activity in a sucrose density gradient and immunolocalize the enzyme to the intralamellar septum of the bi-lobate gill lamellae.
Materials and Methods
Material
All solutions were prepared using Millipore MilliQ ultrapure, apyrogenic water. Tris, ATP ditris salt, pyruvate kinase (PK), phosphoenolpyruvate (PEP), NAD+, NADH, imidazole, N-(2-hydroxyethyl) piperazine-N19-ethanesulfonic acid (HEPES), lactate dehydrogenase (LDH), ouabain, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), nitroblue tetrazolium (NBT), 5-bromo-4-chloro-3-indole phosphate (BCIP), 4′,6-diamidino-2-phenylindole (DAPI), alamethicin, imidazole, sodium orthovanadate, 3-phosphoglyceraldehyde diethyl acetal, ethacrynic acid, oligomycin, thapsigargin, bafilomycin A1 were purchased from the Sigma Chemical Company (Saint Louis, USA). Dimethyl sulfoxide (DMSO) and triethanolamine (TEA) were from Merck (Darmstadt, Germany). The protease inhibitor cocktail (1 mmol L−1 benzamidine, 5 µmol L−1 antipain, 5 µmol L−1 leupeptin 1 µmol L−1, pepstatin A and 5 µmol L−1 phenyl-methane-sulfonyl-fluoride) was from Calbiochem (Darmstadt, Germany). Mouse monoclonal α-5 IgG antibody raised against the α-subunit of chicken (Na+, K+)-ATPase was from the Development Studies Hybridoma Bank, maintained by the University of Iowa (Iowa, USA). Antimouse IgG, alkaline phosphatase conjugate was purchased from the Promega Corporation (Madison, USA). Optimal Cutting Temperature Compound was from Sakura Tissue-Tek (Torrance, USA). Alexa-fluor 488-conjugated goat anti-mouse IgG, was from Invitrogen (Carlsbad, USA); fluoromount-G and paraformaldehyde were from Electron Microscopy Sciences (Hatfield, USA). All other reagents were of the highest purity commercially available.
Crystalline suspensions of LDH and PK in 2.9 mol L−1 ammonium sulfate (200 µL) were centrifuged at 14,000 rpm for 15 min at 4 °C in an Eppendorf Model 5810 refrigerated centrifuge (Hamburg, Germany). The pellet was resuspended in 500 µL of 50 mmol L−1 HEPES buffer, pH 7.5, transferred to a YM-10 Microcon filter (Millipore Corporation, Billerica, USA) and washed five times at 10,000 rpm for 15 min at 4 °C in the same buffer until complete removal of ammonium ions (tested with the Nessler reagent). Finally, the pellet was resuspended to the original volume. For PGK and GAPDH, the suspension was treated as above with 50 mmol L−1 triethanolamine buffer, pH 7.5, containing 1 mmol L−1 dithiothreitol. Ammonium sulfate-depleted PK, LDH, PGK and GAPDH suspensions were used within 2 days. Glyceraldehyde-3-phosphate (G3P) was prepared by hydrolysis of 3-phospho-glyceraldehyde diethyl acetal, barium salt, with 150 µL HCl (d = 1.18 g mL−1) in a boiling water bath for 2 min, after removal of the barium salt with Dowex 50H+ resin, as recommended by the manufacturer (see Sigma Chem. Co. Product Information for Product Number G5376). Final pH was adjusted to 7.0 with 50 µL triethanolamine just before use. When necessary, enzyme solutions were concentrated on Amicon Ultra 10 K centrifugal filters (Millipore Corporation, Billerica, USA). The stock solution of ATP was prepared by dissolving ATP di-Tris salt in water and adjusting the pH to 7.0 with triethanolamine (d = 1.12 g mL−1). The exact concentration was established from the extinction coefficient (ε260 nm,pH 7.0 = 15,400 mol L−1 cm−1) and adjusted to 100 mmol L−1. Concentrated bafilomycin A1 (200 μmol L−1) and thapsigargin (28 μmol L−1) solutions were prepared in DMSO; oligomycin (100 μg mL−1) and aurovertin (5 mmol L−1) were prepared in ethanol. Sodium orthovanadate solution was prepared according to Gordon (1991).
Shrimps
Adult X. kroyeri were caught from Ubatuba Bay (23°26′ S, 45°02′ W), São Paulo State (Brazil) using double rig trawl nets. The shrimps were transported to the laboratory and maintained in tanks containing aerated seawater (33 ‰ salinity, 25 °C) overnight. For each homogenate prepared, 15–30 intermolt specimens of about 10 cm total length were anesthetized by chilling in a freezer (−20 °C) and killed by quickly removing the carapace and appendages and destroying the thoracic ganglion. All gills were rapidly excised and placed in 25 mL ice-cold 20 mmol L−1 imidazole buffer, pH 6.8, containing 250 mmol L−1 sucrose, 6 mmol L−1 EDTA and the protease inhibitor cocktail (homogenization buffer).
Preparation of Microsomal Fractions
The gills were rapidly diced and homogenized in a Potter homogenizer in 20 mmol L−1 imidazole homogenization buffer (20 mL buffer/g wet tissue). After centrifuging, the crude extract at 20,000×g for 35 min at 4 °C, the supernatant was placed on crushed ice and the pellet was re-suspended in an equal volume of the homogenization buffer. After further centrifugation as above, the two supernatants were gently pooled and centrifuged at 100,000×g for 90 min at 4 °C. The resulting pellet containing the microsomal fraction was homogenized in 20 mmol L−1 imidazole buffer, pH 6.8, containing 250 mmol L−1 sucrose (15 mL buffer/g wet tissue). Finally, 0.5-mL aliquots were rapidly frozen in liquid nitrogen and stored at −20 °C. No appreciable loss of (Na+, K+)-ATPase activity was seen after two-months’ storage of the microsomal enzyme prepared as above. When required, the aliquots were thawed, placed on crushed ice and used immediately.
Continuous-Density Sucrose Gradient Centrifugation
An aliquot (1.9 mg) of the ATPase-rich gill microsomal fraction was layered into a 10–50 % (w/w) continuous-density, sucrose gradient in 20 mmol L−1 imidazole buffer, pH 6.8, and centrifuged at 180,000 × g and 4 °C, for 3 h, using a PV50T2 Hitachi vertical rotor. Fractions (0.5 mL) collected from the bottom of the gradient were then assayed for total ATPase activity, ouabain-insensitive ATPase activity (assayed in the presence of 3 mmol L−1 ouabain), protein and refractive index.
Measurement of ATP Hydrolysis
Total ATPase activity was assayed at 25 °C using a PK/LDH coupling system (Rudolph et al. 1979) in which ATP hydrolysis was coupled to NADH oxidation according to Leone et al. (2014). The oxidation of NADH was monitored at 340 nm (ε340 nm, pH 7.5 = 6,200 mol−1 L cm−1) in a Hitachi U-3000 spectrophotometer equipped with thermostatted cell holders. Standard conditions were: 50 mmol L−1 HEPES buffer, pH 7.5, 1 mmol L−1 ATP, containing 2 mmol L−1 MgCl2, 30 mmol L−1 NaCl, 10 mmol L−1 KCl, 0.14 mmol L−1 NADH, 2 mmol L−1 PEP, 82 µg PK (49 U), and 110 µg LDH (94 U) in a final volume of 1 mL.
ATP hydrolysis was also estimated using 3 mmol L−1 ouabain to assess ouabain-insensitive activity. The difference in activity measured in the absence (total ATPase activity) or presence of ouabain (ouabain-insensitive activity) represents the (Na+, K+)-ATPase activity. Alternatively, for K+ and NH4 +, ATPase activity was estimated using a GAPDH/PGK-linked system coupled to the reduction of NAD+ at 340 nm (Leone et al. 2014). Standard conditions were: 50 mmol L−1 TEA buffer, pH 7.5, 1 mmol L−1 NAD+, 0.5 mmol L−1 sodium phosphate, 1 mmol L−1 G3P, 150 µg GAPDH (12 U) and 20 µg PGK (9 U) in a final volume of 1 mL. The two coupling systems gave equivalent results with a difference of less than 10 %.
