Abstract
Purpose
The increased use of proton pump inhibitors (PPIs) in the elderly has raised concerns about potential severe adverse effects. Our systematic review investigated the mortality associated with PPI use in elderly populations.
Methods
We searched MEDLINE, EMBASE, and the Cochrane Library for relevant publications until August 2022. We included randomized controlled trials (RCTs), quasi-RCTs, and observational studies on the association between proton pump inhibitors and mortality in the elderly. To estimate the pooled relative risk (RR) and 95% confidence interval (CI), the inverse-variance random effect model was used. Heterogeneity was assessed using the I2 test. Subgroup analyses were performed by follow-up period, population, and study design.
Results
A total of 4 RCTs and 36 cohort studies were included in the meta-analysis. Four RCTs showed that there was no significant association between PPIs and the risk of death. From 23 observational studies (26 cohorts), the use of proton pump inhibitors was not significantly associated with increased mortality in the elderly (RR 1.14; 95% CI, 0.90–1.45). However, when controlling for covariates from 33 observational studies (41 cohorts), proton pump inhibitors in older adults aged 50 years or more were significantly associated with a 15% higher risk of mortality compared to nonusers (RR 1.15; 95% CI, 1.10–1.20).
Conclusions
Our meta-analysis of RCTs found that PPIs did not show a significant association with increased mortality risk in older adults. However, the meta-analysis of cohort studies and long-term follow-up studies showed a higher increased risk of death with PPI use in older adults. The prescription of PPIs in patients aged 50 years or older should be carefully considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, gastrointestinal diseases have been on the rise in older populations [1]. In this demographic, proton pump inhibitors (PPIs) are commonly used to treat heartburn, gastroesophageal reflux disease, peptic ulcer disease, and Helicobacter pylori infections [2, 3]. In an Irish study among adults aged 40 years or older with intellectual disabilities, the largest population of PPI users was aged 50–64 years (53.4%), and 30.2% were aged 65 years or older [4].
Concerns of potentially severe adverse effects of PPIs have increased, particularly in older adults. A recent review demonstrated that the use of PPI in older adults was associated with an increased risk of osteoporotic-related fractures, Clostridium difficile infection, community-acquired pneumonia, vitamin B12 deficiency, kidney disease, and dementia [5]. These potential side effects are of particular concern to the elderly because this vulnerable population is already more likely to suffer from an increased risk of these conditions and consequently, severe morbidity. As a result, the guidelines recommend avoiding the use of PPIs for longer than 8 weeks in the elderly except in high-risk patients and discontinuing or reducing PPIs in older adults with more than 8-week usage of PPIs for uncomplicated peptic ulcer disease or erosive peptic esophagitis [5,6,7].
Additionally, recent studies have reported that PPIs were associated with increased mortality in the general population and/or patients with cancer [8,9,10,11]. The association between PPIs and mortality in the elderly population is undefined, but it has been shown that they are potentially affected [10, 11]. Since previous studies have yielded inconsistent results for mortality in older adults who take PPIs and the excess risk of death associated with PPI use has not been systematically investigated, pooled estimates that combined relative risks for mortality from each study are needed. Thus, we performed a systematic review and meta-analysis of the association between PPIs and mortality in older adults.
Methods
Literature search and study selection
We conducted searches on MEDLINE, EMBASE, and the Cochrane Library for articles published up to August 24, 2022. To search for relevant articles, we used MeSH terms and text words related to outcome, such as “mortality,” “death,” and “fatality,” and intervention-related search terms, including the drug, brand, and chemical names of “proton pump inhibitor” (i.e., benatoprazole, dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, and tenatoprazole). The search strategy is presented in Supplement Table 1.
All studies that met the following criteria were included: (1) investigated the association between PPIs and the risk of mortality in adults aged 50 years or older; (2) compared PPIs and control such as placebo or active comparator; (3) reported the quantified relative risk of death; (4) conducted randomized controlled trials (RCTs), non-randomized controlled studies, and observational studies; and (5) written in English. If the participants’ age was not clearly mentioned in the inclusion criteria for the study, we included studies with participants of both a mean or median ≥ 66 years of age and an interquartile range of ≥ 56 years. Two independent reviewers examined the study selection, and the third reviewer was consulted when there was a disagreement. We registered the study protocol in PROSPERO (CRD42020179631) and followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [12].
