Abstract
Purpose
Inappropriate prescribing (IP) is common among the elderly and is associated with adverse health outcomes. The role of different patterns of IP in clinical practice remains unclear. The aim of this study is to analyse the characteristics of different patterns of IP in hospitalized older adults.
Methods
This is a prospective observational study conducted in the acute care of elderly (ACE) unit of an acute hospital in Barcelona between June and August 2021. Epidemiological and demographic data were collected, and a comprehensive geriatric assessment (CGA) was performed on admitted patients. Four patterns of inappropriate prescribing were identified: extreme polypharmacy (10 or more drugs), potentially inappropriate medications (PIMs), potential prescribing omissions (PPOs) and anticholinergic burden.
Results
Among 93 admitted patients (51.6% male, mean age of 82.83), the main diagnosis was heart failure (36.6%). Overprescribing patterns (extreme polypharmacy, PIMs, PPOs and anticholinergic burden) were associated with higher comorbidity, increased dependence on instrumental activities of daily living (IADL) and greater prevalence of dementia. Underprescribing (omissions) was associated with important comorbidity, residence in nursing homes, an increased risk of malnutrition, higher social risk and greater frailty. Comparing different patterns of IP, patients with high anticholinergic burden exhibited more extreme polypharmacy and PIMs. In the case of omissions, no association was identified with other IP patterns.
Conclusions
We found statistically significant association between patterns of inappropriate prescribing and clinical and CGA variables such as comorbidity, dependency, dementia or frailty. There is a statistically significant association between patterns of overprescribing among patients admitted to the ACE unit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ageing of the population has resulted in an increased multimorbidity of the elderly, closely related to polypharmacy. Inappropriate prescribing is common in the elderly and is associated with polypharmacy, and both situations are implicated in adverse health outcomes [1,2,3,4,5]. In addition, patients with advanced age and multimorbidity are at increased risk of being prescribed anticholinergic drugs, which increases the risk of cognitive and functional impairment, falls, hospitalization and death [6,7,8,9].
There is no universal definition of polypharmacy, but the most widespread definition refers to the presence of 5 or more medications, with extreme polypharmacy defined as the presence of 10 or more medications [1]. In the case of anticholinergic burden, several scales measure exposure to these drugs, each with its advantages and disadvantages, and there is no consensus on which is the most accurate one [10].
The prevalence of polypharmacy ranges from 7 to 45% among community-dwelling elderly [10, 11]. In Spain, the prevalence of polypharmacy among community-dwelling elderly is as high as 27.3%, with those over 80 years of age experiencing the most significant percentage increase in polypharmacy (3.4%) over a 10-year study period. In addition, up to 50% of the elderly are prescribed anticholinergic/sedative drugs, which may consequently worsen their functional or cognitive capacity [10].
Numerous studies highlight the increased prevalence of inappropriate prescribing in the elderly across community, hospital and residential settings. Inappropriate prescribing encompasses both overprescribing (potentially inappropriate prescribing of medicines (PIM), anticholinergic burden) and underprescribing (potentially omitted prescribing (PPO)) [12,13,14,15,16,17,18,19,20]. A study in our setting showed that in over half of the cases, both problems were present simultaneously [18].
The role of different patterns of inappropriate prescribing in clinical practice remains ill-defined. Little is known about the ability to predict negative outcomes resulting from the combination of polypharmacy, potentially inappropriate medication (PIMs and PPOs) or anticholinergic burden because previous studies have predominantly focused on individual patterns [21, 22].
The aim of this study is to analyse the clinical characteristics of different patterns of inappropriate prescribing and the association between them, prior to admission to an acute care of the elderly (ACE) unit.
Materials and methods
Study design
This is a prospective observational study conducted in the ACE unit of the Sant Rafael acute hospital, a collaborating centre of the Vall d´Hebron Hospital in Barcelona city. The hospital currently provides a 24-bed ACE unit, distributed across three clinical teams. Data was obtained from the medical records of admitted patients to one of these three teams between June 2021 and August 2021.
Study population
We include in our ACE unit all patients from Barcelona northern area, requiring hospital admission for acute medical illness or exacerbation of chronic pathology. The recommended admission criteria was as follows: individuals aged 75 and above, categorized as frail and pre-frail (FRAIL score > 0), with no significant baseline dependence in daily activities of living (Barthel Index > 60), and lacking severe baseline cognitive impairment (global deterioration scale (GDS)-Reisberg < 6).
