Abstract
Nanofluid is the colloidal suspension of nanosized solid particles like metals or metal oxides in some conventional fluids like water and ethylene glycol. Due to its unique characteristics of enhanced heat transfer compared to conventional fluid, it has attracted the attention of research community. The forced convection heat transfer of nanofluid is investigated by numerous researchers. This paper critically reviews the papers published on experimental studies of forced convection heat transfer and pressure drop of Al2O3, TiO2 and CuO based nanofluids dispersed in water, ethylene glycol and water–ethylene glycol mixture. Most of the researchers have shown a little rise in pressure drop with the use of nanofluids in plain tube. Literature has reported that the pumping power is appreciably high, only at very high particle concentration i.e. more than 5 %. As nanofluids are able to enhance the heat transfer at low particle concentrations so most of the researchers have used less than 3 % volume concentration in their studies. Almost no disagreement is observed on pressure drop results of different researchers. But there is not a common agreement in magnitude and mechanism of heat transfer enhancement. Few studies have shown an anomalous enhancement in heat transfer even at low particle concentration. On the contrary, some researchers have shown little heat transfer enhancement at the same particle concentration. A large variation (2–3 times) in Nusselt number was observed for few studies under similar conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cooling is very important for sustaining the desired performance and reliability of wide variety of products, such as computers, power electronics, automobile engines etc. In the modern era, the electronic devices are becoming more and more compact and on the other hand automobile engines are becoming high powered engines. The heat removal from such devices have become a challenge. Many modifications have been made in the past to increase the heat transfer e.g. increasing the surface area or changing the flow pattern. But the fluids like water or oil used for heat removal, itself have poor thermal conductivity. Any modification in the given system will be of limited use unless the fluid will not be modified.
It is well known that by adding very small size metallic particles in the fluid increases its heat carrying capacity. Earlier some micro meter sized particles were used for improving heat transfer characteristics of the fluid. Although, increased heat transfer was observed with these micro particles, but they have poor suspension stability and also clog the micro channels. After the advent of nanotechnology, it is possible to synthesize the particles of the order of 1–100 nm. These nano meter sized particles dispersed in liquid behave more or less like a single phase fluid. Also nanoparticles have better suspension stability than that of micro particles and do not cause any operational problem.
Pure metals have higher thermal conductivity, but during manufacturing stage it is very difficult to avoid oxidation. So it is easy to manufacture and economical to use metal oxide nanoparticles compared to pure metal nanoparticles.
Al2O3, TiO2 and CuO are the most popular metal oxide nanoparticles. Al2O3 nanoparticles are generally spherical in shape and during the usage, they retain their shape. Ease of availability in the purity range of 94–99.8 % is another feature which has attracted the research community to use Al2O3 nanoparticles. Low density of Al2O3 nanoparticles is very demanding because it increases the dispersion stability of nanoparticles in the base fluid. So the low cost, ease of manufacturing, high thermal conductivity and better suspension stability make the Al2O3 nanoparticles most preferred choice of research community [1].
TiO2 nanoparticles are less expensive, nontoxic and have high surface area to volume ratio. The thermal conductivity of TiO2 is less than CuO and Al2O3, but these nanoparticles are highly active in base fluid which increases the Brownian motion, suspension stability and heat transfer of TiO2 nanofluids [2].
CuO have very high thermal conductivity compared to Al2O3 and TiO2 due to which CuO nanoparticles are also used for heat transfer enhancement. On the other hand, CuO has high density which causes early settlement of nanoparticles resulting in poor suspension stability. CuO is toxic and need to be handled carefully [3].
Other nanoparticles used for heat transfer application are SiO2, carbon nanotubes (CNT), Cu nanoparticles, ZnO, Fe2O3 and Fe3O4 etc. Yang et al. [4] investigated heat transfer characteristics of nanofluids prepared from Cu nanoparticles in a viscoelastic base fluid. The base fluid was the aqueous solution of cetyltrimethylammonium chloride (CTAC) and sodium salicylate (Na Sal). The Cu nanoparticles were suspended in different concentrations of 0.25, 0.5 and 1 vol%. The enhancement in heat transfer coefficient was observed to be 9, 18.3 and 37.9 % for 0.25, 0.5 and 1 vol% of Cu nanoparticles respectively. Amrollahi et al. [5] used CNT nanoparticles in distilled water. 25 % increment in heat transfer coefficient for 0.12 wt% was reported at a Reynolds number of nearly 5000.
2 Heat transfer mechanism in nanofluids
Brownian motion, interfacial layer, particle aggregation, thermophoresis, intensification of turbulence are some of the proposed mechanisms responsible for high thermal conductivity of nanofluids.
2.1 Brownian motion
In nanofluids, the nanoparticles are always in random motion called Brownian motion and additional energy transport is possible due to this motion. The higher momentum of nanoparticles are believed to carry and transfer thermal energy more efficiently at greater distances inside the base fluid before they release it in a colder region of the fluid [6].
2.2 Interfacial layer
The liquid layer close to nanoparticle is called interfacial layer. In liquid–solid interfaces (e.g. nanoparticle-base fluid interface), the boundary resistance decreases, hence the overall thermal resistance of the system reduces. In other words, this ordered nanolayer acts like a thermal bridge between solid particle and liquid and results in appreciable increased heat transfer [7].