Quality assessment
Two independent researchers conducted a quality assessment of the included studies. For RCTs, we used version 2 of the Cochrane Risk of Bias (ROB 2) tool, which is composed of five items (bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result), to assess the quality of studies with three levels (low, some concerns, and high risk of bias) [13]. The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) was used to evaluate the quality of non-RCTs. ROBINS-I consists of five items from three domains: pre-intervention, at-intervention, and post-intervention domains [14]. Pre-intervention domain includes bias due to confounding and bias in selection of participants into the study. At-intervention domain includes biases in classification of intervention, and post-intervention domain includes bias due to deviations from intended interventions, bias due to missing data, bias in measurement of the outcome, and bias in selection of the reported result. Four levels were used to assess the risk of bias in included studies: low, moderate, serious, and critical.
Data extraction
We collected information on the study and demographic characteristics (country, study design, data source, study period, mean age, percentage of male participants), exposure (definition and number of PPI users and controls, follow-up period), and outcomes (number of death of PPIs and comparators, relative risk [RR] of mortality) from the included studies. The confounding variables were extracted in regression analysis when applicable.
The primary outcomes were the unadjusted and adjusted estimates of the risk of mortality associated with PPI use. We used the best-adjusted relative risks with a 95% confidence interval (CI) after controlling the confounding variables from each included study for the meta-analysis.
Statistical analysis
To estimate the pooled RRs with CIs, the inverse-variance random effect model was used. We calculated log RR and standard error (SE) using the 95% CIs or P-values reported in the studies. Each study reported a different type of relative risk, such as RR, hazard ratio (HR), or odds ratio (OR). In our meta-analysis, HRs were considered RRs [15, 16], and ORs were converted to RRs using the method described by Zhang and Yu, which uses OR and the incidence of mortality in the control group [17]. The studies that reported OR were included in the meta-analysis when we could calculate RRs from the data on OR and the incidence of mortality in the control group. In addition, we performed subgroup and sensitivity analyses according to follow-up period, population, country, study design, the median age of included studies, and quality of the studies. We conducted a duration analysis of the risk of death among PPI users by follow-up period: ≤ 6 months, > 6 months– ≤ 1 year, > 1– ≤ 2 years, > 2– ≤ 3 years, > 3– ≤ 4 years, and > 4 years. To further evaluate disease-specific mortality, we evaluated the association between PPIs and mortality among patients with cancers, cardiovascular disease, and kidney diseases as well as people who were institutionalized and hospitalized. To assess the impact of different health care systems, the subgroup analysis by countries was conducted: USA/Canada, Europe, Australia, and Asia. The association of subgroups was also examined by patient’s age to take into account differences in mortality by age groups (≤ 75, 76–85, and > 85 years old) as well as by quality assessment of included studies (low, moderate, and serious/critical risk of bias) to incorporate assessment into the analyses. We also analyzed the association in studies that included and did not include Charlson Comorbidity Index (CCI) as a covariate.
Heterogeneity was assessed using the I2 test and Q statistic, with the significance of the Q-statistic test being considered at p < 0.05. Heterogeneity was considered for I2 values of more than 50% [13]. The funnel plot was used to estimate possible publication bias owing to the tendency to publish studies with positive results. We used Review Manager 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Literature search
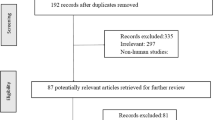
An initial search was performed on 15,202 articles; we identified 13,544 relevant ones after excluding 1658 duplicate articles (Fig. 1). In the screening process, 12,992 articles were removed during the title/abstract review, and 511 articles were excluded during the full-text review due to one of the following reasons: no elderly patients, no PPI therapy, ineligible study design, no comparator group available, no outcomes of interest reported, and non-original studies. Finally, 49 cohorts among 41 studies (four RCTs and 37 observational studies) were included in our systematic review [11, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] since five studies included more than one cohort in their studies [19, 20, 23, 26, 50]. In the meta-analysis, 4 cohorts among 4 RCTs and 44 cohorts among 36 observational studies were included. One study/cohort was not included in the meta-analysis as we could not calculate RR from OR due to a lack of data on the number of deaths [29].