Methods
Four patterns of inappropriate prescribing were defined: extreme polypharmacy, PIMs, PPOs and high anticholinergic burden.
Inappropriate prescribing pattern definitions were as follows:
-
Extreme polypharmacy: 10 or more medications
-
Potentially inappropriate medication (PIM), focusing on the central nervous system with the Screening Tool of Older Persons’ Prescriptions (STOPP-CNS or group D), using STOPP/START version 2 [23]
-
Potential prescribing omissions (PPO) focusing on the cardiovascular group with the Screening Tool to Alert to Right Treatment (START-CV or group A), using STOPP/START version 2
-
High anticholinergic burden using the DBI (drug burden index) [24]
We examined the clinical history and compiled epidemiological and demographic data, encompassing factors such as age, gender, origin, main diagnoses upon admission, length of stay and discharge destination. The assessment of baseline status, concerning specific variables, involved a thorough evaluation of frailty (using the FRAIL scale) [25], basic and instrumental activities of daily living (measured by the Barthel Index and Lawton Index, respectively) [26, 27], cognitive function (measured by the GDS-Reisberg) [28], multimorbidity (Charlson comorbidity index) [29] and geriatric syndromes, including dementia diagnosis at admission, social risk (medical records), dysphagia (medical records), risk of malnutrition with the Short Nutritional Assessment Questionnaire (SNAQ) [30], risk of falls (history of more than one fall in the last year confirmed in the medical history or during the interview) and risk of pressure ulcers (Norton scale) [31].Within the initial 48 h of admission, cognitive status was assessed using the Pfeiffer test, and the risk of delirium was assessed with the 4AT delirium assessment tool [32, 33].
Comprehensive geriatric assessment (CGA) was performed by the hospital geriatrics team, whilst the evaluation of inappropriate prescribing was facilitated by the hospital pharmacy team.
Statistical analysis
The sample was analysed using the average and standard deviation for continuous variables. Frequencies and percentages were used for categorical variables. To compare between subgroups in the present study, the Student’s t-test was used for continuous variables. To compare the results of the categorical variables, the χ2 was applied. When the result was less than 5, Fisher’s test was indicated. The statistically significant value was 0.05.
Results
A total of 93 patients were included, of whom 48 (51.6%) were male, with a mean age of 82.83 (SD 7.53). Eighty-two (88.2%) came from home, and the main diagnosis at admission was heart failure in 34 (36.6%) of patients. Length of stay was 8.46 (SD5.37). The mean of FRAIL was 2.92 (SD 1.23). Most patients exhibited high comorbidity (Charlson index 3.3, SD 2.27). In addition, they showed slight dependence for basic and instrumental activities of daily living. The mean number of drugs at admission was 11.75 (SD 4.71), and 64 (68.8%) patients had extreme polypharmacy. Thirty-nine (42.0%) of patients had one or more STOPP criteria of group D, and 43 (46.3%) of patients had one or more START criteria of group A. Forty-one (44.1%) of patients had high anticholinergic burden. The baseline characteristics of the study population are summarized in Table 1.
We observed differences in the clinical profiles of admitted patients based on two types of inappropriate prescribing: firstly, the overprescribing group characterized by polypharmacy, PIMs and anticholinergic burden, and secondly, the underprescribing group marked by PPOs.
In the first group, patients with extreme polypharmacy exhibited more comorbidity, with a statistically significant difference (3.64 vs 2.55, p = 0.046). Patients with one or more STOPP criteria in group D were more dependent for instrumental activities of daily living (IADL) (Lawton Index 3.49 vs 4.72, p = 0.042) and had a higher diagnosis of dementia than those without criteria. A total of 16 (41%) 6 (11.1%) of patients with one or more STOPP criteria had dementia, compared to 6 (11.1%) of patients without STOPP criteria. They also had a higher likelihood of being discharged home compared to those without such criteria (82.1% vs 68.5%, p = 0.023). No statistically significant differences were found for the presence of comorbidity, dependence or other geriatric syndromes when analysing anticholinergic burden.