2.3 Aggregation
Clustering or aggregation is another phenomenon which takes place in nanofluids. If the clustering is ordered, it may appreciably increase the rate of heat transfer. This mechanism suggests that there is a formation of a linear assembly of nanoparticle chain. This chain provides a faster path for heat transfer through the nanofluid. But if the clustering is not aligned it may rather affect badly the heat transfer characteristics of the nanofluids [8].
2.4 Thermophoresis
One more possible reason of increased heat transfer in nanofluids is thermophoresis. Thermophoresis is particle motion induced by thermal gradients. It is the ordered movement different from Brownian motion. Due to thermophoretic forces, concentration of nanoparticles changes around cooling and heating sides relative to the mean concentration, which enhances the heat transfer coefficient [9].
2.5 Intensification of turbulence
Small sold particles in nanofluid develop turbulent eddies. The effective thermal conductivity of nanofluid is a function of primary properties like temperature & pressure and the motion of nanoparticles in the stationary fluid due to turbulent eddies. So the resultant thermal conductivity of nanofluid is higher compared to base fluid and hence higher heat transfer [9].
2.6 Properties of base fluid and nanoparticles
Some of the commonly used base fluids for heat transfer applications are water, ethylene glycol (EG) and the mixture of water and EG. Water is almost a perfect fluid for heat transfer applications due to its favourable thermo physical properties, safe to use, ease of availability and low cost. But water freezes at 0 °C. Ice crystals cause volume expansion, which may destroy the piping system and other components. Antifreeze is commonly used in water to avoid damage caused by freezing. EG is most common antifreeze. Pure EG freezes at about −12 °C. When dissolved in water, EG disrupts hydrogen bonding and the mixture does not readily crystallise. Therefore the freezing point of water–ethylene glycol mixture is depressed. For example, a 60:40 mixture of EG: water freezes at −45 °C. EG is also used with water for high temperature applications because it increases the boiling point of the mixture. Properties of base fluid is important in the respect that this affects the properties of nanofluid.
The thermo physical properties of water and EG as reported in literature in the temperature range of 30–40 °C is given in Table 1. Pure EG is hardly used for heat transfer applications, rather EG–water mixture is more common. Mixing theory is used for measuring the density, specific heat and viscosity of water–EG mixture [19].
where ρm, Cpm and μm is the density, specific heat and viscosity of the water–EG mixture respectively. ϕw and ϕEG is the volume fraction of water and EG respectively.
The empirical correlation given by Xie et al. [20] is used for measuring the thermal conductivity of the water–EG mixture.
where k m is the thermal conductivity of water–EG mixture.
The properties of nanoparticles also affect the properties of nanofluid. In fact, the high thermal conductivity of nanoparticles compared to conventional fluid is largely responsible for the high heat carrying capacity of nanofluids. The relevant thermo-physical properties such as density, specific heat and thermal conductivity reported in literatures are summarised in Table 2.
3 Properties of nanofluids
Nanofluids have higher thermal conductivity, density and viscosity compared to base fluid but lower specific heat. Density and specific heat of nanofluids is usually evaluated by assuming that nanoparticles are well dispersed within the base fluid. The effective density and specific heat is then calculated using the classical formulae used for two phase flow:
where ρnf and C p,nf is the density and specific heat of nanofluid respectively. Φ is the volume percentage of nanoparticles in base fluid.
Thermal conductivity is the most studied property of nanofluids. Transient hot wire method is oldest and most popular method of measuring the thermal conductivity experimentally. Vakili et al. [30] tested an experimental system with a mixture of 60 wt% ethylene glycol and 40 wt% distilled water and investigated the thermal conductivity of TiO2 based nanofluid at increasing concentration of nanoparticles. The result showed that thermal conductivity of nanofluid increased with increasing concentration. Li and Peterson [31] experimentally observed the thermal conductivity of CuO and Al2O3 nanoparticles in water at volume fractions of 2, 4, 6 and 10 %. Extremely high thermal conductivity was observed for both nanofluids. At 6 vol%, an increment of about 52 % in thermal conductivity was reported for CuO/water nanofluid. With Al2O3 the enhancement in thermal conductivity was 30 % at 10 vol%. A maximum enhancement of 11 % in thermal conductivity was observed by Kole and Dey [32].
Some theoretical models for evaluating the thermal conductivity of nanofluids are also used. Most of the models are extension of Maxwell’s Eq. (7).
Equation (7) is valid for homogeneous and low-volume fraction liquid–solid suspension with uniform size and spherical particles. Maxwell considered only particle volume fraction and thermal conductivity of particles in their model. To account for the shape of the particles, Maxwell’s model was modified by Hamilton and Crosser [33] by introducing a shape factor.
where n is the shape factor. The value of n is given by 3/ψ where ψ is the particle sphericity, defined as surface area of the sphere of same volume as that of given particle to the actual surface area of the particle. The value of ψ is 3 for sphere.
He et al. [34] compared thermal conductivity of TiO2/water nanofluids, measured experimentally, with that of theoretical results predicted from H-C model. It was observed that the predicted values were lower than the values obtained from experiments. Elias et al. [35] compared experimental thermal conductivity of Al2O3 nanofluids with that of Maxwell and H-C model. The base fluid used was a mixture of water and ethylene glycol (50:50 by volume). In the concentration range of 0–1 vol%, the experimental values of thermal conductivity were higher than predicted values. At 1 vol% concentration of Al2O3, the experimental value was 4.26 % higher than the value calculated from Maxwell model and 2.2 % higher than H-C model. Liu et al. [36] also observed that the predicted values of thermal conductivity obtained from Maxwell model and H-C model for CuO/ethylene glycol nanofluids were lower, compared to experimental values.