General characteristics of the included studies
We included four RCTs, 34 cohort studies, and three case–control studies (Table 1). In RCTs, PPIs including omeprazole and pantoprazole were included as interventions with comparators of placebo. In observational studies, most studies included all kinds of PPIs as interventions and non-PPI users as controls including H2RA. The studies were conducted in the USA, Canada, Europe, Australia, and Asia. The study participants included the following: patients with cancer (n = 9), cardiovascular disease (n = 11), kidney disease (n = 4), institutionalization (n = 6), and hospitalization (n = 5). All studies included 2,515,079 participants with a median age ranging from 67 to 96 years and the percentage of males ranging from 18.4 to 99.5%. Study follow-up periods were from 21 days to 13.8 years. When estimating adjusted HRs or ORs, baseline demographics, disease-related clinical factors, comorbidities, and medications were included as confounding variables (Table 1).
Quality assessment
The included RCTs had a low risk of bias from missing outcome data, measurement of the outcome, and selection of the reported result (Supplement Fig. 1). For measurement of the outcome, we determined a low risk because mortality was not affected by any measurement methods. We evaluated some concerns for deviations from intended interventions for half of the included RCTs because PPIs can also be used as over-the-counter (OTC) drugs.
All cohort studies had a low risk of bias from deviations from intended interventions, missing data, measurement of the outcome, and selection of the reported result (Supplement Table 2). Among 37 cohort studies, 10 studies had moderate, serious, or critical risk of bias because appropriate confounding covariates for the adjusted estimates were not included, and 19 had a moderate risk for classification of interventions because OTC use of PPIs could not be captured. The overall risk of bias was assessed as low at 27%, moderate at 57%, and serious or critical risk at 16%.
Proton pump inhibitors and mortality in RCTs
Among 4 RCTs, the death rates were 11.8% in PPI users (1286/10,918) and 11.4% in nonusers (1250/10,928). The PPI use and risk of mortality in unadjusted RR among individuals aged 50 years or older were not significantly associated (RR 1.03; 95% CI, 0.91–1.16), with moderate heterogeneity was moderate (I2 = 45%, p = 0.14 (Fig. 2A). When estimating adjusted risk ratio among 20,859 elderly people from 2 RCTs, the association between PPI users and death was not significantly associated compared to nonusers (aRR, 1.01; 95% CI, 0.94–1.08), with 0% of heterogeneity (I2 = 0%, p = 0.60) (Fig. 2C).
Proton pump inhibitors and mortality in observational studies
In 26 observational studies (29 cohorts), there were 75,675 deaths in 512,263 PPI users (14.8%) and 94,428 deaths in 1,731,521 nonusers (4.9%). We found no significant association between the use of PPI and mortality from unadjusted RR in the elderly (RR 1.14; 95% CI, 0.90 − 1.45) (Fig. 2B). There was significant heterogeneity (I2 = 100%, p < 0.001), and funnel plots showed no evidence of publication bias (Fig. 3A). For adjusted estimates, 41 cohorts from 33 studies of the association between PPIs and death included 2,429,961 individuals aged 50 years or older. The use of PPI was significantly associated with a 15% increased risk of mortality compared to non-use (aRR, 1.15; 95% CI, 1.10–1.20) (Fig. 2D). Significant heterogeneity was detected (I2 = 93%, p < 0.001), and there was no evidence of publication bias based on the funnel plot (Fig. 3B).