Within the underprescribing group, patients with one or more START criteria exhibited higher comorbidity (3.91 vs 2.78, p = 0.016) and were at higher risk of malnutrition (55.8% vs 32.0%, p = 0.023) and social risk (37.2% vs 12.0%, p = 0.004). Patients with one or more START criteria were more frail: 36 (83.7%) of patients with omissions were frail, compared to 29 (59.1%) of those without omissions, with a statistically significant difference. We found also that patients with one or more START criteria were from nursing homes or intermediate care hospitals more than those without START criteria (23.2% vs 2.0%, p = 0.009). Among the four patterns of inappropriate prescribing, there were no statistically significant differences in terms of risk of falls, delirium, dementia, risk of pressure ulcers or dysphagia. These results are summarized in Appendix 1. Summary of the significant results are shown in Table 2.
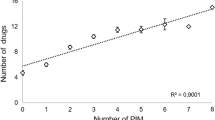
Analysis of the four patterns of inappropriate prescribing mentioned above, we observed differences in the group of overprescribing. In cases involving extreme polypharmacy and PIMs, both groups also displayed a high anticholinergic burden with a statistically significant difference: 34 (53.1%) of patients with extreme polypharmacy had a high anticholinergic burden, with a statistically significant difference. Of the group of patients with one or more STOPP criteria, 23 (59%) had a high anticholinergic burden. In the case of omissions, no association was identified with other inappropriate prescribing patterns. Results are shown in Table 3.
Discussion
In this study, we found that the most important factors associated with inappropriate prescribing depend on the clinical profile of the patients studied. Patients with extreme polypharmacy exhibit more comorbidity. Those with STOPP criteria of group D demonstrate increased dependence on instrumental activities of daily living and higher prevalence of dementia. Patients with one or more STOPP criteria had a higher likelihood of being discharged home compared to those without such criteria. Finally, patients with START criteria in group A were non-dwelling, more frail, with higher comorbidity, higher social risk and higher risk of malnutrition compared to those without such criteria. When analysing patterns of inappropriate prescribing, we found that patients with high anticholinergic burden had more extreme polypharmacy and more STOPP criteria, with no findings on clinical variables related to this pattern. In the case of omissions, no association was identified with other inappropriate prescribing patterns. A statistically significant association was identified among extreme polypharmacy, potentially inappropriate medications (PIMs) of group D of STOPP/START criteria and high anticholinergic burden in patients admitted to the acute care of the elderly unit. Conversely, no statistically significant association was found between the PPOs of group A of STOPP/START criteria with the three other profiles analysed.
A study conducted by Alshammari et al. showed that the strongest predictor of potentially inappropriate prescribing is polypharmacy, followed by Alzheimer’s disease, depression, irritable bowel syndrome, hypothyroidism and chronic kidney disease [34]. This confirms that inappropriate prescribing patterns are influenced by factors associated with comorbidity, frailty and the presence of cognitive impairment, which is consistent with our patient group. The findings of our study highlight the importance of understanding prescribing patterns to assess the risk of inappropriate prescribing of each patient.
Our results are consistent with other research, such as the study conducted by Meid et al. on omissions using the START criteria of the cardiovascular group or group A. They reported a high prevalence of omissions, with noted association between omissions and frailty [35]. Gutiérrez-Valencia et al. in their investigation did not observe an association between frailty and polypharmacy in multivariate analysis. However, they found more START criteria in frail patients, with a tendency towards a higher rate of omissions in frail patients living in nursing homes [36]. In our series, patients with omissions were also from nursing homes or intermediate care hospitals. More of them, however, did not have advanced frailty or dependency for basic activities of daily living. Additionally, they did not present advanced dementia that could justify a more palliative approach to comorbidities. Our findings align with other studies that have reported a similar association between common PPO and older patients, particularly those with cardiovascular diseases such as heart failure or ischaemic heart disease [35, 37, 38]. Few studies have explored the clinical predictors linked to both overprescribing and underprescribing conditions. Limited data exist that establish a relationship between these two variables, with one notable exception being a study conducted in our setting. In this particular study, the prevalence of inappropriate prescribing was reported to be 87.6%, and notably, 54.3% of patients simultaneously exhibited PIM and underprescribing (PPO) [18]. Regarding anticholinergic burden, our series showed an association between high anticholinergic burden and extreme polypharmacy with a statistically significant difference. This finding was also corroborated with STOPP criteria of group D. There were no statistically significant differences in terms of age, comorbidity, frailty, functionality or dementia between patients with high anticholinergic burden and those with low anticholinergic burden, as has been reported in other studies [10, 39, 40].