Yu and Choi [37] presented a modified Maxwell model, in which the effect of nanolayer surrounding the nanoparticles was included. It was assumed in this model that thermal energy transport in nanofluids is diffusive because the average inter particle distance of nanoparticles is much higher than the mean free path of liquid molecules. So the effective thermal conductivity of nanofluid can be given as
where kpe is the equivalent thermal conductivity of the particles and can be calculated as
where γ = kl/kp is the ratio of nanolayer thermal conductivity to the particle thermal conductivity. When kl = kp, the above equation reduces to kpe = kp. β is the ratio of the nanolayer thickness to the original nanoparticle radius. The nanolayer impact is significant for small particles where nanolayer thickness is of the order of nanoparticle radius (β ~ 1). However, for large particles where nanoparticle radius is much larger than nanolayer thickness (β ~ 0), the nanolayer impact is small and Eq. 9 reduces to original Maxwell equation.
A better prediction of nanofluid thermal conductivity enhancement was done by Jang and Choi [10]. They postulated that the nanoparticle produces a convection like effect at nanoscale. They considered four modes of energy transfer in nanofluids; collision between base fluid molecules, thermal diffusion in nanoparticles, collision of nanoparticles with each other due to Brownian motion and collision between base fluid molecules and nanoparticles due to thermal fluctuations.
where β = 0.01 is a constant related to Kapitza resistance, C = 18 × 106 is a proportional constant, dp is the diameter of the nanoparticle, dbf is the diameter of the base fluid molecule and Re p is the Reynolds number of the nanoparticles given as \( \text{Re}_{p} = \frac{{{\text{V}}_{B} {\text{d}}_{p} }}{\upnu} \) where VB is the mean Brownian velocity and can be obtained as \( {\text{V}}_{\text{B}} = \frac{{{\text{k}}_{\text{b}} {\text{T}}}}{{3\uppi \upmu _{\text{bf}} {\text{d}}_{\text{p}} {\text{l}}_{\text{bf}} }} \) where kb = 1.3807 × 10−23 J/K is the Boltzmann constant and lbf is the mean free path of base fluid molecules. For water based nanofluids at a temperature of 27 °C the diameter (dbf) can be taken as 0.384 nm and mean free path (lbf) can be taken as 0.738 nm.
Lee et al. [38] measured experimentally the thermal conductivity of water based Al2O3 nanofluids at different particle concentrations and results were compared with Jang and Choi model (Eq. 11). They showed that the thermal conductivity of nanofluids increases non linearly with increase in particle concentration. They suggested that Jang and Choi model can be used for the estimation of thermal conductivity of Al2O3/water nanofluids for a wide range of particle concentration from 0.01 to 5 vol%.
Corcione et al. [39] proposed a correlation for the thermal conductivity of water and ethylene glycol based Al2O3, CuO, TiO2 and Cu nanofluids.
where Re is the nanoparticles Reynolds number, Pr is the Prandle number of the base fluid, T is the nanofluid temperature, Tfr is the freezing point of the base fluid, kp is the thermal conductivity and ϕ is the volume fraction of the nanoparticles. The ranges of nanoparticle diameter, volume fraction and temperature were taken as 10–150 nm, 0.2–9 % and 294–324 K respectively.
While the thermal conductivity of nanofluids is important for heat transfer applications, viscosity is important for pressure drop and pumping power calculations. Literature on viscosity reported that nanofluids have higher viscosity compared to host fluid and the viscosity increases with increasing particle concentration. Chandrasekar et al. [40] investigated the viscosity of Al2O3/water nanofluids with a particle diameter of 43 nm and at different volume concentrations (0.33–5 %). They observed that the viscosity of the nanofluids increases with increasing particle concentration. A maximum viscosity enhancement of 136 % was obtained at 5 % volume concentration of Al2O3/water nanofluid. Bobbo et al. [41] investigated the viscosity of single walled carbon nanohorn (SWCNH) and titanium dioxide (TiO2) dispersed in water. It was observed that both the nanofluids showed Newtonian behavior at low concentration and the viscosity increases with increasing particle concentration. A maximum viscosity enhancement of 12.9 % for SWCNH/water and 6.8 % for TiO2/water was obtained at 1.0 wt% particle concentration. Vakili et al. [30] measured experimentally the viscosity of TiO2/water nanofluids at a particle concentration ranging from 0.5 to 1.5 vol%. It was observed that the viscosity of nanofluids increases with increasing particle concentration. The increase in viscosity reported was approximately 2, 4.01 and 5.03 % at the nanoparticle concentration of 0.5, 1.0 and 1.5 vol% respectively.
Einstein’s Eq. (13) is a remarkable milestone to calculate the viscosity of dilute suspended solution theoretically and depicted a linear dependence on the volume fraction. Later researchers reported the extension of this equation.
He et al. [34] compared the viscosity of nanofluids measured experimentally with that of predictions of Einstein’s model and observed that Einstein’s model is unable to predict the viscosity of TiO2/water nanofluids. The predicted values were very low compared to the values obtained experimentally in the particle concentration range of 0.24–1.10 vol%.
