Abstract
Purpose
The aim of this study was to evaluate in what measure is dosage adjustment particularly prevalent in pivotal clinical trials of oral targeted therapy drugs approved by the European Medicine Agency as of July 31, 2018, for the treatment of solid tumors.
Methods
We performed a search on the official EMA site on human medicines, using as Keyword Search the ATC Code L01X (other antineoplastic agents); from the list of drugs results, we subsequently excluded antineoplastic drugs for hematological diseases, as well as refused and withdrawn drugs. For all analyzed drugs, we recorded full dosages, dose adjustments with relative reduction percentage, reason for the adjustments, number of patients included in the trial, percentage of patients who reduced their dosage or temporarily discontinued therapy, cause of dose reduction, and presence or absence of reference to a clinical outcome in patients who reduced their dose or discontinued therapy.
Results
We considered 74 pivotal trials on 29 target therapies, of which 56 (76%) provide information on dosage reduction, 41 (55%) on therapy suspension, and 29 (39%) on the dose taken by the sample. Trials that provide information on dosage adjustment include reduction and suspension data widely used to manage side effects; they concern, respectively, 32 and 44% of the samples considered. No trial results take account of the possible role of adjustment in clinical outcomes.
Conclusion
It would be advisable for pivotal clinical trials to give more relevance to dose management, which is a widely used tool for the management of adverse events in clinical practice. To date, such information is lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In clinical trials and in clinical practice, the management of adverse events related to the use of a drug very often involves dose adjustment that consists of temporary therapy suspension or temporary dose reductions, with resumption at full dose upon resolution of the adverse event, or permanent dose reduction until the end of treatment.
As a result, not all patients take the standard or the recommended dose; a percentage of them take a lower dose.
In clinical trials, the correspondence between the recommended dose and the dose delivered to the patient is defined as the relative dose intensity (RDI), expressed as percentage. Some studies have shown that a better RDI corresponds to a better clinical outcome [1, 2]; at the same time, however, it would be useful to have more details about the dose intensities of each arm of the trials [3]. Clinical trials often do not provide data on the number of patients who change their drug dose during chemotherapy. These data should be appropriately explained and described [4]. In target therapies, dose selection is fundamental for therapy optimization in terms of risk/benefit [5]. At present, investigators tend to share and make available data from clinical trials in order to conduct meta- or other statistical analyses that help physicians provide better treatments to patients [6]. For this reason, it is essential to have the most complete results available on all the variables involved in patient management, and, by extension, on treatment efficacy and safety.
The objective of this study was to evaluate how much is dose adjustment prevalent in clinical trials of oral targeted therapy drugs for the treatment of solid tumors, as well as how such adjustment is described and whether dose changes are considered in the results as a factor that may influence a clinical outcome.
Materials and methods
We considered all pivotal clinical trials of oral targeted therapy drugs approved by the European Medicine Agency as of July 31, 2018, for the treatment of solid tumors.
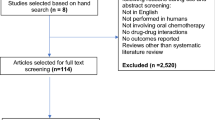
We performed a search on the official EMA site on human medicines, using as Keyword Search the ATC Code L01X (other antineoplastic agents); from the list of drugs results, we subsequently excluded antineoplastic drugs for hematological diseases, as well as refused and withdrawn drugs.
We decided to consider only pivotal clinical trials because they yield data that populate information entered in the Summary of Product Characteristics (SpC) and are taken as reference for dose adjustments in clinical practice. We only considered oral targeted therapy drugs for solid tumors because some studies that evaluate dosage adjustment for conventional chemotherapies already exist, though reviews in the literature that investigate targeted therapies are lacking.
Search for articles: we considered all pivotal articles included in section 5.1 Pharmacodynamic Properties, subsection Clinical Studies, of the SpC.
For all analyzed drugs, we recorded full dosages, dose adjustments with relative reduction percentage, reason for the adjustments, number of patients included in the trial, percentage of patients who reduced their dosage or temporarily discontinued therapy, cause of dose reduction, and presence or absence of reference to a clinical outcome in patients who reduced their dose or discontinued therapy.