ATP hydrolysis was also estimated at 25 °C after 10 min pre-incubation with alamethicin (1 mg/mg protein) to demonstrate the presence of leaky and/or disrupted vesicles. Controls without added enzyme were included in each experiment to quantify the non-enzymatic hydrolysis of substrate. Initial velocities were constant for at least 15 min provided that less than 5 % of the total NADH (or NAD+) was oxidized (or reduced). The reaction rate for each modulator was estimated in duplicate using identical aliquots from the same preparation. For each microsomal preparation, assay linearity was checked using samples containing 5–50 μg protein; total microsomal protein added to the cuvette always fell well within the linear range of the assay. Neither NADH, PEP, LDH, PK, NAD+, G3P, PGK nor GAPDH was rate-limiting over the initial course of the assay, and no activity could be measured in the absence of NADH or NAD+. Mean values were used to fit each corresponding saturation curve, which was repeated three times utilizing different microsomal homogenates (N = 3). One enzyme unit (U) is defined as the amount of enzyme that hydrolyzes 1.0 nmol of ATP per minute, at 25 °C, and (Na+, K+)-ATPase specific activity is given as nmol Pi min−1 mg−1 total protein.
Western Blot Analysis
SDS-PAGE of the gill microsomes from shrimps held in sea water was performed as described by Laemmli (1970) using 4 and 160 µg protein/slot for protein staining and blotting analysis, respectively. After electrophoresis, the gel was split, one half being stained with silver nitrate and the other electro-blotted using a Gibco BRL Mini-V 8–10 system (Gaithersburg, USA) employing a nitrocellulose membrane according to Towbin et al. (1979). The nitrocellulose membrane was blocked for 10 h with 5 % nonfat dry milk powder freshly prepared in 50 mmol L−1 Tris. HCl buffer, pH 8.0, containing 150 mmol L−1 NaCl and 0.1 % Tween 20, with constant agitation. The membrane was incubated for 30 min at 25 °C in a 1: 10 dilution (2.1 µg mL−1) of the α-5 monoclonal antibody. After washing three times in 50 mmol L−1 Tris. HCl buffer, pH 8.0, containing 150 mmol L−1 NaCl and 0.1 % Tween 20, the membrane was incubated for 30 min at 25 °C with an anti-mouse IgG, alkaline phosphatase conjugate, diluted 1: 7,500. The membrane was washed three times in 50 mmol L−1 Tris. HCl buffer, pH 8.0, containing 150 mmol L−1 NaCl and 0.1 % Tween 20, and specific antibody binding was developed in 100 mmol L−1 Tris. HCl buffer, pH 9.5, containing 100 mmol L−1 NaCl, 5 mmol L−1 MgCl2, 0.2 mmol L−1 NBT and 0.8 mmol L−1 BCIP. Controls consisting of membranes incubated with the secondary antibody without previous incubation with the α-5 antibody were included in each experiment. Western blot analysis for each experiment was repeated three times using different gill preparations from separate pools of 15–30 shrimps each. Immunoblots were scanned and imported as JPG files into a commercial software package (Kodak 1D 3.6) where immuno-reaction densities were compared.
Immunofluorescence Microscopy
Fourth, left side gills were dissected and incubated in a fixative solution containing 2.5 % p-formaldehyde in a phosphate buffered saline PBS, (10 mmol L−1 Na2HPO4, 2 mmol L−1 KH2PO4, 137 mmol L−1 NaCl, 2.7 mmol L−1 KCl, 290 mOsm kg−1 H2O), pH 7.4, for 1 h, and then embedded in Optimal Cutting Temperature Compound. Thick cryosections (15 μm) were taken transversely to the gill lamellae long-axes using a Micron model HM 505E Cryostatic Microtome (Walldorf, Germany) at −20 °C and collected on gelatin-coated slides (Bloom 225).
Cryosectioning was performed according to França et al. (2013), using a primary α-5 IgG antibody raised against chicken (Na+, K+)-ATPase α-subunit, diluted to 21 mg mL−1 in PBS (Takeyasu et al. 1988). A goat anti-mouse IgG secondary antibody conjugated with Alexa-fluor 488 was used to provide the fluorescence signal. The sections were observed and photographed using an Olympus BX-50 fluorescence microscope (Olympus America Inc., Melville, USA) equipped with a SPOT RT3 25.4 2 Mb Slider camera (SPOT Imaging Solutions Inc., Sterling Heights, USA) employing phase contrast microscopy and excitation/emission wavelength of 495/519 nm (Alexa-fluor 488) and 358/461 nm (DAPI).
Protein Measurement
Protein concentration was estimated using the Coomassie Blue G dye-binding assay (Read and Northcote 1981) employing bovine serum albumin as the standard.
Estimation of Kinetic Parameters
The kinetic parameters VM (maximum velocity), K0.5 (apparent dissociation constant) and KM (Michaelis–Menten constant), and the nH (Hill coefficient) values for ATP hydrolysis under the different assay conditions were calculated using SigrafW software (Leone et al. 2005). The apparent dissociation constant, KI, of the enzyme-inhibitor complex was estimated as described by Marks and Seeds (1978). The kinetic parameters furnished in the tables are calculated values and represent the mean (±SEM) derived from three different microsomal preparations (N = 3). SigrafW software can be freely obtained from http://portal.ffclrp.usp.br/sites/fdaleone/downlods.
Results
Transverse sections near the tips of gill lamellae from X. kroyeri (Fig. 1a) reveal the lamellar epithelium to consist of an intralamellar septum extending from one marginal channel to the other. A fine epithelium of pillar cell flanges underlies the cuticle. Immunofluorescence labeling reveals the (Na+, K+)-ATPase α-subunit to be located predominantly in the intralamellar septum of the gill lamellae (Fig. 1b). Although irregular in distribution, fluorescent signal is concentrated in the central region of the lamellar transect, declining toward the peripheral marginal channels whose epithelia show very little or no signal.
Immunolocalization of the (Na+, K+)-ATPase α-subunit in cryosections of gill lamellae from Xiphopenaeus kroyeri. a Phase contrast image revealing typical structure of the dendrobranchiate gills with an intralamellar septum extending between the two marginal channels that delimit each gill tip. b Immunofluorescence labeling (Alexa-fluor 488; 495/519 nm) showing distribution of the (Na+, K+)-ATPase α-subunit (green) located predominantly in the intralamellar septum. Nuclei were stained with DAPI (blue). Scale bars = 50 µm
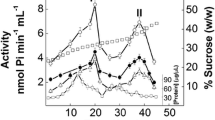
Sucrose density gradient centrifugation of the gill microsomal preparation (Fig. 2) revealed a single protein peak in the light fractions (15–20 % sucrose), and a second component spread evenly over the heavy fractions (30–40 % sucrose). There are two well-defined peaks showing (Na+, K+)-ATPase activity (Fig. 2). The light fraction shows low (Na+, K+)-ATPase activity and is highly contaminated by other ATP hydrolyzing enzymes. The heavy fraction exhibits significant (Na+, K+)-ATPase activity and very low levels of other ATP hydrolyzing enzymes (inset to Fig. 2). Protein recovery from the gradient was greater than 90 %, and no significant loss of (Na+, K+)-ATPase activity was seen when the microsomal fraction was maintained at 4 °C for up to 6 h (data not shown).
Sucrose density gradient centrifugation of a microsomal fraction from the gill epithelium of X. kroyeri. An aliquot containing 1.9 mg protein was layered into a 10–50 % (w/w) continuous sucrose density gradient. Fractions (0.5 mL) were collected from the bottom of the gradient and analyzed for total ATPase activity (filled square); ouabain-insensitive ATPase activity (open square); (Na+, K+)-ATPase activity (filled circle); protein concentration (open circle); sucrose concentration (open triangle)
Western blot analysis identified a single immunoreactive band of the α-5 monoclonal antibody against the α-subunit of the (Na+, K+)-ATPase with an Mr of ≈110 kDa, suggesting the presence of a single α-subunit isoform (Fig. 3). The data also show that the (Na+, K+)-ATPase represents just a small proportion of the total protein content in the gill microsomal fraction from X. kroyeri.
SDS-PAGE and western blot analysis of the microsomal fraction from the gill epithelium of X. kroyeri. Electrophoresis was performed in a 5–20 % polyacrylamide gel using 4 and 160 µg protein/slot for protein staining and blotting analysis, respectively. Lane A silver nitrate-stained SDS-PAGE. Lane B Western blotting against the α-subunit of the (Na+, K+)-ATPase as revealed by an anti-mouse IgG, alkaline phosphatase conjugate
The effect of increasing ATP concentrations on (Na+, K+)-ATPase activity of the gill microsomal preparation is shown in Fig. 4. Under saturating K+ (10 mmol L−1), Na+ (30 mmol L−1) and Mg2+ (2 mmol L−1) concentrations, ATP hydrolysis follows a well-defined saturation curve in the range of 10−7–10−3 mol L−1 ATP, obeying Michaelis–Menten kinetics, with VM = 109.5 ± 3.2 nmol Pi min−1 mg−1 and KM = 0.03 ± 0.003 mmol L−1 (Table 1). At ATP concentrations as low as 10−7 mol L−1, an ATPase activity of ≈10 nmol Pi min−1 mg−1 was measured independently of the presence of ouabain. Ouabain-insensitive ATPase activity is stimulated up to 95 nmol Pi min−1 mg−1 over the same ATP concentration range, which represents around 48 % of total ATPase activity (inset to Fig. 4), suggesting the presence of ATPases other than (Na+, K+)-ATPase. Above 1 mmol L−1, (Na+, K+)-ATPase activity is significantly inhibited by excess free ATP (not shown). Alamethicin has no effect on (Na+, K+)-ATPase activity, suggesting that ATP has free access to the enzyme.