Hwang et al. [42] showed that the dynamic viscosity of Al2O3/water nanofluids was increased by 2.9 % for 0.3 vol% at 21 °C, compared to that of water. They also reported that the Einstein’s model was unable to predict the viscosity of nanofluids.
Brinkman [43] extended the Enstein’s model to be used for moderate particle concentration. He assumed the mixture of solid particles and base fluid as continuum and formulated the theoretical correlation given as
Frankei and Acrivos [44] gave the following correlation for estimating the viscosity of solid–liquid suspensions:
where \( \upphi_{\text{m}} \) is maximum concentration.
Batchelor [45] recognize the effect of Brownian motion of small particles in suspension. He modified Einstein’s model by introducing the effect of Brownian motion. The equation is given as
The model is applicable to isotropic suspension of rigid and spherical particle.
Graham [46] developed the generalized form of Frankei and Acrivos model by introducing the effect of particle size and spacing between the particles in suspension. The proposed correlation is as follows
where h is the inter-particle spacing and rp is the particle radius.
All these theoretical classical models give accurate results at lower concentration but at higher concentration, these fail to predict the viscosity of nanofluids.
Pak and Choi [47] investigated experimentally the viscosity of Al2O3/water and TiO2/water nanofluids using Brookfield rotating viscometer. They observed that with increasing particle concentration, the viscosity of nanofluids increases. Higher values of nanofluids were obtained experimentally compared to Bachelor model.
Tseng and Lin [48] observed the non-Newtonian behaviour of nanofluids. The nanofluids behave like pseudoplastic fluid up to ϕ = 0.1 and above this concentration, the suspension become thixotropic. They proposed the exponential correlation which is valid for TiO2/water nanofluids in the particle concentration range of 0.05–0.12.
Chandrasekar et al. [40] experimentally investigated the viscosity of Al2O3/water nanofluid using Brookfield cone and plate viscometer. They proposed a correlation considering electromagnetic, mechanical and geometrical aspects.
4 Forced convection heat transfer and pressure drop of nanofluids: review and analysis
In almost all industrial sectors, forced convection plays a significant role. Even a small amount of nanoparticles are able to enhance the heat transfer appreciably. Low concentration of nanoparticles is preferred choice for industrial applications because it costs less and almost no pressure drop penalty. Thus most of the researchers have considered less than 1 % nano particle concentration in their studies. The heat transfer performance of nanofluids is analysed by finding the convective heat transfer coefficient and Nusselt number of the nanofluid.
Twenty-nine published papers have been reviewed, in which forced convection of nanofluid is studied experimentally. 13 papers are taken for Al2O3 nanofluid, 6 papers for TiO2 nanofluid, 4 papers for CuO nanofluid, and 6 papers are taken in which comparison of two or more nanofluids is done. The papers with two important boundary conditions have been selected i.e. constant heat flux condition and constant wall temperature condition. The papers published in the years 2006–2015 are considered.
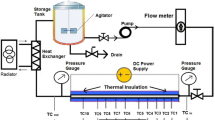
The typical experimental setup with constant heat flux boundary condition is shown in Fig. 1. It consists of a flow loop including a pump, a flow measuring device, a test section with heating element, a cooling unit and a nanofluid reservoir. The constant heat flux condition is achieved by heating a heater wire (generally Nickel-Chrome wire) wrapped on the outer periphery of the test tube. To measure the wall temperature, thermocouples are installed at various axial locations on outer surface of the tube. The constant wall temperature condition at the outer periphery of the test tube is achieved by flowing condensing steam in the annulus of double pipe heat exchanger. The test section is insulated to reduce the heat loss to the surrounding.
The local heat transfer coefficient at any location can be measured experimentally using measured wall temperature and fluid temperature at that location
where \( h_{nf} (x) \) is the local heat transfer coefficient at location x, \( q^{\prime \prime } \) is the heat flux of the test section, T i,w (x) is the inner wall temperature of the tube and T m (x) is the bulk fluid temperature at axial position x.
Inner wall temperature cannot be measured directly, so it is determined from measured outlet wall temperature using heat conduction equation in cylindrical coordinates:
where Di and Do are the inner and outer diameter of the test tube respectively. ks is the thermal conductivity of tube material.
Test section is in general selected as high thermal conductivity pipe to increase the heat transfer e.g. copper (Cu) and stainless steel (SS). If the thickness of pipe is very small, then there is very small difference between inner wall and outer wall temperature at any location i.e. T i,w (x) ≈ T o,w (x).
The bulk temperature T m (x) at any location x can be determined from the following energy balance equation
Substituting above equations in Eq. (20), the local convective heat transfer coefficient can be obtained as
The value local heat transfer coefficient is used to determine the local Nusselt number at that location
The increase in viscosity of the nanofluid compared to base fluid is responsible for extra pumping power penalty at same flow rate due to higher pressure drop in the given length of the pipe. So the pressure drop analysis becomes important while dealing with nanofluids. To determine the pressure drop, convenient way is to calculate friction factor, which is a dimension less form of pressure drop and is defined as
For laminar and turbulent flow, the friction factor can be obtained as:
Darcy friction factor (27) for fully developed laminar flow.
