Abstract
Background
Cysteinyl leukotrienes (LTC4, LTD4, and LTE4) are pro-inflammatory mediators of the 5-lipooxygenase (5-LO) pathway, that play an important role in bronchoconstriction, but can also enhance endothelial cell permeability and myocardial contractility, and are involved in many other inflammatory conditions. In the late 1990s, leukotriene receptor antagonists (LTRAs) were introduced in therapy for asthma and later on, approved for the relief of the symptoms of allergic rhinitis, chronic obstructive pulmonary disease, and urticaria. In addition, it has been shown that LTRAs may have a potential role in preventing atherosclerosis progression.
Purpose
The aims of this short review are to delineate the potential cardiovascular protective role of a LTRA, montelukast, beyond its traditional use, and to foster the design of appropriate clinical trials to test this hypothesis.
Results and Conclusions
What it is known about leukotriene receptor antagonists? |
•Leukotriene receptor antagonist, such as montelukast and zafirlukast, is used in asthma, COPD, and allergic rhinitis. • Montelukast is the most prescribed CysLT1 antagonist used in asthmatic patients. • Different in vivo animal studies have shown that leukotriene receptor antagonists can prevent the atherosclerosis progression, and have a protective role after cerebral ischemia. |
What we still need to know? |
• Today, there is a need for conducting clinical trials to assess the role of montelukast in reducing cardiovascular risk and to further understand the mechanism of action behind this effect. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
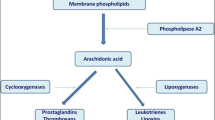
Arachidonic acid pathway
Arachidonic acid (AA) (5, 8, 11, 14-eicosatetraenoic acid) is a polyunsaturated fatty acid, abundant in cell membrane phospholipids and mainly released upon activation of cytosolic phospholipase A2 (cPLA2). Soon after its release, AA is further metabolized by several enzymatic and non-enzymatic pathways, such as cyclooxygenase (COX) and lipoxygenase (LOX) pathways.
The COX enzyme, also known as PGH synthase (PGHS), gives rise to the production of cyclic endoperoxides (PGG2-PGH2), hence to different prostaglandins (PGs), i.e., PGD2, PGE2, PGF2α, and prostacyclin (PGI2), and thromboxane (TXA2). Two isoforms of this enzyme have been identified [1]: COX-1, the constitutive isoform, involved in various processes such as thromboxane synthesis in platelets, PGI2 production in vascular endothelium, and prostanoid generation at gastric mucosal level. COX-2 [2, 3], on the other hand, is the inducible isoform involved in various processes, particularly inflammation. It is expressed in response to pro-inflammatory stimuli (different traumas, heat, cytokines, etc.), causing an increase synthesis of prostanoids that contribute to the classical signs and symptoms of the inflammatory process [4].
Arachidonic acid and 5-lipooxygenase pathway
LOXs are a group of enzymes that metabolize AA into monohydroxylated products, namely hydroperoxyeicosatetraenoic acid (HPETE). These enzymes are classified as 5-LO, 12-LO, and 15-LO according to the site of hydroperoxy group insertion [5].
The 5-LO pathway gives rise, mainly, to the production of leukotrienes (LTs). LTs may be classified in two groups: the dihydroxy acid, represented by leukotriene B4 (LTB4), and peptide leukotrienes or cysteinyl leukotrienes (cysteinyl-LTs) (Fig. 1). LTB4 exerts its action through binding to two officially recognized GPCRs, BLT1 (expressed in leukocytes and monocytes) and BLT2 [6–8], while cysteinyl-LTs bind to CysLT1 and CysLT2 receptors [7–9]. CysLT1 and CysLT2 are also GPCRs that share only a 38% amino acid identity and that are expressed by the cells of the immune system, (B cells, T cells, macrophages, monocytes, etc.) [10], by vascular smooth muscle (SMCs), and by endothelial cells (ECs) [11].
