Abstract
Purpose
The present study was carried out in order to assess the effects of chronic administration of flunitrazepam (as an oral hypnotic) on 24-h blood pressure (BP) and heart rate (HR) in healthy young adults.
Materials and methods
Following a 2-week placebo run-in period, 28 healthy volunteers (13 males and 15 females) between 21 and 30 years were randomized to receive either flunitrazepam 1 mg or placebo (both administered once a day in the evening) for 4 weeks in two cross-over periods; each separated by a 2-week placebo period. At the end of each study period, non-invasive 24-h BP and HR ambulatory monitoring was performed.
Results
Flunitrazepam produced a significant decrease in nighttime systolic blood pressure (SBP) (− 6.4 mmHg) and diastolic blood pressure (DBP) (− 4.1 mmHg) (both P < 0.05 vs placebo) without affecting nocturnal HR. During the morning hours, significantly higher values of SBP (+ 7.4 mmHg, P < 0.01), DBP (+ 3.4 mmHg, P < 0.05) and HR (+ 3.9 beats/min, P < 0.05) were observed in the flunitrazepam group compared to the placebo-treated group. No significant differences were noted between the two groups during afternoon and evening hours.
Conclusions
These results suggest that chronic oral administration of 1 mg flunitrazepam as a hypnotic agent causes a significant nocturnal fall in BP and a transient rebound increase of both BP and HR at awakening in the morning. Mechanisms underlying these cardiovascular effects remain unclear, although the direct vasodilatory effect, which is typical of flunitrazepam (with consequent reflex counter-regulatory responses), and the attenuation of baroreflex sensitivity are likely to play a major role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flunitrazepam is a positive GABAA modulator of GABA neurotransmission that binds benzodiazepine sites on the GABA receptor and strongly enhances GABA receptor-mediated transmission [1,2,3]. Like all benzodiazepines, flunitrazepam mediates inhibitory neurotransmission in the brain producing sedative-hypnotic effects [4, 5]. This feature is greater than that of both diazepam [6, 7] and nitrazepam [8], as flunitrazepam induces sleep more quickly [9] and produces compelling anterograde amnesia [6, 7, 10, 11]. Flunitrazepam activity seems to be particularly significant in the limbic system [12, 13], thus, via the hypothalamus, influencing autonomic function and related cardiovascular changes [14, 15].
The cardiovascular effects of flunitrazepram [15] also seem to be dependent upon its primary peripheral vasodilatory effect. This distinctive action has been demonstrated in both experimental and human studies [16,17,18,19,20,21]. In isolated segments of the rat tail artery, flunitrazepam (not diazepam, however) produced marked relaxation by means of a 15% increase in diameter, even following maximum noradrenaline-induced constriction [16]. This vasodilatory effect has also been confirmed in human studies: a decrease of 15–24% in peripheral resistance was observed [17,18,19,20,21] following flunitrazepam administration. In healthy individuals, acute intravenous flunitrazepam administration consistently led to significant decreases in systolic blood pressure (SBP), diastolic blood pressure (DBP), and plasma noradrenaline (NE) [22,23,24,25]. The only study which evaluated the effect of a single oral flunitrazepam administration as a hypnotic agent in healthy subjects reported a significant fall in SBP values with no change in HR [26]. In patients with CHD, acute flunitrazepam administration produced a 15% reduction in blood pressure (BP) and a slight, transient increase in HR [21, 27]. A vasodilatory effect on the coronary vascular bed, together with a loss of coronary circulation autoregulation, was reported [21].
Flunitrazepam i.v. administration as a premedication in patients undergoing invasive diagnostic procedures, produced an increase in resting HR and a decrease in BP during bronchoscopy [28]. Conversely, no change was reported during gastroscopy [29, 30].
Use of flunitrazepam for induction of anesthesia in varying types of surgical operations constantly led to a significant reduction in BP [17,18,19, 31,32,33,34,35,36,37]; however, in only two studies was this reduction accompanied by an increase in HR [17, 34]. This lack of increase in HR in the presence of a decrease in BP might be related to the fact that, unlike diazepam, which has little effect on the baroreflex [38], flunitrazepam significantly attenuates baroreflex sensitivity [34, 36]. Interestingly, intravenous flunitrazepam administration (in patients who had already been anesthetized using other drugs) produced decreases in BP with unchanged HR [39, 40]. This suggests that these effects resulted from the direct vasodilatory action of flunitrazepam, and not from sleep induction and loss of consciousness.