Effect of ATP concentration on (Na+, K+)-ATPase activity in the microsomal fraction from the gill epithelium of X kroyeri. Activity was assayed continuously at 25 °C using 7.4 µg protein in 50 mmol L−1 HEPES buffer, pH 7.5, as described in the “Materials and Methods” section. A representative curve obtained from one homogenate is given. The experiment was performed using duplicate enzyme aliquots from three (N = 3) different gill microsomal fractions. Inset Ouabain-insensitive ATPase activity (open circle) Total ATPase activity (filled circle)
The modulation by Mg2+ and Na+ of (Na+, K+)-ATPase activity is shown in Fig. 5. Magnesium ions are essential for (Na+, K+)-ATPase activity of the gill microsomal fraction of X. kroyeri (Fig. 5a). Under saturating ATP (1 mmol L−1), Na+ (30 mmol L−1) and K+ (10 mmol L−1) concentrations, increasing Mg2+ concentrations from 10−5 to 3 × 10−3 mol L−1 stimulated (Na+, K+)-ATPase activity up to 109.8 ± 2.1 nmol Pi min−1 mg−1 with a K0.5 = 0.60 ± 0.03 mmol L−1 (Table 1). Cooperative effects (nH = 2.2) resulting from Mg2+ interaction with the enzyme suggest multiple Mg2+ binding sites. No significant (Na+, K+)-ATPase activity was seen at Mg2+ concentrations as low as 10−5 mol L−1. However, ouabain-insensitive ATPase activity, corresponding to ≈48 % of total ATPase activity (≈98 nmol Pi min−1 mg−1), was estimated over the same Mg2+ concentration range (inset to Fig. 5a) and corroborates the presence of ATPases other than (Na+, K+)-ATPase. Above 10 mmol L−1 (Na+, K+)-ATPase activity was significantly inhibited by excess free Mg2+ (not shown).
Effect of Mg2+ and Na+ concentration on (Na+, K+)-ATPase activity in the microsomal fraction from the gill epithelium of X. kroyeri. Activity was assayed continuously at 25 °C using 7.4 µg protein in 50 mmol L−1 HEPES buffer, pH 7.5, as described in the “Materials and Methods” section. A representative curve obtained from one homogenate is given. The experiment was performed using duplicate enzyme aliquots from three (N = 3) different gill microsomal fractions. a Mg2+. b Na+. Insets Ouabain-insensitive ATPase activity (open circle) Total ATPase activity (filled circle)
Under saturating ATP (1 mmol L−1), K+ (10 mmol L−1) and Mg2+ (2 mmol L−1) concentrations, the Na+-dependence of (Na+, K+)-ATPase activity (Fig. 5b) was characterized by positive cooperativity (n = 1.2) with V = 117.6 ± 3.5 nmol Pi min−1 mg−1 and K0.5 = 5.36 ± 0.14 mmol L−1 over the range from 10−5 to 5 × 10−2 mol L−1 (Table 1). Ouabain-insensitive activity was stimulated from ≈80 to ≈95 nmol Pi min−1 mg−1 over the same Na+ concentration range (inset to Fig. 5b), suggesting the presence of a Na+-stimulated ATPase in the microsomal preparation.
The effect of NH4 + on K+-stimulated (Na+, K+)-ATPase activity of X. kroyeri is shown in Fig. 6a. Under saturating ATP (1 mmol L−1), Na+ (30 mmol L−1) and Mg2+ (2 mmol L−1) concentrations, and without NH4 +, stimulation of (Na+, K+)-ATPase activity by K+ (from 10−6 to 2 × 10−2 mol L−1) reached a maximum rate of 112.9 ± 1.4 nmol Pi min−1 mg−1 with K0.5 = 1.32 ± 0.08 mmol L−1, obeying cooperative kinetics (Table 1). (Na+, K+)-ATPase activity of ≈20 nmol Pi min−1 mg−1 was seen at K+ concentrations as low as 10−6 mol L−1, and ouabain-insensitive ATPase activity was not stimulated over the same concentration range, suggesting the absence of a K+-stimulated ATPase in the microsomal fraction (not shown). Notable responses were revealed when ATPase activity was assayed in the presence of both K+ plus NH4 +. Modulation by K+ in the presence of low fixed NH4 + concentrations (5 and 10 mmol L−1) stimulated (Na+, K+)-ATPase activity ≈fivefold (from ≈20 to ≈110 nmol Pi min−1 mg−1) as K+ concentration increased from 10−6 to 5 × 10−2 mol L−1. No synergistic effect was seen under such ionic conditions. However, for the higher NH4 + concentrations (20 and 50 mmol L−1), further addition of K+ (10−6 to 5 × 10−2 mol L−1) inhibited (Na+, K+)-ATPase activity. Activity decreased from ≈200 nmol Pi min−1 mg−1 (with 50 mmol L−1 NH4 + and 10−6 mol L−1 K+) and ≈140 nmol Pi min−1 mg−1 (with 20 mmol L−1 NH4 + and 10−6 mol L−1 K+) to ≈110 nmol Pi min−1 mg−1 (Table 2). This inhibitory effect suggests that even when fully saturated by NH4 +, increasing K+ concentrations displace NH4 + from the K+ binding sites.
Effect of potassium plus ammonium ions on (Na+, K+)-ATPase activity in the microsomal fraction from the gill epithelium of X. kroyeri. Activity was assayed continuously at 25 °C using 7.4 µg protein in 50 mmol L−1 TEA buffer, pH 7.5, 1 mmol L−1 NAD+, 0.5 mmol L−1 sodium phosphate, 1 mmol L−1 G3P, 150 µg GAPDH (12 U) and 20 µg PGK (9 U) in a final volume of 1 mL. Representative curves obtained from one homogenate are given. The experiment was performed using duplicate enzyme aliquots from three (N = 3) different gill microsomal fractions a (filled circle) No NH4 +; (open circle) 5 mmol L−1 NH4 +; (filled triangle) 10 mmol L−1 NH4 +; (open triangle) 20 mmol L−1 NH4 +; (closed diamond) 50 mmol L−1 NH4 +. b (filled circle) No K+; (open circle) 0.5 mmol L−1 K+; (filled triangle) 1 mmol L−1 K+; (open triangle) 5 mmol L−1 K+; (filled diamond) 10 mmol L−1 K+
The effect of K+ on NH4 +-stimulated (Na+, K+)-ATPase activity is shown in Fig. 6b. Under saturating ATP (1 mmol L−1), Na+ (30 mmol L−1) and Mg2+ (2 mmol L−1) concentrations, and without K+, (Na+, K+)-ATPase activity was stimulated by NH4 + (from 10−6 to 7 × 10−3 mol L−1) to maximum values of 200.8 ± 7.1 nmol Pi min−1 mg−1 with K0.5 = 2.70 ± 0.04 mmol L−1, obeying cooperative kinetics (Table 1). A residual (Na+, K+)-ATPase activity of ≈40 nmol Pi min−1 mg−1 was observed for NH4 + concentrations as low as 10−6 mol L−1, but ouabain-insensitive ATPase activity was not stimulated over the same concentration range, suggesting the absence of an NH4 +-stimulated ATPase in the microsomal fraction (not shown). (Na+, K+)-ATPase activity assayed in the presence of fixed K+ concentrations (0.5–5 mmol L−1) also attained maximum values of around 200 nmol Pi min−1 mg−1. Cooperative kinetics was seen in the presence of both ions, and there were no relevant changes in K0.5 values when the enzyme is fully saturated by both ions (Table 2). No synergistic effects were found in the presence of NH4 + plus K+. However, the kinetic response to 10 mmol L−1 NH4 + is remarkable. (Na+, K+)-ATPase activity remaining unchanged at ≈110 nmol Pi min−1 mg−1 over the same K+ concentration range. Likely, as enzyme becomes fully saturated by K+ (10 mmol L−1), NH4 + does not displace K+ from its binding site. Table 2 summarizes the values of the kinetic parameters estimated for the stimulation by K+ plus NH4 + of (Na+, K+)-ATPase activity from the gill epithelium of X. kroyeri.