Results
From an initial search on the EMA site, we identified 125 drugs, of which 8 are in withdrawal status and 6 in refused status; from the remaining 111, we excluded those prescribed for the treatment for hematological diseases, all non-targeted therapies, and all parenteral therapies.
At the end of our screening, we considered 29 drugs: afatinib, alectinib, axitinib, cabozantinib, ceritinib, cobimetinib, crizotinib, dabrafenib, erlotinib, everolimus, imatinib, lenvatinib, lapatinib, nintedanib, niraparib, olaparib, osimertinib, palbociclib, pazopanib, regorafenib, ribociclib, rupacarib, sonidegib, sorafenib, sunitinib, tivozanib, trametinib, vandetanib, vemurafenib.
Based on the SpC, the 29 drugs call for dosage adjustment for the management of side effects, intended both as dose reduction and temporary suspension of treatment. Only 2 drugs (afatinib and axitinib), according to their SpC, allow for the possibility of reducing or increasing dosage vis-à-vis the recommended dose, if the medicine is well tolerated and if patients have not experienced adverse events.
Table 1 shows the recommended dosage, dose increase, dose reduction with the relative percentage compared to the recommended dosage, and administration suspension with relative number of days contemplated in the SpC.
Of the 29 drugs which allow dose reduction, only 6 accommodate a single-dose reduction: crizotinib, everolimus, osimertinib, erlotinib, tivozanib, sonidegib. The remaining treatments provide instead at least 2 reductions. On the other hand, all drugs allow a temporary interruption for the management of side effects.
We identified a range going from a minimum 20% reduction of crizotinib up to a maximum 67% reduction, compared to the recommended dose for cabozantinib, ribociclib, vandetanib, ceritinib, and dabrafenib. The maximum dose reduction occurs between the dose increased to 10 mg twice per day of axitinib and the minimum dose of 2.5 mg twice per day, with a 75% reduction between the former and the latter dose.
We considered 74 pivotal clinical trials, for a total of 17,637 patients evaluated (Table 2). Of the 74 pivotal clinical trials we considered, 56 (76%) provide information on the number of patients who reduced their dose, and 41 (55%) on the number of dose interruptions. Eleven of the 74 trials, namely 14.8%, for a total of 2377 patients (12.7%), do not provide information about the dosage adjustment, reduction and suspension, and amount of drug taken by the sample.
For the remaining trials, although dosage reductions and therapy suspension are allowed, the number or percentage of patients undergoing adjustment is not specified. In 29 of 74 (39%) trials, the authors referred to the dose taken by the sample, considering as an indicator the RDI expressed as absolute or percentage value, the mean or median dose intensity expressed in milligram, or the percent of patients who took more 80 or 90% of the recommended therapy. Dose adjustment is prevalent in clinical trials. From data presented in the articles, it is evident how dose reductions affect 4475 of 13,856 patients (32%); therapy suspension concerns instead 4730 of 10,814 patients (44%).
The causes of dosage adjustment are rooted in the management of adverse events, and, in particular, grade 3 and grade 4 events of hematological and gastrointestinal nature, fatigue, and increase in the level of transaminases.
Table 3 shows the 29 drugs with one or more indications organized in percent of reduction’s rates, for 10% cutoffs.
In reporting results pertaining to treatment efficacy, pivotal clinical trials do not consider subgroup efficacy analysis on the basis of the total dosage taken.
Discussion
Dosage adjustments are mainly related to the management of side effects, and are prevalent in clinical trials. In most trials, the authors were careful to report the number of patients for whom dosage adjustment was necessary. Less than half of the trials report a general measure of drug intake, usually, but not only, expressed as relative dose intensity; as a result, for most trials, it is impossible to determine what amount of medicine was taken by the sample. Consequently, we are prevented from ascertaining how many patients have taken an adjusted versus a recommended dose. No trial allocates patients to subgroups based on drug intake according to a recommended or an adjusted dose; therefore, the trials on file do not provide any data discussing how the clinical response can vary according to the dose administered and, by extension, on the side effects managed with dose adjustment.