The results of all these studies, however, are not conclusive, and comparison of results is not straightforward. This is mostly due to the varied nature of the study populations, distinct experimental and clinical settings, doses and administration routes, coupled with the relatively short duration of cardiovascular-effect monitoring. Moreover, to the best of our knowledge, to date, no study has evaluated the cardiovascular effects of flunitrazepam in healthy subjects who chronically consume the drug to induce sleep (the most frequent use of flunitrazepam).
In this context, the present study was undertaken to assess the effects of chronic evening oral administration of flunitrazepam on 24-h BP and HR in healthy young adults.
Materials and methods
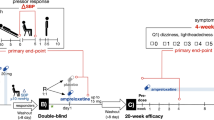
Both male and female healthy volunteers, between 21 and 30 years, with a normal BP, a normal depression and anxiety evaluation and a normal BMI, ECG, and kidney function were eligible for enrolment in this randomized, double-blind cross-over study. Exclusion criteria were the following: history of smoking, shiftwork, diabetes mellitus, hepatic insufficiency, pregnancy, previous cardiovascular accidents, use of any type of drugs, and usual or occasional consumption of alcohol.
The study protocol was approved by the local Ethical Committee, and informed consent was obtained from all the subjects. Following an initial 2-week placebo period, subjects satisfying the inclusion criteria were randomized to flunitrazepam 1 mg or placebo, both administered once a day in the evening (22.00–23.00 h) for 4 weeks in two cross-over periods, each separated by a 2-week placebo period. This wash-out period was considered to be sufficient to prevent any carry-over effect [41]. At the end of each study period, 24-h non-invasive ambulatory BP and HR monitoring was performed using a validated device (Spacelabs 90207, Redmond, Washington) [42] that was programmed to measure BP every 15 min. Each recording was started in the morning and performed throughout a full 24-h period, during which subjects were allowed to follow their normal daily routine. The analysis of 24-h BP recordings were preceded by removal of artifacts, according to previously described editing criteria [43]. Recordings were excluded from the analysis when > 10% of all readings or more than one reading per hour was missed. Computed analysis of the individual recordings provided 24-h, night-time (23.00–07.00 h), morning-time (0.7.00–12.00 h), and afternoon/evening-time (12.00–23.00 h) mean values of SBP, DBP, and HR. At each visit, adverse events spontaneously reported were recorded.
Statistical analysis
The statistical analysis was performed using Analysis of Variance (ANOVA) and by means of the SPSS software package for Windows (version 11.0: Chicago, Illinois, USA); data are presented as means ± standard deviations. Paired tests were also used: a one-sample t test was used to compare values obtained after treatment administration; a two-sample t test was used to compare the change score (treatment-placebo) for a given parameter between the two groups.
Results
A total of 30 healthy young volunteers were recruited, but only 28 of them had the first ambulatory blood pressure recording complying with the quality criteria previously defined. Thus, only these 28 patients were randomized to receive placebo or flunitrazepam and included in the analysis. Their mean clinic SBP/DBP values were 125.2 ± 6.9/80.6 mmHg ± 3.3, and their mean resting HR was 75.4 ± 7.8 beats/min.
The main results of this study are shown in Table 1 and in Figs. 1, 2, and 3. Ambulatory monitoring data showed that 24-h SBP, DBP, and HR mean values were not significantly affected by flunitrazepam. Conversely, when analyzing separately the sub-periods of the day, different behaviors were observed. During nighttime (23.00–07.00 h), both treatments preserved the physiologic reduction of BP and HR. However, after flunitrazepam, SBP values were 6% and DBP 6.8% lower than those observed during placebo (both P < 0.05). Night-time HR mean values were unaffected.
In the morning hours (07.00–12.00 h), i.e., during the 5 h following awakening, both the flunitrazepam and placebo administrations preserved the physiologic increase in HR and BP. Nonetheless, BP and HR mean values were significantly higher under flunitrazepam than under placebo: SBP and DBP were higher by 6.4% (+ 7.4 mmHg, P < 0.01) and by 4.6% (+ 3.4 mmHg, P < 0.05), respectively. HR increased by 5.3% (+ 3.9 beat/min, P < 0.05).
During afternoon/evening-time, SBP, DBP, and HR levels were not different in the two treatment groups.