Additionally, we conducted subgroup and sensitivity analyses of adjusted risk ratios among observational studies. PPI use among the elderly was significantly associated with an increased risk of mortality (by 17–32%) compared to non-use in the follow-up period between more than 6 months and less than 4 years, while the association was not significant in a shorter follow-up of a less than 6 months (aRR, 1.02; 95% CI, 0.45–2.33) and ˃ 4-year follow-up (aRR, 1.06; 95% CI, 0.99–1.14) (Table 2). The mortality risk of PPI users among patients with cancer, cardiovascular diseases, kidney diseases, and institutionalization showed a similar trend. When investigating the risk of death by country, the association between PPIs and increased mortality was significant among studies conducted in Europe (aRR, 1.27; 95% CI, 1.18–1.38), while the association was towards significant among studies conducted in USA/Canada, Asia, and Australia. The significant association between PPI use and mortality appeared consistent among cohort studies (aRR, 1.14; 95% CI, 1.09–1.20) and case–control studies (aRR, 1.31; 95% CI, 1.23–1.40). According to the median age of participants in the included studies, the results of the association between PPI use and mortality remain similar to the base-case analysis results. The lower the risk of bias of the included cohort studies, the larger the RR (aRR, 1.13; 95% CI, 1.01–1.28 in low risk of bias vs aRR, 1.08; 95% CI, 1.02–1.15 in serious/critical risk of bias). The association of risk of death and PPI users among both studies included CCI as a covariate and studies that did not include CCI as a covariate remained significant.
Discussion
This systematic review found a 15% increase in the risk of death among elderly people taking PPI compared to those not taking PPI. In particular, the association between PPI use and mortality was significant in studies among senior adults with cancer, cardiovascular diseases, and kidney diseases as well as studies with longer follow-ups and cohort studies.
Our results are aligned with those of previous studies that included not only adults but also elderly [9, 58]. A previous study using administrative data from Veterans Affairs demonstrated an excess risk of death among adults taking PPIs with a mean age of 61 years [9]. A systematic review also showed that all-cause mortality increased in adults treated with PPI, especially older adults treated with long-term PPI, compared to patients not treated with PPI [58]. Although a clear mechanism is not known for the association between PPIs and the risk of death, a potential biological mechanism was suggested. Prolonged exposure to PPI impairs endothelial function, increases oxidative stress, slows lysosomal acidification and protein accumulation in endothelial cells, and accelerates human endothelial aging by shortening telomere length [59]. PPIs also upregulate the expression of protein levels and mRNA resulting in increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells, which reduces beneficial effects, including antiapoptotic, anti-inflammatory, antioxidant, antiproliferative, and immunomodulatory effects in vascular cells [9, 60, 61]. Elderly patients may be more vulnerable to side effects during long-term PPI use on account of their comorbidities, use of multiple drugs, and poor nutrition [36]. PPIs have also been significantly overused in the elderly, contrary to the recommendation to restrict the PPI treatment period to fewer than 12 weeks [58].
Our subgroup and sensitivity analyses demonstrated that PPI use significantly affected the increased death among older patients with cancer, cardiovascular disease, and kidney disease. These results are similar to those of previous studies that were not limited to the elderly, which correlated PPI usage with excess mortality from cardiovascular disease, chronic kidney disease, and upper gastrointestinal and lung cancers [10, 62, 63] and also suggested an association between PPI usage and increased risk of the aforementioned diseases [62, 64]. The authors suggested a mechanism leading to the excess cause-specific mortality related to PPI use is linked to the exacerbation of underlying disease or the development of a new disease that increases the risk of cause-specific death. This mechanism was also suggested as a unifying mechanism [59]. In addition, inappropriate prescription of high-dose PPI during or after hospitalization is frequent as antithrombotic agents are widely used in elderly patients [36].
Notably, in cohort studies and studies with long-term follow-up (more than 1 year to 3 years), 28–32% of increased risk of death was clearly observed. However, this was not observed when the follow-up period was shortened (less than 6 months). A previous review also showed that there was no immediate apparent increase in all-cause mortality in adults taking PPIs in one RCT, while increased mortality with PPI use was observed in the observational studies followed up to 1 year [58]. Although RCTs have a higher hierarchy of an evidence-based approach than observational studies because of randomization, the findings from observational studies can show the results with all confounders, including unidentified confounders in the real-world population [58]. Our systematic review showed that significant results were obtained in terms of real-world evidence rather than in RCT settings, suggesting that real-world evidence should be considered when applied to clinical settings for patient care, such as pharmacovigilance criteria.