For some pivotal clinical trials, post hoc analyses provide such information: for afatinib [81, 82] and palbociclib [83], no difference between full and reduced dose was found in the clinical outcome, whereas for everolimus, a dose-response model showed that the full dose is more effective than the reduced dose, which, in turn, is more effective than placebo [84]. Although pivotal clinical trials are neither conceived nor designed to evaluate differences in clinical outcomes on the basis of varying drug doses, making such information available would increase the quality of the information, making it more complete and providing a database to produce reviews and meta-analyses on this topic.
As for drug use indices, it would be useful to know with certainty how each patient took the drug and what was his/her clinical outcome. Without sharing such significant data, it would be useful, albeit not exhaustive, to report at minimum, for each arm, the average or total median of the dose taken, the percentage of patients taking more medication than the established cutoff (usually between 80 and 90%) and the RDI.
We believe that sharing such data is fundamental in order to produce dose-response models useful for the optimization of clinical therapy and in clinical practice, as it would provide evidence-based information to support efficacy, or at minimum, dosage adjustments.
Conclusions
It would be advisable for pivotal clinical trials to give more relevance to dosage management, which is a widely used tool for the management of adverse events in clinical practice. This information would help clinicians in managing patients with greater awareness, and in relying on evidence-based data. To date, such information is lacking.
References
Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J (2015) A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol 93(3):203–210
Loibl S, Skacel T, Nekljudova V, Lück HJ, Schwenkglenks M, Brodowicz T, Zielinski C, von Minckwitz G (2011) Evaluating the impact of relative total dose intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer 11(1):131
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw 7(1):99–108
Dale DC, McCarther GC et al (2003) Myelotoxicity and dose intensity of chemotherapy: reporting practices from randomized clinical trials. J Natl Compr Cancer Netw 1(3):440–454
Venkatakrishnan K, Friberg LE et al (2015) Optimizing oncology therapeutics through quantitative translational and clinical pharmacology: challenges and opportunities. Clin Pharmacol Ther 97(1):37–54
Bertagnolli M, Sartor O (2017) Advantages of a truly open-access data-sharing model. N Engl J Med 376(12):1178–1181
Sequist L, Yang V, Yamamoto JC-H et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334
Park K, Tan EK et al (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17(5):577–589
Soria JP, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, Göker E, Georgoulias V, Li W, Isla D, Guclu SZ, Morabito A, Min YJ, Ardizzoni A, Gadgeel SM, Wang B, Chand VK, Goss GD, LUX-Lung 8 Investigators (2015) Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16(8):897–907
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222
Yang JCH, SHIH JY et al (2012) Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 13(5):539–548
Solange P, Camidge DR et al (2017) Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med 377(9):829–838
Ou SHI, Ahn JS, de Petris L, Govindan R, Yang JCH, Hughes B, Lena H, Moro-Sibilot D, Bearz A, Ramirez SV, Mekhail T, Spira A, Bordogna W, Balas B, Morcos PN, Monnet A, Zeaiter A, Kim DW (2016) Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol 34(7):661–668
Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, Chao BH, Borghaei H, Gold KA, Zeaiter A, Bordogna W, Balas B, Puig O, Henschel V, Ou SI, study investigators (2016) Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17(2):234–242
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Géczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ, METEOR Investigators (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1814–1823
Cho BC, Kim DW (2017) ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non–small cell lung cancer (NSCLC). J Thorac Oncol 12(9):1357–1367
Soria JC, Tan DSW (2017) First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389(10072):917–929
Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, Novello S, Bearz A, Gautschi O, Mok T, Nishio M, Scagliotti G, Spigel DR, Deudon S, Zheng C, Pantano S, Urban P, Massacesi C, Viraswami-Appanna K, Felip E (2017) Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18(7):874–886
Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, Vansteenkiste J, Sharma S, de Pas T, Riely GJ, Solomon BJ, Wolf J, Thomas M, Schuler M, Liu G, Santoro A, Sutradhar S, Li S, Szczudlo T, Yovine A, Shaw AT (2016) Intracranial and whole-body response of ceritinib in ALK inhibitor-naïve and previously ALK inhibitor-treated patients with ALK-rearranged non-small-cell lung cancer (NSCLC): updated results from the phase 1, multicentre, open-label ASCEND-1 trial. Lancet Oncol 17(4):452–463