With regard to the adverse effects reported by flunitrazepam-treated volunteers, 25 complained of anterograde amnesia before falling asleep. Twenty-two reported unusual very intense dreams: 11 subjects reported a prevalence of unpleasant dreams or nightmares with aggressive verbal or physical contents; 4 subjects reported a prevalence of pleasant dreams, with frequent sexual contents; 7 subjects did not remember the content of their dreams. Mild difficulty in waking up was reported by 4 subjects and 2 of them also complained of mild somnolence during the first hours of the day. On the contrary, 7 subjects referred to feel particularly active in the morning and more aggressive in dealing with everyday problems during treatment with flunitrazepam. Only 1 subject complained of palpitations in the morning hours.
Discussion
The first main finding of the present study was that chronic oral administration of flunitrazepam (taken in the evening as a hypnotic agent in healthy adults), although not interfering with the physiologic nocturnal fall in BP and HR, led to a significantly greater reduction in nocturnal BP values as compared to placebo. This finding is in accordance with observations made in all human studies to date, where flunitrazepam administration caused an immediate, rapid reduction in BP values [17,18,19,20,21]. However, the findings of these studies are not comparable with our results as those studies were carried out acutely, i.e., following intravenous administration of flunitrazepam to healthy subjects while awake or in patients under stressful conditions. These nocturnal reductions in BP, induced by flunitrazepam in the hours immediately following oral intake, are completely absent in oral administration of diazepam to the same category of subjects [44]. This does not seem to be related to central inhibitory effects [45, 46] but rather to direct vasodilatory effects, which are specific to flunitrazepam [16]. This aspect, however, had never been investigated during nighttime hours following oral administration of the drug.
It should be noted that any drug-related direct vasodilatory action produces an immediate reactive response through counter-regulatory mechanisms, with the activation of arginine, vasopressin, and renin. This is followed by a consequent increase in circulating angiotensin II and sodium retention, as reported with sodium nitroprusside, hydralazine, and nitroglycerine [47,48,49,50,51,52,53,54,55,56]. As a consequence, nocturnal BP behavior under flunitrazepam is likely to be the effect of a balance between the direct vasodilatory effect of flunitrazepam and counter-regulatory forces of activated neurohumoral factors.
Of particular interest is the behavior of nocturnal HR, whose values remained substantially unchanged and similar to placebo values, despite decreases in BP. This lack of HR adaptation to a fall in BP is likely to be related to reductions in baroreflex sensitivity (which is rapidly and greatly affected by flunitrazepam [34,35,36]). In this regard, flunitrazepam differs from diazepam, which does not significantly influence baroreflex sensitivity [38, 39].
The second main finding of this study is that physiological increases in BP and HR following awakening are significantly higher with flunitrazepam than with placebo. This increase in morning BP values has not been observed with diazepam, which, on the contrary, has been reported to cause decreases in SBP [44]. Therefore, increases in morning BP do not seem to be a class effect, but rather a characteristic and specific effect relating to flunitrazepam. The mechanisms underlying this effect are not easily explained; this is also due to a lack of previous similar observations in literature. We can only hypothesize that it could somehow be related to morning reductions in flunitrazepam plasma concentration with the consequent attenuation of direct vasodilatory effects and a prevalence of counter-regulatory neurohumoral responses. Indeed, it has been reported that, when direct vasodilator agents are discontinued, reflex counter-regulatory responses elicited by the agents do not cease immediately, but rather decline slowly over time [47,48,49, 51]. This regards the rebound activation of the sympathetic system, in particular, and of the renin angiotensin system (RAS) to an even greater degree, with consequent persistence of increased levels of angiotensin II (Ang II) and related effects on vascular smooth muscle cells and sodium retention [47,48,49, 51].
Increased values of morning HR could be related, in part, to the above-described counter-regulatory response to vasodilation, and, in part, to rebound activation of the baroreflex system (where reductions in flunitrazepam plasma concentrations might attenuate inhibitory effects of the drug on baroreflex sensitivity). Furthermore, we cannot exclude that, unlike what is found in the periphery, morning concentrations of flunitrazepam in the brain, although reduced, could be high enough to exert a GABA-mediated vagolytic effect, thus contributing to increases in HR.
Adverse events were found to be mild, predictable, and did not necessitate withdrawal of treatment.
To conclude, flunitrazepam, orally administered in the evening to induce sleep, causes a fall in BP which occurs rapidly and persists over the entire sleeping period, without concomitant changes in HR, followed by a transient rebound rise in BP and HR. The mechanisms underlying these cardiovascular effects remain unclear, and their understanding needs further and more detailed pathophysiologic study.