The effect of a wide range of ouabain and orthovanadate concentrations on total ATPase activity in the microsomal fraction from X. kroyeri gills is shown in Fig. 7. Using 1 mmol L−1 ATP, 2 mmol L−1 Mg2+, 30 mmol L−1 Na+, and 10 mmol L−1 K+, increasing ouabain concentrations up to 7 × 10−3 mol L−1 inhibited total ATPase activity by ≈50 % obeying a single ouabain-binding site model (Fig. 7a), with KI = 84.0 ± 2.1 μmol L−1 (inset to Fig. 7a and Table 1). With 50 mol L−1 NH4 +, inhibition increased to ≈80 %, and KI = 28.4 ± 0.7 μmol L−1 was threefold lower compared to that estimated in the absence of NH4 + (Table 1). Low orthovanadate concentrations (≈10−7 mol L−1) did not affect microsomal ATPase activity, but increasing concentrations up to 10−4 mol L−1 resulted in ≈40 % inhibition (79.6 ± 2.4 nmol Pi min−1 mg−1) ATPase activity (Fig. 7b). The calculated KI for orthovanadate inhibition was 0.157 ± 0.001 μmol L−1 (inset to Fig. 7b and Table 1).
Effect of ouabain and orthovanadate on (Na+, K+)-ATPase activity in the microsomal fraction from the gill epithelium of X. Kroyeri. Activity was assayed continuously at 25 °C using 7.4 µg protein in 50 mmol L−1 HEPES buffer, pH 7.5, containing 1 mmol L−1 ATP, 2 mmol L−1 MgCl2, 10 mmol L−1 KCl, 30 mmol L−1 NaCl, 0.14 mmol L−1 NADH, 2 mmol L−1 PEP, 82 µg PK (49 U) and 110 µg LDH (94 U). A representative curve obtained from one homogenate is given. The experiment was performed using duplicate enzyme aliquots from three (N = 3) different gill microsomal fractions. a ouabain: (open cirlce) without NH4 +; (filled circle) with 50 mol L−1 NH4 +. b Orthovanadate. Insets Dixon plot for estimation of KI in which vc is the reaction rate corresponding to (Na+, K+)-ATPase activity
(Na+, K+)-ATPase activity represents ≈50 % of the total ATP hydrolyzing activity present in the gill epithelium. The relative proportions of putative ATPases other than (Na+, K+)-ATPase accounting for the ouabain-insensitive activity in X. kroyeri gill microsomes are given in Table 3. The lack of an additional inhibitory effect by ouabain plus theophylline suggests a minor contribution by neutral phosphatases to the ouabain-insensitive ATPase activity. In contrast, the additional inhibition estimated with ouabain plus aurovertin B and ouabain plus Bafilomycin A1 suggests the presence of ≈22 % mitochondrial F0F1-ATPase and ≈14 % V(H+)-ATPase activity, respectively. The substantial inhibition by ouabain plus ethacrynic acid, corroborated by the stimulation by Na+ of the ouabain-insensitive ATPase activity (see inset to Fig. 5b) is a strong indication of Na+-stimulated ATPase activity (≈10 %). Finally, inhibition by ouabain plus thapsigargin of ouabain-insensitive ATPase activity suggests some Ca2+-ATPase activity (≈6 %).
Discussion
This systematic characterization of the gill (Na+, K+)-ATPase from X. kroyeri discloses two important findings. Firstly, K+ (or NH4 +) modulates stimulation of the (Na+, K+)-ATPase by NH4 + (or K+) without synergistic stimulation of enzyme activity. Secondly, modulation of the enzyme by NH4 + and K+ together shows that both ions bind to the K+ binding site, NH4 + being displaced at increasing K+ concentrations.
Characterization of the Gill Microsomal Fraction
The two peaks of (Na+, K+)-ATPase activity appearing at different sucrose densities suggest that the corresponding light and heavy membrane fractions originate from different regions of the gill epithelium (Furriel et al. 2010; Lucena et al. 2012), possibly the intralamellar septum and pillar cells (Freire and McNamara 1995). Two such peaks occur in gill microsomal fractions from the freshwater crab Dilocarcinus pagei (Furriel et al. 2010), salinity-acclimated blue crab Callinectes ornatus (Garçon et al. 2009), high salinity (45 ‰ S)-acclimated hermit crab Clibanarius vittatus (Lucena et al. 2012), and the freshwater shrimp Macrobrachium rosenbergii (França et al. 2013). In contrast, only a single peak appears in the fractions from M. olfersi and M. amazonicum (Furriel et al. 2000; Santos et al. 2007; Leone et al. 2014) and fresh-caught C. vittatus (Gonçalves et al. 2006) and C. ornatus (Garçon et al. 2007).
The Mr of ≈110 kDa estimated for the X. kroyeri gill (Na+, K+)-ATPase is similar to other decapods (Furriel et al. 2000; Donnet et al. 2001; Masui et al. 2002, 2005; Lucu and Towle 2003; Belli et al. 2009; Garçon et al. 2007, 2009; Lucena et al. 2012; França et al. 2013; Leone et al. 2014). The sole immunoreactive band revealed by Western blotting suggests the presence of a single α-subunit isoform typical of various crustacean species (Furriel et al. 2000; Masui et al. 2002, 2005; Lucu and Towle 2003; Belli et al. 2009; Garçon et al. 2007, 2009; Lucena et al. 2012; França et al. 2013; Leone et al. 2014). This is corroborated by the single titration curve for ouabain inhibition, but differs from the biphasic inhibition curve for D. pagei, for example (Furriel et al. 2010). Immunolocalization reveals the enzyme to be distributed predominantly throughout the intralamellar septum of the gill lamellae as seen in M. rosenbergii (França et al. 2013) and M. amazonicum (Boudour-Boucheker et al. 2014).
Modulation by ATP of the Gill (Na+, K+)-ATPase
ATP hydrolysis by X. kroyeri (Na+, K+)-ATPase (109.5 ± 3.2 nmol Pi min−1 mg−1) is similar to the gill enzyme from various brachyuran crabs acclimated to seawater (Lucu et al. 2000; Corotto and Holliday 1996; Holliday 1985; Lucu and Flik 1999; Masui et al. 2002; Lovett and Watts 1995; Piller et al. 1995; Lucu et al. 2000) but is considerably lower than for freshwater crustaceans (Furriel et al. 2000; Santos et al. 2007; Leone et al. 2012) and in blue crab C. danae (Masui et al. 2002). This may be a characteristic of marine species in general, owing to their elevated ion and water permeabilities and lesser osmoregulatory capacity (Péqueux 1995; Corotto and Holliday 1996) or may be a species-specific characteristic of the X. kroyeri (Na+, K+)-ATPase in particular. While two families of ATP binding sites are present in C. danae (Masui et al. 2002), C. vittatus (Gonçalves et al. 2006), and M. amazonicum (Santos et al. 2007), hydrolysis of ATP by the X. kroyeri (Na+, K+)-ATPase revealed only a single family of sites (KM = 0.03 ± 0.003 mmol L−1) as seen in C. ornatus (Garçon et al. 2007; 2009), Cancer pagurus (Gache et al. 1976), M. olfersi (Furriel et al. 2000), M. rosenbergii (Wilder et al. 2000; França et al. 2013), C. sapidus (Wheatly and Henry 1987) and some euryhaline brachyuran crabs (Holliday 1985; D’Orazio and Holliday 1985; Corotto and Holliday 1996). However, this KM value is ≈tenfold lower than in euryhaline brachyurans (Holliday 1985; D’Orazio and Holliday 1985; Corotto and Holliday 1996). The affinity of the (Na+, K+)-ATPase for ATP seems to be independent of both species’ biotope and tissue. These KM values are similar to those for the low-affinity sites of the vertebrate enzyme (Glynn 1985; Ward and Cavieres 1998).
Modulation by Mg2+ and Na+ of the Gill (Na+, K+)-ATPase
The interaction between the (Na+, K+)-ATPase and Mg2+ is difficult to interpret under physiological conditions since Mg2+ is both a ligand and substrate (Mg-ATP), and the enzyme is inhibited by excess Mg2+ (Glynn 1985; Karlish 2003). Such inhibition may result from binding to a second inhibitory site on the enzyme (Pedemonte and Beaugé 1983). The (Na+, K+)-ATPase affinity for Mg2+ is not dependent on habitat salinity: the affinity of X. kroyeri enzyme for Mg2+ is similar to that of both marine and freshwater crustaceans like C. vittatus (Gonçalves et al. 2006; Lucena et al. 2012), C. danae (Masui et al. 2002; 2009), C. ornatus (Garçon et al. 2007; 2009), M. amazonicum (Santos et al. 2007; Leone et al. 2012), M. rosenbergii (França et al. 2013), M. olfersi (Furriel et al. 2000), and D. pagei (Furriel et al. 2010). Further, as seen in gill enzymes from C. danae (Masui et al. 2002), C. ornatus (Garçon et al. 2007; 2009) and C. vittatus (Gonçalves et al. 2006; Lucena et al. 2012) stimulation of hydrolysis is cooperative, suggesting multiple Mg2+ binding sites.