Crinò L, Ahn MJ, de Marinis F, Groen HJM, Wakelee H, Hida T, Mok T, Spigel D, Felip E, Nishio M, Scagliotti G, Branle F, Emeremni C, Quadrigli M, Zhang J, Shaw AT (2016) Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non–small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol 34(24):2866–2873
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371(20):1867–1876
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371(23):2167–2177
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, de Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O’Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA (2013) Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368(25):2385–2394
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SHI, Kim DW, Salgia R, Fidias P, Engelman JA, Gandhi L, Jänne PA, Costa DB, Shapiro GI, LoRusso P, Ruffner K, Stephenson P, Tang Y, Wilner K, Clark JW, Shaw AT (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13(10):1011–1019
Hauschild AG, Demidov JJ et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380(9839):358–365
Long GV, Trefzer U et al (2012) Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13(11):1087–1095
Ascierto PA, Minor D, Ribas A et al (2013) Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 31(26):3205–3211
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, Souquet PJ, Smit EF, Groen HJM, Kelly RJ, Cho BC, Socinski MA, Pandite L, Nase C, Ma B, D’Amelio A Jr, Mookerjee B, Curtis CM Jr, Johnson BE (2016) Dabrafenib in BRAF V600E–mutant advanced non-small cell lung cancer: an open-label, single arm, multicenter, phase 2 trial. Lancet. Oncol 17(5):642–650
Long GV, Stroyakovskiy D et al (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888
Planchard D, Besse B, Groen HJM et al (2016 July) An open-label phase 2 trial of dabrafenib plus trametinib in patients with previously treated BRAF V600E–mutant metastatic non-small cell lung cancer. Lancet Oncol 17(7):984–993
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, de Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Cappuzzo F, Ciuleanu T et al (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11(6):521–529
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2005) Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med 353(2):123–132
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials Group (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone- receptor–positive advanced breast cancer. N Engl J Med 366(6):520–529
Motzer RJ, Escudier B et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
Yao JC, PAvel M et al (2016) Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 Study. J Clin Oncol Off J Am Soc Clin Oncol 34(32):3906–3913
Yao JC, Pavel M, Lombard-Bohas C, van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K (2016) Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 Study. J Clin Oncol Off J Am Soc Clin Oncol 34(32):3906–3913
Demetri GD, Von Mehren M et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VHC, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CDM, Crowley JJ, Borden EC (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26(4):626–632
Pivot X, Manikhas A et al (2015) CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2 – positive metastatic breast cancer. J Clin Oncol 33(14):1564–1573
Johnston S, Pippen J et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol 27(33):5538–5546
Baselga J, Bradbury I, Eidtmann H, di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, NeoALTTO Study Team (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379(9816):633–640
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de la Sheras B, Zhu J, Sherman SI (2015) Lenvatinib versus placebo in radioiodine- refractory thyroid cancer. N Engl J Med 372(7):621–630
Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The lancet oncology 16(15):1473–1482
Reck M, Kaiser R et al (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15:143–155
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA, ENGOT-OV16/NOVA Investigators (2016) Niraparib maintenance therapy in platinum-sensitive, Recurrent Ovarian Cancer. N Engl J Med 375(22):2154–2164
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366(15):1382–1392
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators (2018) Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med 378(2):113–125
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, AURA3 Investigators (2017) Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 376(7):629–640
Yang JCH, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, Blackhall F, Haggstrom D, Yoh K, Novello S, Gold K, Hirashima T, Lin CC, Mann H, Cantarini M, Ghiorghiu S, Jänne PA (2017) Osimertinib in pretreated T790M-positive advanced non–small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35(12):1288–1296