Colebrrok Eq. (28) for fully developed turbulent flow
Blasius Eq. (29) covers wider range of Reynolds number from transition flow to turbulent flow for the determination of friction factor for smooth pipes.
For smooth pipe flow problems following Nusselt numbers can be used for validating the experimental setup and for comparing the experimental results in the entrance region.
Shah Eqs. (30) and (31) for laminar flow under constant heat flux condition:
Hausen Eq. (32) for laminar flow under constant wall temperature condition:
Equations (31) and (32) takes into account the thermally developing region as well as fully developed region.
Dittus-Boelter (33) and Gnielinski (34) equations for turbulent flow
where f = (0.79 ln Re − 1.64)−2.
Table 3, 4, 5 and 6 summarize the experimental details and important findings of Al2O3, TiO2 and CuO nanofluids respectively. Not all the researchers have performed the pressure drop analysis along with heat transfer analysis. The researchers who investigated the pressure drop analysis have shown a little rise in pressure drop with the use of nanofluids in plain tube. The pumping power was appreciably high, only at very high particle concentration (above 5 vol%). As nanofluids are able to enhance the heat transfer at low concentrations so most of the researchers have used less than 3 % volume concentration in their studies. Almost no disagreement was observed on pressure drop results of different researchers. But, there is no clear consensus on heat transfer performance of nanofluids. Some of the researchers reported an anomalous heat transfer with the use of nanofluids. While a few studies showed no appreciable increment in heat transfer. Also there is no agreement on the mechanism of heat transfer enhancement of nanofluids.
We have performed a detailed critical analysis of the experimental data reported by the researchers for studying the extent of variation in the results of Nusselt number under similar conditions. This is done by carefully extracting the data from the figures in the original papers. The papers which have same boundary condition, same flow regime and same particle type and volume fractions in plain tube were re-plotted as a comparison of Nusselt number in the similar set of conditions.
Figure 2 shows the comparison of 1 vol% Al2O3/water nanofluids for laminar flow in a plain tube under constant wall temperature condition. An anomalous enhancement in heat transfer was observed by Nazari et al. [58] at 1 vol% in laminar flow. At a Reynolds number of approximately 1200, the Nusselt number observed by Nazari et al. [58] was almost twice as reported by Heris et al. [49] and Heyat et al. [21]. One of the important factor which affects the heat transfer characteristics of the nanofluids is the particle size. In general, with the decrease in particle size, the heat transfer increases because of increased Brownian motion of the small size particles. But as shown in Fig. 2, irrespective of the same particle size (40 nm) used by Heyat et al. [21] and Nazari et al. [58], the deviation between the Nusselt number results of the two is very large under similar conditions.
A comparative analysis of Nusselt number at different Reynolds number for TiO2/water nanofluid at 1 vol% under constant wall temperature condition for turbulent flow in plain tube is shown in Fig. 3. A large variation in the results of Hojjat et al. [67] and Arani and Amani [60] was observed. At a Reynolds number of nearly 48,500, the Nusselt number reported by Arani and Amani [60] was almost three times higher than reported by Hojjat et al. [67] under the similar conditions of flow, boundary condition and particle concentration. While at a Reynolds number of nearly 14,700, the Nusselt number observed by Arani and Amani [60] was approximately 1.7 times than reported by Nasiri et al. [25].
Figure 4 shows the heat transfer characteristics of CuO nanofluids, at 0.1 vol% under constant heat flux boundary condition for turbulent flow in plain tube. Much deviation in results of Suresh et al. [64] and Naik et al. [65] was observed. At nearly 6000 Reynolds number, almost twice Nusselt number was measured by Naik et al. [65] compared to the results of Suresh et al. [64]. Here it can be commented that the other factors like time of sonication or agitation, stability of nanofluids, surfactant additions, pH variation, the quantity of dissolved solids in the base fluid (TDS) etc. may be playing the important role for heat transfer variations.
By changing the flow behaviour of nanofluids e.g. using inserts in the plain tube, further enhancement in heat transfer can be obtained. Some of the studies [50–52, 55, 57, 62, 65, 68, 69] using inserts for heat transfer enhancement, are also reviewed in this paper. Inserts in the plain tube flattens the velocity profile near the wall and increases the turbulence and heat transfer. All studies showed an appreciable increase in heat transfer using inserts but on the other hand a large penalty of pumping power was also reported.
The comparative analysis of the two or more nanofluids is summarised in Table 6. Comparison is made by the researchers either using different nanoparticles in same base fluid or same nanoparticle in different base fluids. Literature shows that size of the nanoparticle and thermal conductivity play an important role in heat transfer enhancement. In general for same size of nanoparticles, the CuO nanofluids was reported to have higher heat transfer followed by Al2O3 and TiO2. Lower size nanoparticles have high chaotic movement and better stability, resulting in higher heat transfer. Higher thermal conductivity nanoparticles can carry and transfer the heat from hotter region to colder region, more effectively. Hojjat et al. [67] observed that despite the high thermal conductivity of CuO nanofluids, the heat transfer characteristics of Al2O3 and CuO was similar due to small size Al2O3 nanoparticles.