Our systematic review has strengths that, to our best knowledge, this is the first meta-analysis study to explore the association between PPI use and mortality in the older adult population with a large sample size. The significance of the pooled estimates was higher in high-quality studies with a low overall risk of bias. Our diverse subgroup results categorized by the follow-up period, disease, study design, country, and risk of bias provide comprehensive and detailed information. Notably, our results clearly showed that PPI use was significantly associated with increased death in older patients with cancer, cardiovascular disease, and kidney disease. In addition, we provide real-world evidence based on results from cohort studies and long-term follow-up studies.
Our study has several limitations. Primarily, we were not able to investigate the impact of mortality by the duration or dosage of PPI use due to a lack of data. However, our best available evidence supports the association the PPIs and mortality in elderly people. Secondly, the level of heterogeneity was low in RCTs, and it may be caused by study design or the small number of included studies. However, the pooled estimates in observational studies have significant heterogeneity although we conducted several subgroup analyses to find the cause. It may be caused by various populations, different levels of controlling covariates, and unknown factors. Despite the diversity of included studies, results were consistent across subgroups. Thirdly, half of the cohort studies in the meta-analysis showed a moderate risk of bias in the classification of intervention due to self-administrated OTC without prescriptions in most countries. However, the significant association between PPI use and mortality remained consistent in studies with low, moderate, and serious/critical risk of bias. Furthermore, the relative estimates can be considered conservative as individuals who may take OTCs may be included as both PPI users and nonusers. Finally, we cannot identify the causal relationship between PPI use and the risk of mortality since most of the included studies investigated the association between PPI use and the risk of mortality. Also, there was no clear association between PPIs and death from the four RCTs in the meta-analysis.
Conclusions
Our meta-analysis showed that the association between PPIs and the risk of mortality of RCTs was inconsistent with the association from observational studies in the elderly. The meta-analysis of RCTs showed that PPI use was not powered to detect an increased risk of death compared to nonusers. However, the increased risk of death was identified in observational studies and studies with long-term follow-ups, and the association was consistent in the subgroup population, including among patients with cancer, cardiovascular disease, and kidney disease. Our findings highlight the need to increase awareness of increased mortality due to PPI use and to restrict PPI prescriptions to elderly people, wherein the benefits outweigh the potential risks.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Franceschi M, Di Mario F, Leandro G, Maggi S, Pilotto A (2009) Acid related disorders in the elderly. Best Pract Res Clin Gastroenterol 23:839–848
Thomson AB, Sauve MD, Kassam N, Kamitakahara H (2010) Safety of the long-term use of proton pump inhibitors. World J Gastroenterol 16(19):2323–2330
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL (2015) Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 314:1818–1831
AlMutairi H, O’Dwyer M, McCarron M, McCallion P, Henman MC (2018) The use of proton pump inhibitors among older adults with intellectual disability: a cross sectional observation study. Saudi Pharm J 26:1012–1021
Maes ML, Fixen DR, Linnebur SA (2017) Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf 8(9):273–297
American Geriatrics Society 2015 Beers Criteria Update Expert Panel (2015) American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2227–2246
O’Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44:213–218
Brown JP, Tazare JR, Williamson E, Mansfield KE, Evans SJ, Tomlinson LA et al (2021) Proton pump inhibitors and risk of all-cause and cause-specific mortality: a cohort study. Br J Clin Pharmacol 87(8):1–12
Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z (2017) Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 7(6):e015735
Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z (2019) Estimates of all-cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 365:l1580
Sharma M, Holmes HM, Mehta HB, Chen H, Aparasu RR, Sgug YCT et al (2019) The concomitant use of tyrosine kinase inhibitors and proton pump inhibitors: prevalence, predictors, and impact on survival and discontinuation of therapy in older adults with cancer. Cancer 125(7):1155–1162
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151(4):W65–W94
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al (2023) Cochrane handbook for systematic reviews of interventions: the Cochrane Collaboration, Version 6.3. Available from: https://training.cochrane.org/handbook. Assessed 1 Jan 2023
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Davies HT, Crombie IK, Tavakoli M (1998) When can odds ratios mislead? BMJ 316:989–991
Stare J, Maucort-Boulch D (2016) Odds ratio, hazard ratio and relative risk. Metod Zv 13:59–67
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691
Adelborg K, Sundbøll J, Schmidt M, Bøtker HE, Weiss NS, Pedersen L et al (2018) Use of histamine H2 receptor antagonists and outcomes in patients with heart failure: a nationwide population-based cohort study. Clin Epidemiol 10:521–530
Adelborg K, Sundbøll J, Schmidt M, Bøtker HE, Weiss NS, Pedersen L et al (2020) Use of acid-suppressant medications after diagnosis increases mortality in a subset of gastrointestinal cancer patients. Dig Dis Sci 65(9):2691–2699
Baek YH, Kang EJ, Hong S, Park S, Kim JH, Shin JY (2022) Survival outcomes of patients with nonsmall cell lung cancer concomitantly receiving proton pump inhibitors and immune checkpoint inhibitors. Int J Cancer 150(8):1291–1300
Baik SH, Fung KW, McDonald CJ (2022) The mortality risk of proton pump inhibitors in 1.9 million US seniors: an extended cox survival analysis. Clin Gastroenterol Hepatol 20(4):e671-681
Bakhshwin D, Alotaibi M, Ali AS, Althomali A, Alsuwat A, Alhamyani A et al (2022) Mortality predictors among COVID-19 elderly in Taif, Saudi Arabia. Infect Drug Resist 15:3213–3223
Bell JS, Strandberg TE, Teramura-Gronblad M, Laurila JV, Tilvis RS, Pitkälä KH (2010) Use of proton pump inhibitors and mortality among institutionalized older people. Arch Intern Med 170(17):1604–1605
Bradley ES, Howe E, Wu X, Haran JP (2019) Proton pump inhibitors and 180-day mortality in the elderly after Clostridium difficile treatment. Gut Pathog 11:29
Brozek W, Reichardt B, Zwerina J, Dimai HP, Klaushofer K, Zwettler E (2017) Use of proton pump inhibitors and mortality after hip fracture in a nationwide study. Osteoporos Int 28(5):1587–1595
Cetin H, Wurm R, Reichardt B, Tomschik M, Silvaieh S, Parvizi T et al (2020) Increased risk of death associated with the use of proton-pump inhibitors in patients with dementia and controls - a pharmacoepidemiological claims data analysis. Eur J Neurol 27(8):1422–1428
Cholin L, Ashour T, Mehdi A, Taliercio JJ, Daou R, Arrigain S et al (2021) Proton-pump inhibitor vs. H2-receptor blocker use and overall risk of CKD progression. BMC Nephrol 22(1):264
de Francisco ALM, Varas J, Ramos R, Merello JI, Canaud B, Stuard S, Optimizing Results in Dialysis (ORD) group et al (2017) Proton pump inhibitor usage and the risk of mortality in hemodialysis patients. Kidney Int Rep 3(2):374–384
Farhat N, Birkett N, Haddad N, Fortin Y, Momoli F, Wen SW et al (2020) Risk of adverse cardiovascular events following a myocardial infarction in patients receiving combined clopidogrel and proton pump inhibitor treatment: a nested case-control study. Drugs Real World Outcomes 7(3):191–203
Hálfdánarson ÓÖ, Pottegård A, Lund SH, Ogmundsdottir MH, Ogmundsdottir HM, Zoega H (2020) Use of proton pump inhibitors and mortality among Icelandic patients with prostate cancer. Basic Clin Pharmacol Toxicol 126(6):484–491
Hasselgren G, Lind T, Lundell L, Aadland E, Efskind P, Falk A et al (1997) Continuous intravenous infusion of omeprazole in elderly patients with peptic ulcer bleeding. Results of a placebo-controlled multicenter study. Scand J Gastroenterol 32(4):328–333