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Diéras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C, Randolph S, Koehler M, Cristofanilli M, PALOMA3 Study Group (2015) Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 373(3):209–219
Sternberg CN, Davis ID et al (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6):1061–1068
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, de Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369(8):722–731
Van der Graaf WTA, Blay JY et al (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379(9829):1879–1886
Grothey A, Van Cutsem E et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312
Li J, Shukui Q et al (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 16(6):619–629
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O’Malley DM, Kristeleit RS, Ma L, Bell-McGuinn KM, Brenton JD, Cragun JM, Oaknin A, Ray-Coquard I, Harrell MI, Mann E, Kaufmann SH, Floquet A, Leary A, Harding TC, Goble S, Maloney L, Isaacson J, Allen AR, Rolfe L, Yelensky R, Raponi M, McNeish IA (2017) Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 18(1):75–87
Kristeleit R, Shapiro GI, Burris HA, Oza AM, LoRusso P, Patel MR, Domchek SM, Balmaña J, Drew Y, Chen LM, Safra T, Montes A, Giordano H, Maloney L, Goble S, Isaacson J, Xiao J, Borrow J, Rolfe L, Shapira-Frommer R (2017) A phase I – II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2 -mutated ovarian carcinoma or other solid tumors. Clin Cancer Res 23:4095–4106
Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kudchadkar R, Trefzer U, Gogov S, Pallaud C, Yi T, Mone M, Kaatz M, Loquai C, Stratigos AJ, Schulze HJ, Plummer R, Chang ALS, Cornélis F, Lear JT, Sellami D, Dummer R (2015) Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol 16(6):716–728
Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, Justice R, Pazdur R (2009) Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 14(1):95–100
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ, DECISION investigators (2014) Sorafenib in locally advanced or metastatic, radioactive iodine-refractory, differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet 384(9940):319–328
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, Mckinley A, Ou WB, Fletcher JA, Fletcher CDM, Huang X, Cohen DP, Baum CM, Demetri GD (2008) Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 26(33):5352–5359
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368(9544):1329–1338
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24
Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295(21):2516–2524
Kulke MH, Lenz HJ et al (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26(20):3403–3410
Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, Kogan M, Alekseev BY, Sternberg CN, Szczylik C, Cella D, Ivanescu C, Krivoshik A, Strahs A, Esteves B, Berkenblit A, Hutson TE (2013) Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol Off J Am Soc Clin Oncol 31(30):3791–3799
Flaherty KT, Robert C, Hersey P et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367(2):107–114
Wells SA Jr, Robinson BG et al (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30(2):134–141
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur G, BRIM-3 Study Group (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A (2012) Survival in BRAF V600–mutant advanced melanoma treated with vemurafenib. N Engl J Med 366(8):707–714
McArthur GA, Maio M et al (2016) Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol 28(3):634–641
Kato T, Yoshioka H, Okamoto I, Yokoyama A, Hida T, Seto T, Kiura K, Massey D, Seki Y, Yamamoto N (2015) Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci 106(9):1202–1211
Yang JCH, Sequist LV, Zhou C, Schuler M, Geater SL, Mok T, Hu CP, Yamamoto N, Feng J, O’Byrne K, Lu S, Hirsh V, Huang Y, Sebastian M, Okamoto I, Dickgreber N, Shah R, Märten A, Massey D, Wind S, Wu YL (2016) Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 27(11):2103–2110
Verma S, Bartlett CH et al (2016) Palbociclib in combination with fulvestrant in women with hormone receptor-positive/her2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 21(10):1165–1175
Stein A, Wang W, Carter AA, Chiparus O, Hollaender N, Kim H, Motzer RJ, Sarr C (2012) Dynamic tumor modeling of the dose–response relationship for everolimus in metastatic renal cell carcinoma using data from the phase 3 RECORD-1 trial. BMC Cancer 12(1):311
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lasala, R., Santoleri, F., Romagnoli, A. et al. Dosage adjustments in pivotal clinical trials with oral targeted therapies in solid tumors conducted in Europe. Eur J Clin Pharmacol 75, 697–706 (2019). https://doi.org/10.1007/s00228-018-02621-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-02621-w