In the same way that there is a lack of literature evaluating different patterns of inappropriate prescribing (IP), there is less literature about interventions to improve adverse health effects. There are several studies with interventions in different healthcare settings. A recent clinical trial involving frail older adults without advanced cognitive impairment demonstrated that interventions promoting pharmacological deprescribing could effectively reduce PIMs at 6 months following. However, this reduction was not sustained at 12 months, nor did it translate into a decrease in hospitalizations [41]. In the OPERAM study, the researchers evaluated the effect of a pharmacotherapy optimization intervention in older patients with multimorbidity and polypharmacy. They successfully reduced potentially inappropriate prescribing without observing any impact on drug-related hospital admissions during a 12-month follow-up period. [42].
A systematic review found positive outcomes related to medication review in frail older people, although only in two of the 25 studies were analysed [43]. Another systematic review exploring deprescribing in frail individuals revealed some evidence of positive effects on clinical outcomes such as function, frailty, cognitive status and depression. This review found that mortality and hospitalization rates did not increase after interventions [44]. In our setting, a study carried out within an integrated health intervention in primary care, focusing on polypharmacy and inappropriate prescribing (IP) in the elderly, indicates sustained improvements in inappropriate prescribing even 6 months after the intervention. Moreover, individuals who experienced a reduction in the STOPP criteria during the intervention demonstrated not only a decrease in polypharmacy but also a reduction in falls by the end of the intervention [6]. Authors in the field emphasize the importance of adopting an individualized and multidisciplinary approach, identifying individuals at higher risk for adverse outcomes of polypharmacy. It is noteworthy that there is no conclusive evidence suggesting that the number of medications, rather than inappropriate prescribing, is directly responsible for these adverse outcomes. Potentially inappropriate prescribing is particularly problematic when patients are discharged from hospital. One study found that at least one third of patients have IP at discharge [45]. This is an important moment in drug therapy management, offering an opportunity to proactively prevent inappropriate prescribing and provide support for follow-up care transitions. They also recommend tight communication and collaboration between healthcare settings to ensure a safe transition of care [46, 47]. A recent systematic review underlines the importance of that interventions, strategies and tools designed to minimize iatrogenic risks for multimorbid older patients by reducing the number of drugs they consume. The reviewers considered three main recommendations in implementing measures to improve appropriateness: prescription, acceptance by the patient and continuous monitoring of adherence and risk–benefit profile [48]. Implementing such measures is crucial for achieving meaningful improvements in medications management for the older population with multiple comorbidities.
The available literature which examines the adverse outcomes of polypharmacy in the elderly is complex, extensive and unclear. Most of the studies only consider polypharmacy itself with a diverse range of medications and disease profiles. These studies neglected considerations of inappropriate prescribing [49]. Therefore, the present study is important as it demonstrates the necessity for a different approach to the assessment of inappropriate prescribing. It highlights the importance of developing suitable interventions according to the clinical profile of the patient, aiming to improve prescribing and reduce adverse health events. Further investigations are needed to analyse the different patterns of inappropriate prescribing and their relationship with different items of the CGA. Such studies would be able to provide clinical and prognostic differences among patients based on the specific patterns they exhibit.
The present study boasts strengths in its innovative approach, establishing associations between different patterns of inappropriate prescribing and other clinical and drug prescription variables unique of each patient. Additionally, the application of comprehensive geriatric assessment as part of the evaluation for inappropriate prescribing is also noteworthy. However, it is important to acknowledge the limitations of this study, including a small sample size and a single-centre setting, which implies that the findings may not be extrapolated to the general population. Another limitation is the utilization of an abbreviated form of the STOPP/START criteria, focusing our attention on potentially inappropriate medications affecting the central nervous system and potentially prescribing omissions related to the cardiovascular system.
Conclusions
In the present study, we found an association between different patterns of inappropriate prescribing and clinical and CGA characteristics of admitted patients. There is an association between patterns of overprescribing and dependency and dementia, whilst underprescribing was associated with frailty, risk of malnutrition and social risk. Both patterns of inappropriate prescribing are associated with comorbidity. We also found an association between extreme polypharmacy, PIMs and anticholinergic burden with no association with PPOs, with a statistically significant difference.
