Abstract
This paper provides the method for preparation of nanofluids and measuring thermal properties of nanofluids by various methods. In this paper, we have focused on effect of variation of temperature, particle size and volume fraction of nanofluid on properties of nanofluid such as density, viscosity, thermal conductivity and heat transfer coefficient. We have made comparison between CuO, ZnO and SiO2 nanoparticles with particle size 30–50 nm and 50–70 nm and with three volume fractions 0.5, 1 and 1.5%. The heat transfer coefficient (h) of all nanofluids is determined from unsteady state heat transfer apparatus. The results indicate that the thermal conductivity and h of nanofluid enhance with increase in %vol. fraction and decrease in particle size. CuO–DI water nanofluid with 1.5% volume fraction and 30–50 nm particle size has higher heat transfer coefficient, and thermal conductivity is enhanced by 59.59% than de-ionized water. Hence, CuO–DI water nanofluid (1.5% vol. fraction and 30–50 nm particle size) reaches steady state faster than other nanofluids.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Preparation of nanofluid
- Properties of nanofluid: Heat transfer coefficient
- Thermal conductivity
- Viscosity
- Density and specific heat
- Unsteady state heat transfer behaviour (Unsteady state heating curves) of nanofluid
1 Introduction
Nanofluids have higher thermal properties than current heat transfer fluids. Hence, nanofluid has future scope in heat transfer fluids. Metal nanoparticles have more thermal conductivity than other nanoparticles but have less dispersion properties and poor stability. Oxide nanoparticles have better dispersion properties and stable suspension. Particle size and volume fraction of particles affect the properties of nanofluid. Researchers have given different correlations for properties of nanofluids. We have to observe important conclusions of some researchers.
Bashirnezhad et al. [1] concluded that viscosity increases with increase in volume fraction of nanoparticles and decrease in temperature. Author reported the future scope for doing experimental studies by acknowledging temperature, nanoparticle size, volume fraction, sonication time and base fluid.
Liu et al. [2] observed that thermal conductivity of Cu–water nanofluid at 0.1% volume fraction is increased by 23.8%. Researcher performed the experiment on nanofluids without use of surfactant. Due to poor dispersion properties of nanofluid, thermal conductivity depends on time interval.
Gupta et al. [3] provided the correlations for various properties of nanofluid. Researcher reviewed the factors affecting the thermophysical properties of nanofluid. Researcher has mentioned the future scope for cost effective and efficient nanofluid.
Albadr et al. [4] used the Al2O3 nanoparticles of 30 nm particle size. The researcher concluded that heat transfer coefficient (h) increases with increase in mass flow rate and volume fraction. But friction factor increases with increase in vol. concentration of nanoparticles, which results in pressure drop.
Li et al. [5] summarized the recent improvement in research of stationary nanofluid. The researcher mentioned that long-term stability of nanofluid is a key issue.
2 Nanofluid
Nanofluid contains nanometre-sized particles made of metal, oxides, carbides, borides, nitrides, etc. There are two ways for the preparation of nanofluids.
2.1 Preparation of Nanofluid
2.1.1 One Step Method
The manufacturing of nanoparticles and dispersion in a base fluid are done simultaneously. Some general one step methods are as follows:
-
(a)
Direct evaporation
-
(b)
Chemical vapour condensation
-
(c)
Chemical precipitation.
2.1.2 Two Step Method
The nanoparticles are first manufactured and then dispersed into the base fluid. Example of two step method is gas condensation. We have preferred the two step method for the preparation of nanofluid. In this method, first of all, we have calculated the mass of nanoparticles for 0.5, 1 and 1.5% vol. fraction of nanoparticles into the base fluid which is de-ionized water.
where ρp = Density of Nanoparticles (gm/cm3).
Surfactant used: Sodium Dodecyl Sulphate (SDS).
We can use different surfactants such as Sodium Dodecyl Sulphate (SDS), Ammonium Cetyl Cetyl (CTAB) and Dodecyl Benzen Sulphonate (SDBS). But SDS is more effective for stability of oxide nanoparticles in base fluid.
2.2 Process of Nanofluid Reparation
This measured quantity of nanoparticles and surfactant are mixed with base fluid in magnetic stirrer for 30 min. After magnetic stirring, the fluid is transferred to ultrasonic bath for 2 h. Ultrasonic waves disperse the nanoparticles into the base fluid and form uniform mixture. Use of ultrasonic bath more than 2 h does not show more effect of dispersion of nanoparticles (Fig. 1).
2.3 Stability of Nanofluid
The long-term stability of nanofluid is the key issue. Metal oxide nanofluids are more stable than metallic nanofluids. Nanofluid becomes stable after the proper dispersion of nanoparticles due to existence of Brownian motion. Due to Brownian motion, nanoparticles are in motion and get dispersed properly in base fluid stability can be analysed by the following methods (Fig. 2):
-
(a)
Visual Inspection
-
(b)
Zeta Potential (pH Value).
3 Properties of Nanofluids
After the preparation of nanofluid, it is necessary to find out the following properties of nanofluid. Some important properties are as follows:
-
1.
Density
-
2.
Viscosity
-
3.