Each (Na+, K+)-ATPase α-subunit isoform may have a different apparent affinity for Na+ and K+ when using ATP as a substrate (Blanco and Mercer 1998; Crambert et al. 2000; Blanco et al. 2005). Although a striking correlation exists between apparent affinity for Na+ and habitat (Péqueux 1995), affinities are unaffected by acclimation in fresh-caught, 21 ‰S- and 33 ‰S-acclimated C. ornatus (Garçon et al. 2007, 2009) and fresh-caught and 15 ‰S-acclimated C. danae (Masui et al. 2002, 2009). In fact, specific activity rather than apparent affinity may be the most reliable parameter for predicting the osmoregulatory ability of a given species in a particular medium (Péqueux 1995). The apparent affinity for Na+ (K0.5 = 5.36 ± 0.014 mmol L−1) of the X. kroyeri (Na+, K+)-ATPase is similar to C. danae (Masui et al. 2002), C. ornatus (Garçon et al. 2007; 2009), C. vittatus (Gonçalves et al. 2006), M. amazonicum (Santos et al. 2007; Leone et al. 2012), and D. pagei (Furriel et al. 2010), but is about threefold less than for M. rosenbergii (França et al. 2013) and 45 ‰S-acclimated C. vittatus (Lucena et al. 2012). Further, the cooperative kinetics seen for the X. kroyeri enzyme are similar to C. danae (Masui et al. 2002), C. ornatus (Garçon et al. 2007, 2009), D. pagei (Furriel et al. 2010), M. amazonicum (Leone et al. 2012), M. rosenbergii (França et al. 2013) and M. olfersi (Furriel et al. 2000) but contrast with the Michaelis–Menten behavior seen for C. vittatus (Gonçalves et al. 2006; Lucena et al. 2012).
Modulation by K+ and NH4 + of the Gill (Na+, K+)-ATPase
The apparent affinity of the X. kroyeri (Na+, K+)-ATPase for K+ (K0.5 = 1.32 ± 0.082 mmol L−1) is similar to marine species like C. vittatus (Gonçalves et al. 2006), C. danae (Masui et al. 2002) and C. ornatus (Garçon et al. 2007; Garçon et al. 2009) but is about twofold less than in 45 ‰S-acclimated C. vittatus (Lucena et al. 2012) and freshwater crab D. pagei (Furriel et al. 2010), and roughly twofold greater than in the freshwater shrimps M. amazonicum (Leone et al. 2012), M. rosenbergii (França et al. 2013), and M. olfersi (Furriel et al. 2000). The negative cooperativity (nH = 0.6) seen for stimulation by K+ without NH4 + contrasts with the Michaelis–Menten behavior of the enzyme from M. olfersi (Furriel et al. 2000), M. amazonicum (Leone et al. 2012), M. rosenbergii (França et al. 2013), and C. vittatus (Gonçalves et al. 2006), and with the positive cooperativity for C. danae (Masui et al. 2002; Masui et al. 2009), C. ornatus (Garçon et al. 2007; 2009) and D. pagei (Furriel et al. 2010) gill enzymes.
NH4 + can substitute for K+ in sustaining ATP hydrolysis by the crustacean gill (Na+, K+)-ATPase (Holliday 1985; Weihrauch et al. 2004; Masui et al. 2002; Furriel et al. 2004; Lucena et al. 2012; Leone et al. 2014). NH4 + stimulated activity up to values ≈50 % greater than did K+, as also seen in vertebrate (Robinson 1970; Wall 1996) and other crustacean gill enzymes, although with a lower affinity (Holliday 1985; Masui et al. 2002; Furriel et al. 2004; Gonçalves et al. 2006; Garçon et al. 2007; Santos et al. 2007; Furriel et al. 2010; Lucena et al. 2012; França et al. 2013; Leone et al. 2014). Except for the freshwater crab D. pagei (Furriel et al. 2010), the synergistic stimulation by K+ and NH4 + of the crustacean gill (Na+, K+)-ATPase, first described in C. danae (Masui et al. 2002, 2005), appears to be a phenomenon widespread among the decapod Crustacea (Furriel et al. 2004; Gonçalves et al. 2006; Garçon et al. 2007, 2009; Santos et al. 2007; Lucena et al. 2012; França et al. 2013; Leone et al. 2014). The absence of a synergistic effect induced by K+ plus NH4 + in X. kroyeri suggests that both ions compete for the same site on the enzyme molecule.
Like K+, NH4 + can be actively transported by the vertebrate (Na+, K+)-ATPase (Wall 1996). Organic cations such as acetamidinium and formamidinium can act as K+ surrogates in the (Na+, K+)-ATPase cycle and are transported in exchange for Na+ (Ratheal et al. 2010). However, active ammonia excretion in the crab Cancer pagurus is completely inhibited by ouabain, a specific inhibitor of the (Na+, K+)-ATPase; this suggests that NH4 + is likely transported as a K+ congener (Weihrauch et al. 1999). The 80 % increase in ouabain inhibition of the (Na+, K+)-ATPase in the presence of NH4 +, reflected in a threefold lower KI, also suggests that NH4 + is likely transported as a K+ congener. The significantly higher affinity for ouabain in the presence of NH4 + compared to K+ also suggests that NH4 + facilitates E1–E2 conversion toward to E2. Discrepantly, K+ binding, which facilitates dephosphorylation to E2P, is antagonistic to ouabain binding that prevents ion transport (Crambert al. 2004; Nesher et al. 2007). Likely, the significantly lower rate of E2P dephosphorylation in the presence of NH4 + compared to K+ is sufficient maintain the enzyme cycle.
With few exceptions, crustaceans are ammoniotelic (Weihrauch et al. 1998, 1999). Most ammonia excretion occurs via the gills (Weihrauch et al. 1999); less than 2 % is eliminated via the urine in C. sapidus (Cameron and Batterton 1978). Much of the ammonia content is excreted as NH4 + (Weihrauch et al. 1998, 1999), and the sensitivity of active transepithelial NH4 + ion fluxes to basolateral ouabain strongly suggests involvement of the (Na+, K+)-ATPase (Weihrauch et al. 1998, 1999; Lucu et al. 1989). According to Weihrauch et al. (1998), the basolateral (Na+, K+)-ATPase participates directly in NH4 + translocation from the hemolymph into the gill epithelial cells where it can be counter exchanged for Na+ via an apical Na+/NH4 + antiporter, contributing to Na+ uptake (Mangum and Towle 1977; Armstrong et al. 1981; Weihrauch et al. 1999).
Ambient ammonia in the aquatic environment is usually low as a consequence of bacterial nitrification of ammonia to nitrite and nitrate followed by absorption by autotrophs (Weihrauch et al. 1999). In unpolluted, oxygenated sea water, NH4 + concentrations rarely exceed 5 μmol L−1 (Koroleff 1983) in contrast to hemolymph concentrations of approximately 100 μmol L−1 in various brachyuran species adapted to different salinities (Weihrauch et al. 1999). Body surface permeabilities can play a key role in cell NH4 + titers. In seawater, C. pagurus gills exhibit high permeabilities and constitute little or no barrier to NH4 + influx (Weihrauch et al. 1999). Active ammonia excretion by C. pagurus is twofold greater than in the diadromous crab Eriocher sinensis, reflecting its leaky epithelium, suggesting that an efficient mechanism of active ammonia excretion compensates for ammonia influx in marine crustaceans (Weihrauch et al. 1999). In X. kroyeri, the increased VM in the presence of NH4 + may guarantee appropriate cytosolic NH4 + concentrations, constituting a rapid response underlying the excretion of accumulated NH4 +.