5 Conclusions
The literature review on experimental studies of forced convection show that nanofluids significantly improve the heat transfer characteristics of the forced convection system. The nanoparticle concentration extremely affects the heat transfer coefficient and Nusselt number. Pressure drop is appreciably high only at very high particle concentration (more than 5 vol%). Most of the literature reported increased heat transfer with particle concentration of nanofluids. But some of the researchers also showed adverse effect of higher particle concentration on heat transfer characteristics of nanofluid. In general, CuO nanoparticles have better heat transfer characteristics compared to TiO2 and Al2O3 due to high thermal conductivity of CuO nanoparticles. But the size of nanoparticles also play an important role. Lower the size, higher the chaotic movement of the nanoparticles within the fluid and higher the heat transfer coefficient. Following points are worth noting for nanofluids:
-
1.
Although the thermal conductivity of pure metal nanofluids is higher compared to those of metal oxide nanofluids, but the oxide nanoparticles are commonly used due to ease of manufacturing and stabilization compared to pure metallic particles. Al2O3 is the most commonly used nanoparticle.
-
2.
Nanoparticles are able to enhance heat transfer even at very low concentrations. Higher percentage of nanoparticles may badly affect the heat transfer performance. So there is an optimum concentration at which heat transfer is maximum.
-
3.
Although most of the studies show higher heat transfer with nanofluid but there is not a common consensus among the research communities. There is a lack of agreement of results obtained by different researchers.
-
4.
Mechanism responsible for changes in properties of nanofluid is still unclear. Many issues like thermal conductivity, Brownian motion, turbulence in naofluids, interfacial layer etc. need more research for clear understanding.
-
5.
Poor suspension properties of nanofluid may hinder the devolvement in the field of nanofluids.
Abbreviations
- cp :
-
Specific heat (J/kg K)
- h:
-
Heat transfer coefficient (W/m2 K)
- k:
-
Thermal conductivity (W/m K)
- L:
-
Characteristic length (m)
- d:
-
Inner diameter of the tube
- p:
-
Pitch of the twisted tape/wire coil/helical insert (m)
- Re:
-
Reynolds Number
- Nu:
-
Nusselt number
- Pr:
-
Prandtl number
- f:
-
Darcy friction factor
- q:
-
Heat flux (W/m2)
- T:
-
Temperature (K)
- V:
-
Velocity (m/s)
- EG:
-
Ethylene glycol
- CNT:
-
Carbon nanotubes
- AR:
-
Aspect ratio (ratio of width to height of longitudinal insert)
- TR:
-
Twist ratio (ratio of diameter of pipe to the pitch of twisted tape/wire coil/helical insert)
- ρ:
-
Density
- µ:
-
Viscosity
- φ:
-
Volume concentration
- bf:
-
Base fluid
- nf:
-
Nanofluid
- np:
-
Nanoparticle
- f:
-
Fluid condition
- w:
-
Wall condition/water properties
- x:
-
Axial direction
- m:
-
Two fluid mixing condition
References
Sridhara V, Satapathy LN (2011) Al2O3-based nanofluids: a review. Nanoscale Res Lett 6:1–16
Mital GS, Manoj T (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56(16):1639–1657
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Yang JC, Li FC, He YR, Huang YM, Jiang BC (2013) Experimental study on the characteristics of heat transfer and flow resistance in turbulent pipe flows of viscoelastic-fluid-based Cu nanofluid. Int J Heat Mass Transf 62:303–313
Amrollahi A, Rashidi AM, Lotfi R, Meibodi ME, Kashefi K (2010) Convection heat transfer of functionalized MWNT in aqueous fluids in laminar and turbulent flow at the entrance region. Int Commun Heat Mass Transf 37:717–723
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84(21):4316–4318
Xie H, Fujii M, Zhang X (2005) Effect of interfacial nanolayer on the effective thermal conductivity of nanoparticle–fluid mixture. Int J Heat Mass Transf 48:2926–2932
Prasher R, Phelan PE, Bhattacharya P (2006) Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions (nanofluid). Nano Lett 6(7):1529–1534
Buongiorno J (2006) Convective transport in nanofluids. J Heat Transf 128:240–250
Jang SP, Choi SUS (2007) Effects of various parameters on nanofluid thermal conductivity. J Heat Transf 129:617–623
Peyghambarzadeh SM, Hashemabadi SH, Hoseini SM, Jamnani MS (2011) Experimental study of heat transfer enhancement using water/ethylene glycol based nanofluids as a new coolant for car radiators. Int Commun Heat Mass Transf 38:1283–1290
Utomo AT, Poth H, Robbins PT, Pacek AW (2012) Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int J Heat Mass Transf 55:7772–7781
Pantzali MN, Mouza AA, Paras SV (2009) Investigating the efficacy of nanofluids as coolants in plate heat exchangers (PHE). Chem Eng Sci 64:3290–3300
Ghozatloo A, Rashidi A, Shariaty-Niassar M (2014) Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp Thermal Fluid Sci 53:136–141