Thermal Conductivity
-
4.
Specific Heat.
3.1 Density
Density of nanofluid can be determined from following two methods:
Method 1
Pak and Cho correlation
where ρnf = Density of nanofluid in gm/cm3
ρbf = Density of base fluid
Ф = Volume fraction.
Method 2
Experimental Method
where W2 = Weight of jar with 50 cc of nanofluid
W1 = Weight of empty jar
ρnf = Density of nanofluid in kg/m3 (Fig. 3).
3.2 Viscosity
Method 1
Einstein correlation
where μnf = Density of nanofluid in kg/ms
μbf = Density of base fluid in kg/ms
\( \varPhi \) = Volume fraction.
Method 2
Redwood Viscometer
where γ = Kinematic viscosity of nanofluid m2/s
t = Time for collecting 50 cc of nanofluid in sec
A and B = Redwood constants
A = 0.264 and B = 190.
where µ = Density of nanofluid in kg/ms (Fig. 4).
3.3 Thermal Conductivity
Method 1
Wasp Correlation
where Knf= Thermal conductivity of nanofluid in W/mK
Kbf = Thermal conductivity of base fluid in W/mK
Ф = Volume fraction.
Method 2
Ultrasonic Interferometer
where N = Avogadro’s Number = 6.02 ∗ 1023
KB = Boltzmann’s Constant = 1.3807 × 10−23 × 10−23 J/K
vs = Ultrasound Velocity in m/s
V = Molar Volume = \( \frac{m}{\rho } \) (Fig. 5).
3.4 Specific Heat
Pak and Choi have given the following correlation for specific heat:
where \( C_{{{\text{P}}_{\text{nf}} }} \) = Specific Heat of Nanofluid.
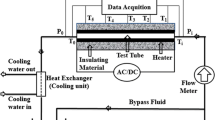
4 Experimental Apparatus
See (Fig. 6).
Specification:
-
1.
Electric Heater: 500 W (1.5 Lit)
-
2.
Digital Temperature Indicator: 0 °C–199.9 °C
-
3.
Thermocouples: Cr–Al type (K-Type)
-
4.
Specimens Material: Copper (Dia—30 mm, Length—30 mm) (K = 386 W/m K).
After performing the experiments for different nanofluids, we can get the heat transfer coefficient, and also, we can compare these nanofluids by drawing unsteady state heating curves. Following formulas are required to calculate the heat transfer coefficient:
-
1.
Grashof Number
-
2.
Prandtl Number
-
3.
Rayleigh Number
-
4.
Nusselt Number
-
5.
Heat Transfer Coefficient
5 Results and Discussion
-
1.
Heat transfer coefficient of the nanofluid increases with increase in temperature, increase in volume fraction and decrease in particle size.
-
2.
Thermal conductivity increases with increase in volume fraction, increase in temperature and decrease in particle size.
-
3.
Thermal conductivity of CuO–DI water nanofluid (with particle size 30–50 nm, 1.5% Vol. fraction) is 59.59% more than the de-ionized water.
-
4.
Viscosity of nanofluid increases with increase in volume fraction and decrease in temperature.
-
5.
Density of nanofluid increases with decrease in particle size, decrease in volume fraction and decrease in temperature.
-
6.
Specific heat increases with increase in temperature, increase in particle size and decrease in volume fraction.
6 Conclusion and Future Scope
In this paper, we studied the thermophysical properties of nanofluids. We can conclude that CuO–DI water nanofluid with 30–50 nm particle size and 1.5% volume fraction has higher heat transfer coefficient. Due to this, CuO–DI water nanofluid reaches the steady state faster than other fluids.
At higher volume fraction, nanofluid has better thermal properties, but stability of nanofluid is a key issue. Further work is required related to the stability of nanofluid. New types of nanofluids having higher thermal properties should be found.
References
Bashirnezhad K, Bazri S, Safaei MR (2016) Viscosity of nanofluids: a review of recent experimental studies. Int Commun Heat Mass Transfer 73
Liu MS, Lin MCC, Tsai CY, Wang CC (2006) Enhancement of thermal conductivity with cu for nanofluids using chemical reduction method. Int J Heat Mass Transfer 49
Gupta M, Singh V, Kumar R, Said Z (2017) A review on thermophysical properties of nanofluids and heat transfer applications. Renew Sustain Energy Rev 74
Albadr J, Tayal S, Alasadi M (2013) Heat transfer through heat exchanger using Al2O3 nanofluid at different concentrations. Case Stud Therm Eng 1
Li Y, Zhou J, Tung S, Schneider E, Xi S (2009) A review on development of nanofluid preparation and characterization. Powder Technol 196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhumkar, Y., Acharya, A.R., Pise, A.T. (2021). Experimental Investigation of Unsteady State Heat Transfer Behaviour of Nanofluid. In: Akinlabi, E., Ramkumar, P., Selvaraj, M. (eds) Trends in Mechanical and Biomedical Design. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4488-0_45
Download citation
DOI: https://doi.org/10.1007/978-981-15-4488-0_45
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4487-3
Online ISBN: 978-981-15-4488-0
eBook Packages: EngineeringEngineering (R0)