Jun T, Ozbek U, Dharmapuri S, Hardy-Abeloos C, Zhu H, Lin JY et al (2021) Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol 13:17588359211010936
Juurlink DN, Gomes T, Mamdani MM, Gladstone DJ, Kapral MK (2011) The safety of proton pump inhibitors and clopidogrel in patients after stroke. Stroke 42(1):128–132
Liabeuf S, Lambert O, Metzger M, Hamroun A, Laville M, Laville SM, Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD REIN) study group et al (2021) Adverse outcomes of proton pump inhibitors in patients with chronic kidney disease: the CKD-REIN cohort study. Br J Clin Pharmacol 87(7):2967–2976
Lo CH, Ni P, Yan Y, Ma W, Joshi AD, Nguyen LH et al (2022) Association of proton pump inhibitor use with all-cause and cause-specific mortality. Gastroenterology 163(4):852–61.e2
Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Buttò V et al (2013) Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med 173(7):518–523
Mahabaleshwarkar RK, Yang Y, Datar MV, Bentley JP, Strum MW, Banahan BF et al (2013) Risk of adverse cardiovascular outcomes and all-cause mortality associated with concomitant use of clopidogrel and proton pump inhibitors in elderly patients. Curr Med Res Opin 29(4):315–323
Maret-Ouda J, Santoni G, Xie S, Rosengren A, Lagergren J (2022) Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc Drugs Ther 36(6):1121–1128
Marker S, Krag M, Perner A, Wetterslev J, Lange T, Wise MP, SUP-ICU Trial Investigators et al (2019) Pantoprazole in ICU patients at risk for gastrointestinal bleeding-1-year mortality in the SUP-ICU trial. Acta Anaesthesiol Scand 63(9):1184–1190
Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, COMPASS Investigators et al (2019) Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology 157(3):682-691.e2
Muñoz-Torrero JFS, Zamorano J, Rico-Martín S, Rivas MD, Bacaicoa MA, Robles R, FRENA Investigators et al (2020) Proton pump inhibitors and risk for recurrent ischemic events or death in outpatients with symptomatic artery disease. Atherosclerosis 292:84–89
Nayan M, Juurlink DN, Austin PC, Macdonald EM, Finelli A, Kulkarni GS, Canadian Drug Safety and Effectiveness Research Network (CDSERN) et al (2018) Medication use and kidney cancer survival: a population-based study. Int J Cancer 142(9):1776–1785
Oudit GY, Bakal JA, McAlister FA, Ezekowitz JA (2011) Use of oral proton pump inhibitors is not associated with harm in patients with chronic heart failure in an ambulatory setting. Eur J Heart Fail 13(11):1211–1215
Pani A, Pastori D, Senatore M, Romandini A, Colombo G, Agnelli F et al (2020) Clinical and pharmacological characteristics of elderly patients admitted for bleeding: impact on in-hospital mortality. Ann Med 52(7):413–422
Pegoli MA, Dedigama M, Mangoni AA, Russell PT, Grzeskowiak LE, Thynne T (2017) Proton pump inhibitors and risk of readmission and mortality in older patients discharged from a tertiary hospital to residential aged care facilities. Ther Adv Drug Saf 8(4):137–139
Rassen JA, Choudhry NK, Avorn J, Schneeweiss S (2009) Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation 120(23):2322–2329
Roberts G, Pegoli M, Grzeskowiak L, Benger S, Forbes H, Hunt K et al (2021) Hospital admission as a deprescribing triage point for patients discharged to Residential Aged Care Facilities. Age Ageing 50(5):1600–1606
Rooney MR, Bell EJ, Alonso A, Pankow JS, Demmer RT, Rudser KD et al (2021) Proton pump inhibitor use, hypomagnesemia and risk of cardiovascular diseases: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Gastroenterol 55(8):677–683
Sturm L, Muller L, Schultheiss M, Binder B, Huber JP, Thimme R et al (2021) Proton pump inhibitor therapy is associated with reduced survival following first-time transarterial chemoembolization in patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol 33(1S Suppl 1):e247–e253
Teramura-Grönblad M, Bell JS, Pöysti MM, Strandberg TE, Laurila JV, Tilvis RS et al (2012) Risk of death associated with use of PPIs in three cohorts of institutionalized older people in Finland. J Am Med Dir Assoc 13(5):488.e9-e13