References
Tallman JF, Thomas JW, Gallager DW (1978) GABAergic modulation of benzodiazepine binding site sensitivity. Nature 274(5669):383–385
Treweek JB, Roberts AJ, Janda KD (2010) Superadditive effects of ethanol and flunitrazepam: implications of using immunopharmacotherapy as a therapeutic. Mol Pharm 7(6):2056–2068
Leonard ST, Gerak LR, Delatte MS, Moerschbaecher JM, Winsauer PJ (2009) Relative potency and effectiveness of flunitrazepam, ethanol, and beta-CCE for disrupting the acquisition and retention of response sequences in rats. Behav Pharmacol 20(1):33–44
Collard J (1972) Le contrôle de l’insomnie par une nouvelle benzodiazepine: le flunidazepam ou Ro 5-4200 (investigations princeps de phase I et contrôlée de phase II). Acta Psychiatr Belg 72(6):721–735
Bixler EO, Kales A, Soldatos CR, Kales JD (1977) Flunitrazepam, an investigational hypnotic drug: sleep laboratory evaluations. J Clin Pharmacol 17(10 Pt 1):569–578
Dundee JW, Varadarajan CR, Gaston JH, Clarke RS (1976) Clinical studies of induction agents XLIII: Flunitrazepam. Br J Anaesth 48(6):551–555
George KA, Dundee JW (1977) Relative amnesic actions of diazepam, flunitrazepam and lorazepam in man. Br J Clin Pharmacol 4(1):45–50
Schuler M, Gaillard JM, Tissot R (1972) Comparison of the sleep-inducing effect of Ro 5-4200 and nitrazepam. Psychopharmacologia 27(2):123–130
Monti JM, Altier H (1973) Flunitrazepam (Ro 5-4200) and sleep cycle in normal subjects. Psychopharmacologia 32(4):343–349
McKay AC, Dundee JW (1980) Effect of oral benzodiazepines on memory. Br J Anaesth 52(12):1247–1257
Richardson FJ, Manford ML (1979) Comparison of flunitrazepam and diazepam for oral premedication in older children. Br J Anaesth 51(4):313–317
Randall LO, Kappell B (1973) Pharmacological activity of some benzodiazepines and their metabolites. In: Garrattini S, Mussini E, Randall LO (eds) The Benzodiazepines, Raven, New York, pp 27–51
Mattila MA, Larni HM (1980) Flunitrazepam: a review of its pharmacological properties and therapeutic use. Drugs 20(5):353–374
du Cailar J, Kienlen J (1978) Pharmacology of flunitrazepam. Ann Anesthesiol Fr 19(11–12):1O–9O
DiMicco JA, Abshire VM, Shekhar A, Wilbe JH Jr (1987) Role of GABAergic mechanisms in the central regulation of arterial pressure. Eur Heart J 8(Suppl B):133–138
Pasch T, Bugsch LA (1979) Influence of narcotic analgesics, droperidol, diazepam, and flunitrazepam on the smooth muscles of small arteries. Anaesthesist 28(6):283–289
Coleman AJ, Downing JW, Moyes DG, O'Brien A (1973) Acute cardiovascular effects of Ro5-4200: a new anaesthetic induction agent. S Afr Med J 47(9):382–384
Rifat K, Bolomey M (1975) Les effets cardiovasculaires du rohypnol® utilize comme agent d’induction anesthésique. Ann Anesthesiol Fr 16(3):135–144
Haldemann G, Hossli G, Schaer H (1977) Die anaesthesie mit rohypnol (flunitrazepam) und fentanyl beim geriatrischen patienten. Anaesthesist 26:168–171
Tarnow J, Hess W, Schmidt D, Eberlein HJ (1979) Narkoseeinleitung bei patienten mit koronarer herzkrankheit: flunitrazepam, diazepam, ketamine, fentanyl. A haemodynamic study. Anaesthesist 28(1):9–19
Nitenberg A, Marty J, Blanchet F, Zouioueche S, Baury A, Desmonts JM (1983) Effects of flunitrazepam on left ventricular performance, coronary haemodynamics and myocardial metabolism in patients with coronary artery disease. Br J Anaesth 55(12):1179–1184
Korttila K (1975) The effect of diazepam, flunitrazepam and droperidol with an analgesic on blood pressure and heart rate in man. Arzneimittelforschung 25(8):1303–1306
Fantera A, Fioritti P, Iannarone C, Salzani MC, Ciaschi A (1982) Variazioni respiratorie, cardiocircolatorie ed ossimetriche indotte da flunipazepam nell’uomo. Acta Anaesthesiol Ital 33:507–516