Inappropriate prescribing is a complex challenge in older adults, and further investigation is needed to elucidate the different patterns of IP and their relationship with clinical characteristics. These investigations are likely to improve outcomes in both hospitalized and community-dwelling older adults. The present study demonstrated the importance of a CGA to identify different risks associated with inappropriate prescribing.
Availability of data and materials
The data that support the findings of this study are available upon reasonable request from the corresponding author.
References
Palmer K, Villani ER, Vetrano DL et al (2019) Association of polypharmacy and hyperpolypharmacy with frailty states: a systematic review and meta-analysis. Eur Geriatr Med 10(1):9–36. https://doi.org/10.1007/s41999-018-0124-5
Martinot P, Landré B, Zins M, Goldberg M, Ankri J, Herr M (2018) Association between potentially inappropriate medications and frailty in the early old age: a longitudinal study in the GAZEL cohort. J Am Med Dir Assoc 19(11):967–973.e3. https://doi.org/10.1016/j.jamda.2018.07.008. Epub 2018 Aug 30. PMID: 30172683.
Gutiérrez-Valencia M, Izquierdo M, Malafarina V et al (2017) Impact of hospitalization in an acute geriatric unit on polypharmacy and potentially inappropriate prescriptions: a retrospective study. Geriatr Gerontol Int 17(12):2354–2360. https://doi.org/10.1111/ggi.13073
Wallace E, McDowell R, Bennett K, Fahey T, Smith SM (2017) Impact of potentially inappropriate prescribing on adverse drug events, health related quality of life and emergency hospital attendance in older people attending general practice: a prospective cohort study. J Gerontol A Biol Sci Med Sci 72(2):271–277. https://doi.org/10.1093/gerona/glw140
Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D (2011) Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 171(11):1013–1019. https://doi.org/10.1001/archinternmed.2011.215
San-José A, Pérez-Bocanegra C, Agustí A et al (2021) Integrated health intervention on polypharmacy and inappropriate prescribing in elderly people with multimorbidity: results at the end of the intervention and at 6 months after the intervention. Med Clin (Barc) 156(6):263–269
Salahudeen MS, Duffull SB, Nishtala PS (2015) Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 25;15:31. https://doi.org/10.1186/s12877-015-0029-9
Welsh TJ, van der Wardt V, Ojo G, Gordon AL, Gladman JRF (2018) Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging 35(6):523–538. https://doi.org/10.1007/s40266-018-0549-z
Wouters H, Hilmer SN, Gnjidic D et al (2020) Long-term exposure to anticholinergic and sedative medications and cognitive and physical function in later life. J Gerontol A Biol Sci Med Sci 75(2):357–365. https://doi.org/10.1093/gerona/glz019
Martínez Arrechea S, Ferro Uriguen A, Beobide Telleria I, González Bueno J, Alaba Trueba J, Sevilla SD (2021) Prevalence of prescription of anticholinergic/sedative burden drugs among older people with dementia living in nursing homes. Rev Esp Geriatr Gerontol 56(1):11–17
Hsu HF, Chen KM, Belcastro F, Chen YF (2021) Polypharmacy and pattern of medication use in community-dwelling older adults: a systematic review. J Clin Nurs 30(7–8):918–928. https://doi.org/10.1111/jocn.15595
Bruin-Huisman L, Abu-Hanna A, van Weert HCPM, Beers E (2017) Potentially inappropriate prescribing to older patients in primary care in the Netherlands: a retrospective longitudinal study. Age Ageing 46(4):614–619. https://doi.org/10.1093/ageing/afw243
Cruz-Esteve I, Marsal-Mora JR, Galindo-Ortego G et al (2017) Análisis poblacional de la prescripción potencialmente inadecuada en ancianos según criterios STOPP/START (estudio STARTREC) [Potentially inappropriate prescribing in older Spanish population according to STOPP/START criteria (STARTREC study)]. Aten Primaria 49(3):166–176. https://doi.org/10.1016/j.aprim.2016.02.013