Effect of Inhibitors on Gill (Na+, K+)-ATPase Activity
KI values for ouabain inhibition of the X. kroyeri (Na+, K+)-ATPase (28.4 ± 2.1 and 84 ± 0.7 μmol L−1 with and without NH4 +, respectively) lie within the range known for various crustaceans (D’Orazio and Holliday 1985; Holliday 1985; Lucu 1990; Corotto and Holliday 1996; Masui et al. 2002; Gonçalves et al. 2006; Garçon et al. 2007, 2009; Furriel et al. 2010; Lucena et al. 2012; Leone et al. 2012; França et al. 2013). The threefold lower KI estimated with NH4 + may derive from the fact that this ion apparently shifts the conformational E1–E2 equilibrium to the E2 conformation. Thus, the stable orthovanadate trigonal bipiramidal structure might compete with the stable transitional phosphate analog apparently producing a stable intermediate with the E2 conformation causing inhibition (Pick 1982). Yoda and Yoda (1982) suggest that different phosphorylated intermediates occurring between E1P and E2P and the E2P phosphorylated enzyme subconformations (Fedosova et al. 1998) might explain such alterations in KI values.
The residual ≈50 % ATPase activity found with 3 mM ouabain suggests that the (Na+, K+)-ATPase represents only half the total ATPase activity of the microsomal preparation from the X. kroyeri gill epithelium (see Table 3). Further, the additional 6 % inhibition seen with 3 mmol L−1 ouabain plus 50 μmol L−1 orthovanadate likely reveals neutral phosphatases or P-ATPases other than (Na+, K+)-ATPase. A Ca2+-ATPase in the crustacean gill epithelium is well known (Gonçalves et al. 2006; Santos et al. 2007; Lucena et al. 2012; Leone et al. 2014).
The inhibition by 10 μmol L−1 aurovertin strongly suggests an F0F1-ATPase in the gill microsomal fraction, as also seen in M. olfersi (Furriel et al. 2000; Gonçalves et al. 2006; Santos et al. 2007; Garçon et al. 2009; França et al. 2013; Leone et al. 2014) owing to the elevated mitochondrial density in the intralamellar septum (Freire and McNamara 1995; McNamara and Lima 1997). The significant fraction of ouabain-insensitive ATPase activity revealed by bafilomycin A1, indicates the presence of a V(H+)ATPase (Bowman et al. 1988) in X. kroyeri. A gill V(H+)-ATPase has been characterized kinetically in microsomal gill preparations from M. amazonicum (Faleiros et al. 2010; Lucena et al. 2015) and D. pagei (Firmino et al. 2011). In contrast, V(H+)-ATPase activity is very reduced in the blue crabs C. danae (Masui et al. 2002) and C. ornatus (Garçon et al. 2009), and in M. amazonicum after high salinity acclimation (Faleiros et al. 2010).
The presence of a Na+-ATPase in the gill epithelium of X. kroyeri revealed by ouabain plus ethacrynic acid is corroborated by the Na+-stimulated ouabain-insensitive ATPase activity (≈15 nmol Pi min−1 mg−1, Fig. 5b). While Na+-ATPase activity is found in the gill epithelia of various crustaceans (Proverbio et al. 1990; Moretti et al. 1991; Santos et al. 2007; Garçon et al. 2009; Lucena et al. 2012; França et al. 2013; Leone et al. 2014), it is absent from M. olfersi (Furriel et al. 2000) and C. danae gill preparations (Masui et al. 2002).
Concluding, the pivotal role of the (Na+, K+)-ATPase in osmoregulation and in the excretion of nitrogen end products in aquatic crustaceans is well known (Péqueux 1995; Weihrauch et al. 1999). Since both processes occur mainly in the gill epithelia (Péqueux 1995; Weihrauch et al. 1999), our kinetic characterization of the (Na+, K+)-ATPase from the gill epithelium of X. kroyeri, a pelagic marine shrimp, together with findings from other crustaceans from various habitats constitutes a valuable tool to better comprehend the biochemical adjustments of crustaceans to biotopes of different salinity. Many of the kinetic characteristics exhibited by the X. kroyeri enzyme are similar to those of marine crabs. However, some kinetic properties are more closely allied with those of estuarine and freshwater decapods. Considering that X. kroyeri inhabits estuarine regions during its juvenile phase (Dall et al. 1990), it seems likely that the adult shrimps conserve this kinetic profile after migration to marine coastal waters.
References
Armstrong DA, Strange K, Crowe J, Knight A, Simmons M (1981) High salinity acclimation by the prawn Macrobrachium rosenbergii—uptake of exogenous ammonia and changes in endogenous nitrogen compounds. Biol Bull 160:349–365
Belli NM, Faleiros RO, Firmino KCS, Masui DC, Leone FA, McNamara JC, Furriel RPM (2009) Na, K-ATPase activity and epithelial interfaces in gills of the freshwater shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). Comp Biochem Physiol 152A:431–439
Blanco G, Mercer RW (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275:F633–F650
Blanco G, Wagoner K, Sanchez G, Enders GC (2005) Ontogeny of the Na, K-ATPase alpha 4 isoform during rat male germ cell development. FASEB J 19:A1153–A1173
Boudour-Boucheker N, Boulo V, Charmantier-Daures M, Grousset E, Anger K, Charmantier G, Lorin-Nebel C (2014) Differential distribution of V-type H(+)-ATPase and Na, K-ATPase in the brachial chamber of the palaemonidae shrimp Macrobrachium amazonicum. Cell Tissue Res 357:195–206
Bowman EJ, Siebers A, Altendorf K (1988) Bafilomycins: a class of inhibitors of membrane ATPase from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85:7972–7976
Cameron JN, Batterton CV (1978) Antennal gland function in the freshwater crab Callinectes sapidus: water, electrolyte acid-base and ammonia excretion. J Comp Physiol 123B:143–148
Corotto FS, Holliday CW (1996) Branchial Na, K-ATPase and osmoregulation in the purple shore crab Hemigrapsus nudus (Dana). Comp Biochem Physiol 113A:361–368
Costa RC, Fransozo A, Melo GAS, Freire FAM (2003) An illustrated key for Dendrobranchiata shrimps from the northern coast of São Paulo State, Brazil. Biota Neotrop 3:1–12
Crambert G, Hasler U, Beggah AT, Yu CL, Modyanov NN, Horisberger JD, Lelievre L, Geering K (2000) Transport and pharmacological properties of nine different human isozymes. J Biol Chem 275:1976–1986
Crambert G, Schaner D, Roy S, Geering K (2004) New molecular determinants controling the accessibility of ouabain to its binding site in human Na,K-ATPase alpha isoforms. Mol Pharmacol 65:335–341
D’Orazio SE, Holliday CW (1985) Gill Na+, K+-ATPase and osmoregulation in the sand fiddler crab, Uca pugilator. Physiol Zool 58:364–373
Dall W, Hill BJ, Rothlisberg PC, Sharples DJ (1990) The biology of the Penaeidae. In: Blaxter JHS, Southward AJ (eds) Advances in marine biology, vol 27. Academic Press, San Diego, pp 1–489
Donnet C, Arystarkhova E, Sweadner KJ (2001) Thermal denaturation of the Na, K-ATPase provides evidence for alpha-alpha oligomeric interaction and γ subunit association with the C-terminal domain. J Biol Chem 276:7357–7365
Faleiros RO, Goldman MHS, Furriel RPM, McNamara JC (2010) Differential adjustment in gill Na+/K+- and V-ATPase activities and transporter mRNA expression during osmoregulatory acclimation in the cinnamon shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). J Exp Biol 15:3894–3905
Fedosova NU, Cornelius F, Klodos I (1998) E2P phosphoforms of Na, K-ATPase. I Comparison of phosphointermediates formed from ATP and Pi by their reactivity toward hydroxylamine and vanadate. Biochemistry 37:13634–13642
Firmino KCS, Faleiros RO, Masui DC, McNamara JC, Furriel RPM (2011) Short- and long-term salinity-induced modulation of V-ATPase activity in the posterior gills of the true freshwater crab, Dilocarcinus pagei (Brachyura, Trichodactylidae). Comp Biochem Physiol 160B:24–31
França JL, Pinto MR, Lucena MN, Garçon DP, Valenti WC, McNamara JC, Leone FA (2013) Subcellular localization and kinetic characterization of a gill (Na+, K+)-ATPase from the giant freshwater prawn Macrobrachium rosenbergii. J Memb Biol 246:529–543