Suzuki N, Beppu S, Okumura H, Uematsu H, Kondo T, Kubota Y (1985) Sedative effects and cardiorespiratory influences of intravenous flunitrazepam premedication. Anesth Prog 32(3):98–103
Duka T, Ackenheil M, Noderer J, Doenicke A, Dorow R (1986) Changes in noradrenaline plasma levels and behavioural responses induced by benzodiazepine agonists with the benzodiazepine antagonist Ro 15-1788. Psychopharmacology 90(3):351–357
Zinzen E, Clarijs JP, Cabri J, Vanderstappen D, Van den Berg TJ (1994) The influence of triazolam and flunitrazepam on isokinetic and isometric muscle performance. Ergonomics 37(1):69–77
Lepage JY, Blanloeil Y, Pinaud M, Helias J, Auneau C, Cozian A, Souron R (1986) Hemodynamic effects of diazepam, flunitrazepam, and midazolam in patients with ischemic heart disease: assessment with a radionuclide approach. Anesthesiology 65(6):678–683
Korttila K, Saarnivaara L, Tarkkanen J, Himberg JJ, Hytönen M (1978) Effect of age on amnesia and sedation induced by flunitrazepam during local anaesthesia for bronchoscopy. Br J Anaesth 50(12):1211–1218
Nimmo WS, Forrest JA, Heading RC, Finlayson ND, Prescott LF (1978) Premedication for upper gastrointestinal endoscopy: a comparative study of flunitrazepam, diazepam and neuroleptanalgesia. Endoscopy 10(3):183–186
Yoshizawa T, Miwa H, Kojima T, Kawakubo Y, Namihisa A, Ohtaka K, Ohkura R, Nishira Y, Toriumi E, Nishira T, Sato N (2003) Low-dose flunitrazepam for conscious sedation for EGD: a randomized double-blind placebo-controlled study. Gastrointest Endosc 58(4):523–530
Stovner J, Endresen R, Osterud A (1973) Intravenous anaesthesia with a new benzodiazepine Ro 5-4200. Acta Anaesthesiol Scand 17(3):163–169
Clarke RS, Lyons SM (1977) Diazepam and flunitrazepam as induction agents for cardiac surgical operations. Acta Anaesthesiol Scand 21(4):282–292
Morel D, Forster A, Gardaz JP, Suter PM, Gemperle M (1981) Comparative haemodynamic and respiratory effects of midazolam and flunitrazepam as induction agents in cardiac surgery. Arzneimittelforschung 31(12a):2264–2267
Wajima Z (1991) Large dose of flunitrazepam attenuates baroreflex control of heart rate in man. J Anesth 5(1):10–16
Rolly G, Lamote P, Cosaert P (1974) Hemodynamic studies of flunitrazepam or RO 05-4200 injection in man. Acta Anaesthesiol Belg 25(3):359–370
Gauzit R, Balagny D, Marty J, Couderc E, Farinotti R (1987) Effets du flunitrazépam sur le contrôle baroréflexe de la fréquence cardiaque et sur l’activité adrénergique. Ann Fr Anesth Reanim 6(4):347–351
Pluskwa F, Bonnet F, Abhay K, Touboul C, Rey B, Marcandoro J, Becquemin JB (1989) Comparaison des profils tensionnels sous flunitrazépam/fentanyl/N2O vs anesthésie péridurale cervicale por chirurgie carotidienne. Ann Fr Anesth Reanim 8(1):26–32
Taneyama C, Goto H, Kohno N, Benson KT, Sasao J, Arakawa K (1993) Effects of fentanyl, diazepam, and the combination of both on arterial baroreflex and sympathetic nerve activity in intact and baro-denervated dogs. Anesth Analg 77(1):44–48
Seitz W, Hempelmann G, Piepenbrock S (1977) Zur kardiovaskulären wirkung von flunitrazepam (Rohypnol®, RO-5-4200). Anaesthesist 26(5):249–256
Hennart D, d'Hollander A, Primo-Dubois J (1982) The haemodynamic effects of flunitrazepam in anaesthetized patients with valvular or coronary artery lesions. Acta Anaesthesiol Scand 26(3):183–188
Senn S (1993) Cross-over trials in clinical research, 1st edn John Wiley, New York, pp 10–53
Groppelli A, Omboni S, Ravogli A, Villani A, Parati G, Mancia G (1991) Validation of the SpaceLabs 90202 and 90207 devices for ambulatory blood pressure monitoring by comparison with intra-arterial resting and ambulatory measurements. J Hypertens Suppl 9:S334–S335