Khedkar RS, Sonawane SS, Wasewar KL (2013) Water to nanofluids heat transfer in concentric tube heat exchanger: experimental study. Procedia Eng 51:318–323
Tiwari AK, Ghosh P, Sarkar J (2013) Performance comparison of the plate heat exchanger using different nanofluids. Exp Thermal Fluid Sci 49:141–151
Ho CJ, Wei LC, Li ZW (2010) An experimental investigation of forced convective cooling performance of a microchannel heat sink with Al2O3/water nanofluid. Appl Therm Eng 30:96–103
Oh DW, Jain A, Eaton JK, Goodson KE, Lee JS (2008) Thermal conductivity measurement and sedimentation detection of aluminum oxide nanofluids by using the 3ω method. Int J Heat Fluid Flow 29:1456–1461
Bhanvase BA, Sarode MR, Putterwar LA, Abdullah KA, Deosarkar MP, Sonawane SH (2014) Intensification of convective heat transfer in water/ethylene glycol based nanofluids containing TiO2 nanoparticles. Chem Eng Process 82:123–131
Xie H, Wang J, Xi T, Liu Y, Al F (2002) Dependence of the thermal conductivity of nanoparticle-fluid mixture on the base fluid. J Mater Sci Lett 21:1469–1471
Heyhat MM, Kowsary F, Rashidi AM, Momenpour MH, Amrollahi A (2013) Experimental investigation of laminar convective heat transfer and pressure drop of water-based Al2O3 nanofluids in fully developed flow regime. Exp Thermal Fluid Sci 44:483–489
Fotukian SM, Esfahany MN (2010) Experimental investigation of turbulent convective heat transfer of dilute γ-Al2O3/water nanofluid inside a circular tube. Int J Heat Fluid Flow 31:606–612
Darzi AAR, Farhadi M, Sedighi K (2013) Heat transfer and flow characteristics of Al2O3–water nanofluid in a double tube heat exchanger. Int Commun Heat Mass Transf 47:105–112
Nassan TH, Heris SZ, Noie SH (2010) A comparison of experimental heat transfer characteristics for Al2O3/water and CuO/water nanofluids in square cross-section duct. Int Commun Heat Mass Transf 37:924–928
Nasiri M, Etemad SG, Bagheri R (2011) Experimental heat transfer of nanofluid through an annular duct. Int Commun Heat Mass Transf 38:958–963
Heyhat MM, Kowsary F, Rashidi AM, Esfehani SAV, Amrollahi A (2012) Experimental investigation of turbulent flow and convective heat transfer characteristics of alumina water nanofluids in fully developed flow regime. Int Commun Heat Mass Transf 39:1272–1278
Peyghambarzadeh SM, Hashemabadi SH, Naraki M, Vermahmoudi Y (2013) Experimental study of overall heat transfer coefficient in the application of dilute nanofluids in the car radiator. Appl Therm Eng 52:8–16
Fotukian SM, Esfahany MN (2010) Experimental study of turbulent convective heat transfer and pressure drop of dilute CuO/water nanofluid inside a circular tube. Int Commun Heat Mass Transf 37:214–219
Sajadi AR, Kazemi MH (2011) Investigation of turbulent convective heat transfer and pressure drop of TiO2/water nanofluid in circular tube. Int Commun Heat Mass Transf 38:1474–1478
Vakili M, Mohebbi A, Hashemipour H (2013) Experimental study on convective heat transfer of TiO2 nanofluids. Heat Mass Transf 49:1159–1165
Li CH, Peterson GP (2006) Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J Appl Phys 99:084314 (1–8)
Kole M, Dey TK (2010) Thermal conductivity and viscosity of Al2O3 nanofluid based on car engine coolant. J Phys D Appl Phys 43:315501 (1–10)
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two-component systems. Ind Eng Chem Fund 1:187–191
He Y, Jin Y, Chen H, Ding Y, Cang D, Lu H (2007) Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int J Heat Mass Transf 50:2272–2281
Elias MM, Mahbubul IM, Saidur R, Sohel MR, Shahrul IM, Khaleduzzaman SS, Sadeghipour S (2014) Experimental investigation on the thermo-physical properties of Al2O3 nanoparticles suspended in car radiator coolant. Int Commun Heat Mass Transf 54:48–53
Liu MS, Lin MCC, Huang IT, Wang CC (2006) Enhancement of thermal conductivity with CuO for nanofluids. Chem Eng Technol 29(1):72–77
Yu W, Choi SUS (2003) The role of interfacial layers in the enhanced thermal conductivity of nanofluids: a renovated Maxwell model. J Nanopart Res 5:167–171
Lee JH, Hwang KS, Jang SP, Lee BH, Kim JH, Choi SUS, Choi CJ (2008) Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf 51:2651–2656
Corcione M (2011) Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers Manag 52:789–793
Chandrasekar M, Suresh S, Bose AC (2010) Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp Thermal Fluid Sci 34:210–216
Bobbo S, Fedele L, Benetti A, Colla L, Fabrizio M, Pagura C, Barison S (2012) Viscosity of water based SWCNH and TiO2 nanofluids. Exp Thermal Fluid Sci 36:65–71
Hwang KS, Jang SP, Choi SUS (2009) Flow and convective heat transfer characteristics of water-based Al2O3 nanofluids in fully developed laminar flow regime. Int J Heat Mass Transf 52:193–199
Brinkman HC (1952) The viscosity of concentrated suspensions and solutions. J Chem Phys 20:571
Frankel NA, Acrivos A (1967) On the viscosity of a concentrated suspension of solid spheres. Chem Eng Sci 22:847–853