Tomisaki I, Harada M, Minato A, Nagata Y, Kimuro R, Higashijima K et al (2022) impact of the use of proton pump inhibitors on pembrolizumab effectiveness for advanced urothelial carcinoma. Anticancer Res 42(3):1629–1634
Tran T, Assayag D, Ernst P, Suissa S (2021) Effectiveness of proton pump inhibitors in idiopathic pulmonary fibrosis: a population-based cohort study. Chest 159(2):673–682
Tsai IJ, Lai TS, Shiao CC, Huang TM, Wang CH, Tsao CH et al (2020) Proton pump inhibitors augment the risk of major adverse cardiovascular events and end-stage renal disease in patients with acute kidney injury after temporary dialysis. Clin Pharmacol Ther 107(6):1434–1445
Wang X, Liu Q, Halfdanarson ÓÖ, Zoega H, Sadr-Azodi O, Engstrand L et al (2021) Proton pump inhibitors and survival in patients with colorectal cancer: a Swedish population-based cohort study. Br J Cancer 125(6):893–900
Wilson N, Gnjidic D, March L, Sambrook P, Hilmer SN (2011) Use of PPIs are not associated with mortality in institutionalized older people. Arch Intern Med 171(9):866
Wu H, Jing Q, Wang J, Guo X (2011) Pantoprazole for the prevention of gastrointestinal bleeding high-risk patients with acute coronary syndromes. J Crit Care 26(4):434.e1-e6
Xie J, Chen Q, He D (2022) Pre-existing proton pump inhibitor treatment and short-term prognosis of acute myocardial infarction patients. Front Cardiovasc Med 9:919716
Ben-Eltriki M, Green CJ, Maclure M, Musini V, Bassett KL, Wright JM (2020) Do proton pump inhibitors increase mortality? A systematic review and in-depth analysis of the evidence. Pharmacol Res Perspect 8(5):e00651
Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP (2016) Proton pump inhibitors accelerate endothelial senescence. Circ Res 118:e36-42
Kucuk HF, Akyol H, Kaptanoglu L, Kurt N, Barisik NO, Bingul S et al (2006) Effect of proton pump inhibitors on hepatic regeneration. Eur Surg Res 38(3):322–328
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119
Shiraev TP, Bullen A (2018) Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ 27(4):443–450
Song HJ, Rhew K, Lee YJ, Ha IH (2021) Acid-suppressive agents and survival outcomes in patients with cancer: a systematic review and meta-analysis. Int J Clin Oncol 3(1):34–50
Li T, Xie Y, Al-Aly Z (2018) The association of proton pump inhibitors and chronic kidney disease: cause or confounding? Curr Opin Nephrol Hypertens 27(3):182–187
Funding
This work was supported by the Postdoctoral Research Program of Sungkyunkwan University (2017).
Author information
Authors and Affiliations
Contributions
HJS was involved in study concept and design, literature search, data extraction, data analysis, data interpretation, and manuscript writing. HJS was involved in data interpretation and manuscript writing. XJ was involved in literature search, data extraction, and data interpretation. NJ, YJL, and IHH were involved in literature search and data interpretation. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Registration: The PROSPERO registration number is CRD42020179631.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Little is known about the excess risk of death associated with proton pump inhibitor use in the elderly.
• We conducted a systematic review and meta-analysis of randomized controlled trials and observational studies regarding the association between proton pump inhibitors and the risk of death in adults aged 50 years or older.
• Our meta-analysis found a 15% increased risk of death in elderly people with PPI use compared to nonusers. Longer follow-up and cohort studies revealed an increased risk of death among older patients with cancers, cardiovascular disease, and kidney disease.
• Awareness of the increased mortality with PPI use should be raised, and the need to limit PPI prescriptions to the elderly where the benefits outweigh the potential risks should be emphasized.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H.J., Seo, HJ., Jiang, X. et al. Proton pump inhibitors associated with an increased risk of mortality in elderly: a systematic review and meta-analysis. Eur J Clin Pharmacol 80, 367–382 (2024). https://doi.org/10.1007/s00228-023-03606-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03606-0