Freire CA, McNamara JC (1995) Fine structure of the gills of the fresh-water shrimp Macrobrachium olfersii (Decapoda): effect of acclimation to high salinity medium and evidence for involvement of the lamellar septum in ion uptake. J Crustacean Biol 15:103–116
Freire CA, Onken H, McNamara JC (2008) A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp Biochem Physiol 151A:272–304
Furriel RPM, McNamara JC, Leone FA (2000) Characterization of (Na+, K+)-ATPase in gill microsomes of the freshwater shrimp Macrobrachium olfersii. Comp Biochem Physiol 126B:303–315
Furriel RPM, Masui DC, McNamara JC, Leone FA (2004) Modulation of gill (Na+, K+)-ATPase activity by ammonium ions: putative coupling of nitrogen excretion and ion uptake in the freshwater shrimp Macrobrachium olfersii. J Exp Zool 301A:63–74
Furriel RPM, Firmino KCS, Masui DC, Faleiros RO, Torres AH, McNamara JC (2010) Structural and biochemical correlates of Na+, K+-ATPase driven ion uptake across the posterior epithelium of the true freshwater crab Dilocarcinus pagei (Brachyura, Trichodactylidae). J Exp Zool 313A:508–523
Gache C, Rossi B, Lazdunski M (1976) (Na+, K+)-Activated adenosinetriphosphatase of axonal membranes: cooperativity and control. Eur J Biochem 65:293–306
Garçon DP, Masui DC, Mantelatto FLM, McNamara JC, Furriel RPM, Leone FA (2007) K+ and NH4 + modulate gill (Na+ K+)-ATPase activity in the blue crab Callinectes ornatus: fine tuning of ammonia excretion. Comp Biochem Physiol 147A:145–155
Garçon DP, Masui DC, Mantelatto FLM, McNamara JC, Furriel RPM, Leone FA (2009) Hemolymph ionic regulation and adjustments in gill (Na+, K+)-ATPase activity during salinity acclimation in the swimming crab Callinectes ornatus (Decapoda Brachyura). Comp Biochem Physiol 154A:44–55
Garty H, Karlish SJD (2006) Role of FXYD proteins in ion transport. Ann Rev Physiol 68:431–459
Geering K (2008) Functional roles of NaK-ATPase subunits. Curr Opin Nephrol Hypertens 17:526–532
Glynn IM (1985) The (Na+, K+)-transporting adenosine triphosphatase. In: Martonosi AN (ed) The enzymes of biological membranes, vol 10. Plenum Press, New York, pp 35–114
Gonçalves RR, Masui DC, McNamara JC, Mantelatto FLM, Garçon DP, Furriel RPM, Leone FA (2006) A kinetic study of the gill (Na+ K+)-ATPase and its role in ammonia excretion in the intertidal hermit crab Clibanarius vittatus. Comp Biochem Physiol 145A:346–356
Gordon JA (1991) Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol 201:477–482
Heckler GS, Costa RC, Fransozo A, Rosso R, Shimizu RM (2014) Long-term patterns of spatial and temporal distribution in the seabob shrimp Xiphopenaeus kroyeri (Decapoda: Penaeidae) population in Southeastern Brazil. J Crustacean Biol 34:326–333
Heller C (1862) Beiträge zur näheren Kenntnis der Macrouren. Sitzundberichten Math-Phys. Kl. K. Bayer. Akad. Wiss. Muench 45:389–425
Holliday CW (1985) Salinity-induced changes in gill Na, K ATPase activity in the mud fiddler crab Uca pugnax. J Exp Zool 233:199–208
Horisberger JD (2004) Recent insights into the structure and mechanism of the sodium pump. Physiology 19:377–387
Jorgensen PL, Hakansson KO, Karlish SJD (2003) Structure and mechanism of Na, K-ATPase: functional sites and their interactions. Ann Rev Physiol 65:817–849
Kaplan JH (2002) Biochemistry of Na, K-ATPase. Ann Rev Biochem 71:511–535
Karlish SJ (2003) Investigating the energy transduction mechanism of P-type ATPases with Fe2+-catalyzed oxidative cleavage. Ann NY Acad Sci 986:39–49
Kirschner LB (2004) The mechanism of sodium chloride uptake in hyperregulating aquatic animals. J Exp Biol 207:1439–1452
Koroleff F (1983) Methods of seawater analysis. In: Grasshoff K, Ehrhart M, Kremling K (eds) Determination of ammonia. Verlag Chemie, Weinheim, pp 150–151
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 227:680–685
Lee CE, Kiergaard M, Gelembiuk GW, Eads BD, Posavi M (2011) Pumping ions: rapid parallel evolution of ionic regulation following habitat invasions. Evolution 65:2229–2244
Leone FA, Baranauskas JA, Furriel RPM, Borin IA (2005) SigrafW: an easy-to-use program for fitting enzyme kinetic data. Biochem Mol Biol Ed 33:399–403
Leone FA, Masui DC, Bezerra TMS, Garçon DP, Valenti VC, Augusto AS, McNamara JC (2012) Kinetic analysis of gill (Na+, K+)-ATPase activity in selected ontogenetic stages of the Amazon River shrimp Macrobrachium amazonicum (Decapoda Palaemonidae): interactions at ATP- and cation-binding sites. J Memb Biol 245:201–215
Leone FA, Bezerra TMS, Garçon DP, Lucena MN, Pinto MR, Fontes CFL, McNamara JC (2014) Modulation by K+ and NH4 + of microsomal (Na+, K+)-ATPase activity in selected ontogenetic stages of the diadromous river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). PLoS One 9(2):e8925. doi:10.1371/journalpone008925
Lovett DL, Watts SA (1995) Changes in polyamine levels in response to acclimation salinity in gills of the blue crab Callinectes sapidus Rathbun. Comp Biochem Physiol 110B:115–119
Lucena MN, Garçon DP, Mantelatto FLM, Pinto MR, McNamara JC, Leone FA (2012) Hemolymph ion regulation and kinetic characteristics of the gill (Na+K+)-ATPase in the hermit crab Clibanarius vittatus (Decapoda, Anomura) acclimated to high salinity. Comp Biochem Physiol 161B:380–391
Lucena MN, Pinto MR, Garçon DP, McNamara JC, Leone FA (2015) A kinetic characterization of the gill V(H+)-ATPase in juvenile and adult Macrobrachium amazonicum, a diadromous palaemonid shrimp. Comp Biochem Physiol 181B:15–25
Lucu C (1990) Ionic regulatory mechanisms in crustacean gill epithelia. Comp Biochem Physiol 97A:297–306
Lucu C, Flik G (1999) Na+ K+-ATPase and Na+/Ca2+ exchange activities in gills of hyperregulating Carcinus maenas. Am J Physiol 276:R490–R499
Lucu C, Towle DW (2003) Na+ K+-ATPase in gills of aquatic crustacean. Comp Biochem Physiol 135A:195–214
Lucu C, Devescovi M, Siebers D (1989) Do amiloride and ouabain affect ammonia fluxes in perfused Carcinus gill epithelia. J Exp Zool 249:1–5
Lucu C, Devescovi M, Skaramuca M, Kozul V (2000) Gill Na, K-ATPase in the spiny lobster Palinurus elephas and other marine osmoconformers adaptiveness of enzymes from osmoconformity to hyperregulation. J Exp Mar Biol Ecol 246:163–178
Mangum CP, Towle DW (1977) Physiological adaptation to unstable environments. Am Sci 65:67–75
Mantelatto FL, Avelar WEP, Silva DML, Tomazelli AC, Lopez JLC, Shuhama T (1999) Heavy metals in the shrimp Xiphopenaeus kroyeri (Heller, 1862) (Crustacea, Penaeidae) from Ubatuba Bay, São Paulo, Brazil. Bull Environ Contam Toxicol 62:152–159
Marks MJ, Seeds NW (1978) A heterogeneous ouabain-ATPase interaction in mouse brain. Life Sci 23:2735–2744
Masui DC, Furriel RPM, McNamara JC, Mantelatto FLM, Leone FA (2002) Modulation by ammonium ions of gill microsomal (Na+, K+)-ATPase in the swimming crab Callinectes danae: a possible mechanism for the regulation of ammonia excretion. Comp Biochem Physiol 132C:471–482
Masui DC, Furriel RPM, Silva ECC, Mantelatto FLM, McNamara JC, Barrabin HM, Scofano HM, Fontes CFL, Leone FA (2005) Gill microsomal (Na+, K+)-ATPase from the blue crab Callinectes danae: interactions at cationic sites. Int J Biochem Cell Biol 37:2521–2535
Masui DC, Mantelatto FLM, McNamara JC, Furriel RPM, Leone FA (2009) (Na+, K+)-ATPase activity in gill microsomes from the blue crab, Callinectes danae, acclimated to low salinity: novel perspectives on ammonia excretion. Comp Biochem Physiol 153A:141–148
McNamara JC, Faria SC (2012) Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: a review. J Comp Physiol 182B:997–1014