Parati G, Bosi S, Castellano M, Di Rienzo M, Germanò G, Lattuada S, Mormino P, Mos L, Omboni S, Palatini P, Ravogli A, Rizzoni D, Verdecchia P, Zito M (1995) Guidelines for 24-h non-invasive ambulatory blood pressure monitoring. Report from a working group of the Italian Society of Hypertension. High Blood Press 4:168–174
Costa A, Bosone D, Zoppi A, D’Angelo A, Ghiotto N, Guaschino E, Cotta Ramusino M, Fogari R (2017) Effect of diazepam on 24 hour blood pressure and heart rate in healthy volunteers. Pharmacology 101:85–91
Marty J, Gauzit R, Lefevre P, Couderc E, Farinotti R, Henzel C, Desmonts JM (1986) Effects of diazepam and midazolam on baroreflex control of heart rate and on sympathetic activity in humans. Anesth Analg 65(2):113–119
Melsom M, Andreassen P, Melsom H, Hansen T, Grendahl H, Hillestad LK (1976) Diazepam in acute myocardial infarction. Clonical effects and effects on catecholamines, free fatty acids and cortisol. Br Heart J 38:804–810
Cottrell JE, Illner P, Kittay MJ, Steele JM Jr, Lowenstein J, Turndorf H (1980) Rebound hypertension after sodium nitroprusside-induced hypotension. Clin Pharmacol Ther 27(1):32–36
Abukhres MM, Ertel RJ, Dixit BN, Vollmer RR (1979) Role of the renin-angiotensin system in the blood pressure rebound to sodium nitroprusside in the conscious rat. Eur J Pharmacol 58(3):247–254
Khambatta HJ, Stone JG, Khan E (1979) Hypertension during anesthesia on discontinuation of sodium nitroprusside-induced hypotension. Anesthesiology 51(2):127–130
Watanabe H, Kakihana M, Ohtsuka S, Sugishita Y (1998) Efficacy and rebound phenomenon related to intermittent nitroglycerin therapy for the prevention of nitrate tolerance. Jpn Circ J 62(8):571–575
Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO (1991) Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation 84(6):2336–2345
Imhof PR, Rennwald D, Müller P, Howald H, Burkart F (1989) Circulatory counter-regulations induced by continuous administration of nitroglycerin. Methods Find Exp Clin Pharmacol 11(6):409–414
Engelman RM, Hadji-Rousou I, Breyer RH, Whittredge P, Harbison W, Chircop RV (1984) Rebound vasospasm after coronary revascularization in association with calcium antagonist withdrawal. Ann Thorac Surg 37(6):469–472
Weil JV, Chidsey CA (1968) Plasma volume expansion resulting from interference with adrenergic function in normal man. Circulation 37(1):54–61
Takagi H, Dustan HP, Page IH (1961) Relationships among intravascular volume, total body sodium, arterial pressure, and vasomotor tone. Circ Res 9:1233–1239
Dustan HP, Tarazi RC, Bravo EL (1972) Dependence of arterial pressure on intravascular volume in treated hypertensive patients. N Engl J Med 286(16):861–866
Author information
Authors and Affiliations
Contributions
R. Fogari, A. Costa, and D. Bosone contributed to the conception and design of the study.
M. Cotta Ramusino, N. Ghiotto, and G. Perini performed clinical activity, contacted the subjects involved in the research, and collected the data under the supervision of D. Bosone.
A. Costa, A. Zoppi, and A. D’Angelo performed the analysis and the interpretation of the data.
The paper was drafted by R. Fogari and A. Zoppi.
All the authors approved the final version.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bosone, D., Fogari, R., Zoppi, A. et al. Effect of flunitrazepam as an oral hypnotic on 24-hour blood pressure in healthy volunteers. Eur J Clin Pharmacol 74, 995–1000 (2018). https://doi.org/10.1007/s00228-018-2466-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2466-9