Ryan C, O’Mahony D, Kennedy J et al (2013) Potentially inappropriate prescribing in older residents in Irish nursing homes. Age Ageing 42(1):116–120. https://doi.org/10.1093/ageing/afs068
McMahon CG, Cahir CA, Kenny RA, Bennett K (2014) Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing 43(1):44–50. https://doi.org/10.1093/ageing/aft114
Gallagher P, Lang PO, Cherubini A et al (2011) Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 67(11):1175–1188. https://doi.org/10.1007/s00228-011-1061-0
Dalleur O, Spinewine A, Henrard S, Losseau C, Speybroeck N, Boland B (2012) Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging 29(10):829–837. https://doi.org/10.1007/s40266-012-0016-1
San-José A, Agustí A, Vidal X et al (2014) Inappropriate prescribing to older patients admitted to hospital: a comparison of different tools of misprescribing and underprescribing. Eur J Intern Med 25(8):710–716. https://doi.org/10.1016/j.ejim.2014.07.011
San-José A, Agustí A, Vidal X et al (2015) Inappropriate prescribing to the oldest old patients admitted to hospital: prevalence, most frequently used medicines, and associated factors. BMC Geriatr 15:42. https://doi.org/10.1186/s12877-015-0038-8
Molist-Brunet N, Sevilla-Sánchez D, González-Bueno J et al (2021) Therapeutic optimization through goal-oriented prescription in nursing homes. Int J Clin Pharm 43(4):990–997. https://doi.org/10.1007/s11096-020-01206-x
Lu WH, Wen YW, Chen LK, Hsiao FY (2015) Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: a retrospective cohort study. CMAJ 187(4):E130–E137. https://doi.org/10.1503/cmaj.141219
Okudur SK, Dokuzlar O, Aydin AE, Kocyigit SE, Soysal P, Isik AT (2021) The evaluation of relationship between polypharmacy and anticholinergic burden scales. North Clin Istanb 11;8(2):139–144. https://doi.org/10.14744/nci.2020.17136. PMID: 33851077; PMCID: PMC8039107.
O'Mahony D (2020) STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol 13(1):15–22. https://doi.org/10.1080/17512433.2020.1697676. Epub 2019 Nov 30. PMID: 31790317.
Hilmer SN, Mager DE, Simonsick EM et al (2007) A drug burden index to define the functional burden of medications in older people. Arch Intern Med 167(8):781–787 (PubMed PMID: 17452540)
Morley JE, Malmstrom TK, Miller DK (2012) A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 16(7):601–608. https://doi.org/10.1007/s12603-012-0084-2
Sainsbury A, Seebass G, Bansal A, Young JB (2005) Reliability of the Barthel Index when used with older people. Age Ageing 34(3):228–232. https://doi.org/10.1093/ageing/afi063
Graf C (2008) The Lawton instrumental activities of daily living scale. Am J Nurs 108(4):52–63. https://doi.org/10.1097/01.NAJ.0000314810.46029.74
Reisberg B, Ferris SH, de Leon MJ, Crook T (1988) Global deterioration scale (GDS). Psychopharmacol Bull 24(4):661–663
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE (2005) Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 82(5):1074–81 https://doi.org/10.1093/ajcn/82.5.1074. PMID: 16280441.
Park SH, Lee HS (2016) Assessing predictive validity of pressure ulcer risk scales-a systematic review and meta-analysis. Iran J Public Health 45(2):122–133
Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23(10):433–441. https://doi.org/10.1111/j.1532-5415.1975.tb00927.x
Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, Ryan T, Cash H, Guerini F, Torpilliesi T, Del Santo F, Trabucchi M, Annoni G, MacLullich AM (2014) Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 43(4):496–502. https://doi.org/10.1093/ageing/afu021. Epub 2014 Mar 2. Erratum in: Age Ageing. 2015 Jan;44(1):175. PMID: 24590568; PMCID: PMC4066613.
Alshammari H, Al-Saeed E, Ahmed Z, Aslanpour Z (2022) Prevalence and predictors of potentially inappropriate medications among patients aged ≥65 years on hospital admissions in Kuwait. Clin Interv Aging. 6;17:1025–1036. https://doi.org/10.2147/CIA.S328693. PMID: 35822127; PMCID: PMC9271279.