McNamara JC, Lima AG (1997) The route of ion and water movements across the gill epithelium of the freshwater shrimp Macrobrachium olfersii (Decapoda, Palaemonidae): evidence from ultrastructural changes induced by acclimation to saline media. Biol Bull 192:321–331
Moretti R, Martin M, Proverbio T, Proverbio F, Martin T (1991) Ouabain-insensitive Na-ATPase activity in homogenates from different animal tissues. Comp Biochem Physiol 98:623–626
Morris S (2001) Neuroendocrine regulation of osmoregulation and the evolution of air-breathing in decapod crustaceans. J Exp Biol 204:979–989
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P (2007) Crystal structure of the sodium-potassium pump. Nature 450:1043–1050
Nesher M, Spolansky U, Rosen H, Lichtstein D (2007) The endogenous digitalis- like compounds—A new family of steroid hormones. Life Sci 80:2093–2107
Onken H, Tresguerres M, Luquet CM (2003) Active NaCl absorption across posterior gills of hyperosmoregulating Chasmagnathus granulatus. J Exp Biol 206:1017–1023
Palmgren MG, Nissen P (2011) P-Type ATPases. Annu Rev Biophys 40:243–266
Pedemonte CH, Beaugé L (1983) Inhibition of (Na+, K+)-ATPase by magnesium-ions and inorganic-phosphate and release of these ligands in the cycles of ATP hydrolysis. Biochim Biophys Acta 748:245–253
Péqueux A (1995) Osmotic regulation in crustaceans. J Crust Biol 15:1–60
Pick U (1982) The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J Biol Chem 257:6111–6119
Piller SC, Henry RP, Doeller JE, Kraus DW (1995) A comparison of the gill physiology of two euryhaline crab species, Callinectes Sapidus and Callinectes similis: energy production, transport-related enzymes and osmorregulation as a function of acclimation salinity. J Exp Biol 198:349–358
Pires AMS (1992) Structure and dynamics of benthic megafauna on the continental shelf offshore of Ubatuba, southeastern Brazil. Mar Ecol Prog Ser 86:63–76
Poulsen H, Morth P, Egebjerg J, Nissen P (2010) Phosphorylation of the Na+K+-ATPase and the H+K+-ATPase. FEBS Lett 584:2589–2595
Proverbio T, Zanders IP, Marin R, Rodrigues JM, Proverbio F (1990) Effects of Na+ and/or K+ on the Mg2+-dependent ATPase activities in shrimp (Macrobrachium amazonicum) gill homogenates. Comp Biochem Physiol 97B:383–390
Ratheal IM, Virgin GK, Yu H, Roux B, Gatto C, Artigas P (2010) Selectivity of externally facing ion-binding sites in the Na/K pump to alkali metals and organic cations. PNAS 107:18718–18723
Read SM, Northcote DH (1981) Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Analyt Biochem 116:53–64
Robinson JD (1970) Interactions between monovalent cations and the (Na+ + K+)- dependent adenosine triphosphatase. Arch Biochem Biophys 139:17–27
Robinson JD (1982) Tryptic digestion of the (Na + K)-ATPase is both sensitive to and modifies K+ interactions with the enzyme. J Bioenerg Biomemb 14:319–333
Rudolph FB, Baugher BW, Beissner RS (1979) Techniques in coupled enzyme assays. Methods Enzymol 63:22–42
Sáez AG, Lozano E, Zaldívar-Riverón A (2009) Evolutionary history of Na, K-ATPases and their osmoregulatory role. Genetica 136:479–490
Santos LCF, Belli NM, Augusto A, Masui DC, Leone FA, McNamara JC, Furriel RPM (2007) Gill (Na+, K+)-ATPase in diadromous freshwater palaemonid shrimps: species specific kinetic characteristics and α-subunit expression. Comp Biochem Physiol 148A:178–188
Shindo Y, Morishita K, Kotake E, Miura H, Carninci P, Kawai J, Hayashizaki Y, Hino A, Kanda T, Kusakabe Y (2011) FXYD6: a Na, K-ATPase regulator is expressed in type II taste cells. Biosci Biotech Biochem 75:1061–1066
Silva ECC, Masui DC, Furriel RP, McNamara JC, Barrabin H, Scofano HM, Perales J, Teixeira-Ferreira A, Leone FA, Fontes CFL (2012) Identification of a crab gill FXYD2 protein and regulation of crab microsomal Na K-ATPase activity by mammalian FXYD2 peptide. Biochim Biophys Acta 1818:2588–2597
Skou JC, Esmann M (1992) The (Na+, K+)-ATPase. J Bioenerg Biomemb 24:249–261
Takeyasu K, Tamkun M, Renaud KJ, Fambrough DM (1988) Ouabain-sensitive (Na+- K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the a-subunit of an avian sodium pump. J Biol Chem 263:4347–4354
Taylor HH, Taylor EW (1992) Gills and lungs: the exchange of gases and ions. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates, decapod Crustacea, vol 10. Wiley-Liss, New York, pp 203–293
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Towle DW, Kays WT (1986) Basolateral localization of Na+ + K+-ATPase in gill epithelium of two osmoregulating crabs, Callinectes-sapidus and Carcinus maenas. J Exp Zool 239:311–318
Towle DW, Weihrauch D (2001) Osmoregulation by gills of euryhaline crabs: molecular analysis of transporters. Am Zool 41:770–780
Wall SM (1996) Ammonium transport and the role of the Na, K-ATPase Miner. Electrol Metab 22:311–317
Ward DG, Cavieres JD (1998) Affinity labeling of two nucleotide sites on Na, K-ATPase using 2′(3′)-O-(2,4,6-trinitrophenyl) 8-azidoadenosine 5′-[alpha-P-32] diphosphate (TNP-8N(3)-[alpha-P-32]ADP) as a photoactivatable probe. Label incorporation before and after blocking the high affinity ATP site with fluorescein isothiocyanate. J Biol Chem 273:33759–33765
Weihrauch D, Towle DW (2000) Na+/H+-exchanger and Na+/K+/2Cl–cotransporter are expressed in gills of the euryhaline Chinese crab Eriocheir sinensis. Comp Biochem Physiol 126:S158–S198
Weihrauch D, Becker W, Postel U, Riestenpatt S, Siebers D (1998) Active excretion of ammonia across the gills of the shore crab Carcinus maenas and its relation to osmoregulatory ion uptake. J Comp Physiol 168B:364–376
Weihrauch D, Becker W, Postel U, Luck-Kopp S, Siebers D (1999) Potential of active excretion of ammonia in three different haline species of crabs. J Comp Physiol 169B:25–37
Weihrauch D, Morris S, Towle DW (2004) Ammonia excretion in aquatic and terrestrial crabs. J Exp Biol 207:4491–4504
Wheatly MG, Henry RP (1987) Branchial and antennal gland Na+, K+-dependent ATPase and carbonic anhydrase activity during salinity acclimation of the euryhaline crayfish Pacifastacus leniusculus. J Exp Biol 133:73–86
Wilder MN, Huong DTT, Atmomarsono M, Tran TTH, Phu TQ, Yang WJ (2000) Characterization of Na/K-ATPase in Macrobrachium rosenbergii and the effects of changing salinity on enzymatic activity. Comp Biochem Physiol 125A:377–388
Williams AB (1984) Shrimps, lobsters and crabs of the Atlantic Coast of the Eastern United State, Maine to Florida. Smithsonian Institute Press, Washington
Yoda A, Yoda S (1982) Interaction between ouabain and the phosphorylated intermediate of (Na+, K+)-ATPase. Mol Pharmacol 22:700–705
Acknowledgments
This work constitutes part of a Ph D thesis by LAR. We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2010/17534-0; 2002/08178-/9; 2010/50188-8), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 470830/2011-5), and Instituto Nacional de Ciência e Tecnologia (INCT) Adapta/Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM- 573976/2008-2) for financial support received. FAL (302776/2011-7), JCM (300662/2009-2) and FLM (302748/2010-5) received research scholarships from CNPq. DPG (2010/06395-9) and MNL (2010/16115-3) received Ph D scholarships from FAPESP. MRP received post-doctoral scholarships from CNPq (560501/2010-2). We thank Nilton Rosa Alves for technical assistance. This laboratory (FAL) is integrated with the Amazon Shrimp Network (Rede de Camarão da Amazônia) and with ADAPTA (Centro de Estudos de Adaptações da Biota Aquática da Amazônia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leone, F.A., Lucena, M.N., Rezende, L.A. et al. A Kinetic Characterization of (Na+, K+)-ATPase Activity in the Gills of the Pelagic Seabob Shrimp Xiphopenaeus kroyeri (Decapoda, Penaeidae). J Membrane Biol 248, 257–272 (2015). https://doi.org/10.1007/s00232-014-9765-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-014-9765-6