5-LO pathway. Leukotrienes are produced via 5-LO pathway. They are classified in leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysteinyl-LTs: LTC4, LTD4, LTE4). Abbreviations: cPLA 2 cytosolic phospholipase A2, AA arachidonic acid, 5-LO 5-lipoxygenase, 5-HPETE 5-hydroperoxyeicosatetraenoic, 5-HETE 5- hydroxyeicosatetraenoic acid, LTA 4 leukotriene A4, LTB 4 leukotriene B4, LTC 4 leukotriene C4, LTD 4 leukotriene D4, LTE 4 leukotriene E4
LTs are responsible for a series of activities such as contraction of bronchial SMC, accumulation of leukocytes at the inflammatory sites, increase in permeability of postcapillary venules, and the formation of edema [7]. In particular, a number of reviews highlight that cysteinyl-LTs, (LTC4, LTD4, and LTE4), besides their preeminent role in asthma and in inflammation [11, 12], can also enhance the cardiovascular (CV) risk [13–16]; they are, indeed, important mediators of the inflammatory process that characterize several CV pathologies [17]. Over the years, cysteinyl-LTs have been demonstrated to be a potent coronary artery vasoconstrictor [18], to stimulate proliferation of arterial SMCs [19], and to increase platelet-activating factor (PAF) synthesis [20], von Willebrand factor secretion [21], and P-selectin surface expression [22] in cultured ECs.
Myocardial infarction (MI), stroke, atherosclerosis, and aortic aneurysms are some of the CV events characterized by an increase generation of LTs. Urinary excretion of LTE4 has been reported to be increased in patients with cardiac ischemia [23, 24], as well as in coronary artery disease and after coronary artery bypass surgery [25], or in the urine of rabbits after coronary artery ligation [26]. In addition, transcellular biosynthesis of LTs takes place when neutrophils adhere to coronary ECs and, following activation, induce formation of cysteinyl-LTs and a slow increase in coronary tone and vascular edema responsible of cardiac damage [27, 28]. Interestingly, two human genetic studies have correlated polymorphism of the 5-LO pathway with relative risk for MI, stroke, and atherosclerosis focusing attention to LTs in the pathogenesis of these diseases [29, 30].
A first report on targeted gene disruption reveals the role of CysLT1 in the enhanced vascular permeability of mice undergoing acute inflammatory responses [31], while transcripts of FLAP and LT receptors have been identified in specimens of arterial walls of patients affected by atherosclerosis of the aorta and of coronary and carotid arteries [32]. More recently, CysLT1 receptor expression in human aortic valve myofibroblasts has been demonstrated to correlate with severity of stenosis [33], while lipopolysaccharide stimulation induces its perinuclear expression in human coronary artery vascular SMCs, a localization that correlates with an increase in mRNA levels for plasminogen activator inhibitor (PAI)-2, a well-known pro-atherosclerotic gene [34].
In addition, cysteinyl-LTs have been also associated with neuronal injury after brain ischemia [35, 36], apparently through the activation of both CysLT1 and CysLT2 receptors [37–40].
Thus, a number of studies reported that cysteinyl-LTs and their receptors play a prominent role in the increase of vasoconstriction, reduction of coronary blood flow, atherosclerosis, hypertension, myocardial ischemia, and stroke [17, 41–45].
Leukotriene modifiers
After vast evidences that cysteinyl-LTs are correlated with the pathophysiology of asthma and allergic rhinitis (AR) [46–50], leukotriene receptor antagonists (LTRAs) were developed in the late 1990s and were approved for the use in asthmatic patient [51–58]. The first LTRAs developed, i.e., montelukast, pranlukast, zafirlukast, and pobilukast are, mainly, inhibitors of CysLT1 receptor [59], while newer compounds are either non selective inhibitor of both CysLT1 and CysLT2 (BAY u9773) [60, 61], or more selective inhibitors of CysLT2 (Bay CysLT2 [62, 63] and HAMI 3379 [64]).
While it is not been approved by EMA (European Medicines Agency), the 5-LO inhibitor zileuton is on the market in the USA for the treatment of asthma. However, 4.6% of patients receiving zileuton had serum alanine transaminase elevations [65], and this side effect combined with a relatively low potency of the drug and its unfavorable therapeutic regimen has limited its use in the clinical practice.
However, 5-LO and FLAP inhibitors, due to their broader pharmacological profile, have been tested in animal models of CV diseases, with varied results. Indeed, significant reduction of infarct size, inflammation, atherosclerotic lesion, and intima-media thickness has been observed with some of these compounds [28, 66–72], while the 5-LO inhibitor and LTRA REV-5901 [73] failed to reduce the extent of MI size following occlusion-reperfusion injury [74]. Furthermore, studies using 5-LO-deficient mice did not reveal any difference in MI area or atherosclerosis vs. controls [75, 76], while two 5-LO inhibitors showed opposite effects in apoE−/− [77] and in LDLR−/− mice [78].