Batchelor GK (1977) The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech 83:97–117
Graham AN (1981) On the viscosity of suspensions of solid spheres. Appl Sci Res 37:275–286
Pak BC, Cho YI (1998) Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf 11:151–170
Tseng WJ, Lin KC (2003) Rheology and colloidal structure of aqueous TiO2 nanoparticle Suspensions. Mater Sci Eng, A 355:186–192
Heris SZ, Esfahany MN, Etemad SG (2007) Experimental investigation of convective heat transfer of Al2O3/water nanofluid in circular tube. Int J Heat Fluid Flow 28:203–210
Sundar LS, Sharma KV (2010) Heat transfer enhancements of low volume concentration Al2O3 nanofluid and with longitudinal strip inserts in a circular tube. Int J Heat Mass Transf 53:4280–4286
Chandrasekar M, Suresh S, Bose AC (2010) Experimental studies on heat transfer and friction factor characteristics of Al2O3/water nanofluid in a circular pipe under laminar flow with wire coil inserts. Exp Thermal Fluid Sci 34:122–130
Sundar LS, Sharma KV (2010) Turbulent heat transfer and friction factor of Al2O3 nanofluid in circular tube with twisted tape inserts. Int J Heat Mass Transf 53:1409–1416
Liu D, Yu L (2011) Single-phase thermal transport of nanofluids in a minichannel. J Heat Transf 133:031009 (1–11)
Prajapati OS, Rajvanshi AK (2012) Effect of Al2O3–water nanofluids in convective heat transfer. Int J Nanosci 1(1):1–4
Suresh S, Selvakumar P, Chandrasekar M, Raman VS (2012) Experimental studies on heat transfer and friction factor characteristics of Al2O3/water nanofluid under turbulent flow with spiraled rod inserts. Chem Eng Process 53:24–30
Sahin B, Gültekin GG, Manay E, Karagoz S (2013) Experimental investigation of heat transfer and pressure drop characteristics of Al2O3–water nanofluid. Exp Thermal Fluid Sci 50:21–28
Esmaeilzadeh E, Almohammadi H, Nokhosteen A, Motezaker A, Omrani AN (2014) Study on heat transfer and friction factor characteristics of γ-Al2O3/water through circular tube with twisted tape inserts with different thicknesses. Int J Therm Sci 82:72–83
Nazari M, Ashouri M, Kayhani MH, Tamayol A (2015) Experimental study of convective heat transfer of a nanofluid through a pipe filled with metal foam. Int J Therm Sci 88:33–39
Kayhani MH, Soltanzadeh H, Heyhat MM, Nazari M, Kowsary F (2012) Experimental study of convective heat transfer and pressure drop of TiO2/water nanofluid. Int Commun Heat Mass Transf 39:456–462
Arani AAA, Amani J (2012) Experimental study on the effect of TiO2–water nanofluid on heat transfer and pressure drop. Exp Thermal Fluid Sci 42:107–115
Rayatzadeh HR, Avval MS, Mansourkiaei M, Abbassi A (2013) Effects of continuous sonication on laminar convective heat transfer inside a tube using water–TiO2 nanofluid. Exp Thermal Fluid Sci 48:8–14
Azmi WH, Sharma KV, Sarma PK, Mamat R, Anuar S (2014) Comparison of convective heat transfer coefficient and friction factor of TiO2 nanofluid flow in a tube with twisted tape inserts. Int J Therm Sci 81:84–93
Asirvatham LG, Vishal N, Gangatharan SK, Lal DM (2009) Experimental study on forced convective heat transfer with low volume fraction of CuO/water nanofluid. Energies 2:97–119
Suresh S, Chandrasekar M, Sekhar SC (2011) Experimental studies on heat transfer and friction factor characteristics of CuO/water nanofluid under turbulent flow in a helically dimpled tube. Exp Thermal Fluid Sci 35:542–549
Naik MT, Fahad SS, Sundar LS, Singh MK (2014) Comparative study on thermal performance of twisted tape and wire coil inserts in turbulent flow using CuO/water nanofluid. Exp Thermal Fluid Sci 57:65–76
Heris SZ, Etemad SG, Esfahany MN (2006) Experimental investigation of oxide nanofluids laminar flow convective heat transfer. Int Commun Heat Mass Transf 33:529–535
Hojjat M, Etemad SG, Bagheri R, Thibault J (2011) Turbulent forced convection heat transfer of non-Newtonian nanofluids. Exp Thermal Fluid Sci 35:1351–1356
Suresh S, Venkitaraj KP, Selvakumar P (2011) Comparative study on thermal performance of helical screw tape inserts in laminar flow using Al2O3/water and CuO/water nanofluids. Superlattices Microstruct 49:608–622
Suresh S, Venkitaraj KP, Selvakumar P, Chandrasekar M (2012) A comparison of thermal characteristics of Al2O3/water and CuO/water nanofluids in transition flow through a straight circular duct fitted with helical screw tape inserts. Exp Thermal Fluid Sci 39:37–44
Mojarrad MS, Keshavarz A, Ziabasharhagh M, Raznahan MM (2014) Experimental investigation on heat transfer enhancement of alumina/water and alumina/water–ethylene glycol nanofluids in thermally developing laminar flow. Exp Thermal Fluid Sci 53:111–118
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khurana, D., Choudhary, R. & Subudhi, S. A critical review of forced convection heat transfer and pressure drop of Al2O3, TiO2 and CuO nanofluids. Heat Mass Transfer 53, 343–361 (2017). https://doi.org/10.1007/s00231-016-1810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1810-9