Meid AD, Quinzler R, Freigofas J, et al (2015) Medication underuse in aging outpatients with cardiovascular disease: prevalence, determinants, and outcomes in a prospective cohort study. PLoS One 10(8):e0136339. https://doi.org/10.1371/journal.pone.0136339
Gutiérrez-Valencia M, Izquierdo M, Lacalle-Fabo E et al (2018) Relationship between frailty, polypharmacy, and underprescription in older adults living in nursing homes. Eur J Clin Pharmacol 74(7):961–970. https://doi.org/10.1007/s00228-018-2452-2
de Groote P, Isnard R, Assyag P, Clerson P, Ducardonnet A, Galinier M, Jondeau G, Leurs I, Thébaut, J.-.-F. and Komajda, M. (2007) Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail 9:1205–1211. https://doi.org/10.1016/j.ejheart.2007.09.008
Manias E, Maier A, Krishnamurthy G (2019) Inappropriate medication use in hospitalised oldest old patients across transitions of care. Aging Clin Exp Res 31(11):1661–1673. https://doi.org/10.1007/s40520-018-01114-1
Wong HL, Weaver C, Marsh L, Mon KO, Dapito JM, Amin FR et al (2023) Polypharmacy and cumulative anticholinergic burden in older adults hospitalized with fall. Aging Medicine
Sevilla-Sánchez D, Molist-Brunet N, González-Bueno J, Solà-Bonada N, Espaulella-Panicot J, Codina-Jané C (2018) Prevalence, risk factors and adverse outcomes of anticholinergic burden in patients with advanced chronic conditions at hospital admission. Geriatr Gerontol Int 18(8):1159–1165
Mortsiefer A, Löscher S, Pashutina Y et al (2023) Family conferences to facilitate deprescribing in older outpatients with frailty and with polypharmacy: the COFRAIL cluster randomized trial. JAMA Netw Open 6(3):e234723. https://doi.org/10.1001/jamanetworkopen.2023.4723
Blum M R, Sallevelt B T G M, Spinewine A, O’Mahony D, Moutzouri E, Feller M et al (2021) Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial BMJ 374 :n1585. https://doi.org/10.1136/bmj.n1585
Pazan F, Petrovic M, Cherubini A et al (2021) Current evidence on the impact of medication optimization or pharmacological interventions on frailty or aspects of frailty: a systematic review of randomized controlled trials [published correction appears in Eur J Clin Pharmacol. Oct;77(10):1593–1594. Eur J Clin Pharmacol 77(1):1–12. https://doi.org/10.1007/s00228-020-02951-8
- Ibrahim K, Cox NJ, Stevenson JM, Lim S, Fraser SDS, Roberts HC (2021) A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr 21(1):258. Published. https://doi.org/10.1186/s12877-021-02208-8
Mucalo I, Hadžiabdić MO, Brajković A et al (2017) Potentially inappropriate medicines in elderly hospitalised patients according to the EU(7)-PIM list, STOPP version 2 criteria and comprehensive protocol. Eur J Clin Pharmacol 73(8):991–999. https://doi.org/10.1007/s00228-017-2246-y
Grischott T, Rachamin Y, Senn O, Hug P, Rosemann T, Neuner-Jehle S (2023) Medication review and enhanced information transfer at discharge of older patients with polypharmacy: a cluster-randomized controlled trial in swiss hospitals. J Gen Intern Med 38(3):610–618. https://doi.org/10.1007/s11606-022-07728-6
Vasilevskis EE, Shah AS, Hollingsworth EK et al (2023) Deprescribing medications among older adults from end of hospitalization through postacute care: a Shed-MEDS randomized clinical trial. JAMA Intern Med 183(3):223–231. https://doi.org/10.1001/jamainternmed.2022.6545
Lunghi C, Trevisan C, Fusaroli M et al (2022) Strategies and tools for supporting the appropriateness of drug use in older people. Pharmaceuticals (Basel) 15(8):977. https://doi.org/10.3390/ph15080977
Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B (2020) Adverse outcomes of polypharmacy in older people: systematic review of reviews. Vol. 21, JAMDA Elsevier Inc p. 181–7.
Author information
Authors and Affiliations
Contributions
M.Z. wrote the entire manuscript and all the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This is a prospective observational study that has the approval of the Ethics Committee of Vall d’Hebron Hospital and adheres to the Declaration of Helsinki (1964) and its later amendments (current version dating from 2013).
Consent to participate
All patients provided informed consent to participate in this study.
Consent for publication
All patients provided informed consent that their patient-related data may be used for publications.
Competing interests
All authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuleta, M., San-José, A., Gozalo, I. et al. Patterns of inappropriate prescribing and clinical characteristics in patients at admission to an acute care of the elderly unit. Eur J Clin Pharmacol 80, 553–561 (2024). https://doi.org/10.1007/s00228-024-03627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03627-3