A prospective clinical trial with DG-031 conducted in patients with the at-risk variants of FLAP and LTA4-H showed a significant reduction of biomarkers associated with increased risk of MI [79]. More recently, in two small clinical trials in patient with recent acute coronary syndrome, reduction in non-calcified plaque volume [80] and a slower plaque progression [81] have been observed with the new 5-LO inhibitor VIA-2291 (atreleuton), proving that a reduction in LT production may influence atherosclerosis in humans.
However, 5-LO inhibition will also cause inhibition of specialized pro-resolving lipid mediators such as lipoxin (LXs) and resolvins [82], which may contribute to the resolution of the inflammatory process at the base of many cardiovascular diseases [83, 84]. For example, CGEN-855A, an ALX/FPR2 peptide agonist, provided protection against ischemia-reperfusion injury through inhibition of PMN recruitment to the myocardium [85]. Furthermore, an epi-lipoxin stable analogs inhibited human EC proliferation and migration [86], while inducing protection against pro-oxidant insults [87] and suppression of NAD(P)H oxidase-mediated ROS generation [88], strongly indicating that LXs may play a protective role against the development and progression of CVDs. In addition, resolving E1 (RvE1) has been demonstrated to reduce ADP-stimulated P-selectin mobilization and actin polymerization and may contribute to both resolution of vascular inflammation and ADP-dependent platelet activation [89]. Taken together, as it is also the case for the COX pathway, these results suggest that altering the equilibrium between pro-inflammatory and anti-inflammatory AA metabolites might not be the most appropriate pharmacological strategy to pursue. Rather, a selective inhibition of a specific target (i.e., receptor), avoiding disruption of the intricate inter-eicosanoid balance and its physiological consequences, should provide additional selectivity to the therapeutic intervention and reduce unwanted side effects [90].
Leukotriene receptor antagonist, montelukast
LTRAs such as montelukast, zafirlukast, or pranlukast can be used in AR [91] or other pathological situations such as urticaria, allergic fungal conjunctivitis, paranasal sinus disease, and atopic dermatitis [58]. In this context, montelukast, a potent antagonist of the CysLT1 receptor subtype, is currently used in patients with different levels of asthma severity or chronic obstructive pulmonary disease (COPD) as an add-on therapy to inhaled corticosteroids. In addition, montelukast alleviates the symptoms present in AR, like eye and throat irritation, and it is commonly prescribed as a monotherapy or in association with antihistamine drugs [92, 93]. Adachi et al. [94] demonstrated an improvement of FEV1 in acute asthma in patients taking intravenous montelukast compared to placebo, irrespectively of the concurrent use of inhaled corticosteroids [95]. These results are in line with the Montelukast Asthma Study Group demonstrating an improvement of FEV1 in patients taking oral or intravenous montelukast compared to placebo [96, 97]. Moreover, data from the Rubeinstein et al. study demonstrated an improvement of nocturnal symptoms, a reduction of the number and duration of hospitalizations, and emergency visit in COPD patients taking montelukast [98], while similar results were also obtained in pulmonary function and quality of life tests [99]. While LTRAs as monotherapy improved asthma control compared with placebo both in adults and children [100] and some recent clinical trials seem to suggest that they are equivalent to an inhaled glucocorticoid as first-line controller therapy at least in the short period [101], the more recent systematic review of randomized clinical trials available concluded that as monotherapy, inhaled corticosteroids display superior efficacy to LTRAs in adults and children with persistent asthma [102]. Montelukast is a well-tolerated anti-asthmatic drug that can also be used in children [103–105] for its anti-inflammatory and bronchoprotective action and is usually administrated in the oral dosage form.
In this context, several studies retain a beneficial effect of montelukast in cystic fibrosis [106, 107] in the respiratory syncitial virus (RSV) bronchiolitis [108], and in the treatment of sepsis [109]. Montelukast and zafirlukast have also been reported to inhibit the activation of NF-κB in a human monocyte/macrophage cell line [110–112].
Montelukast is metabolized by the cytochromes P450, 2C9, and 3A4 in the liver [113]. Currently, no biomarker to assess the response to montelukast exists, considering that a number of patients, particularly children, do not have a better response to montelukast treatment compared to inhaled corticosteroids [114]. Moreover, several studies have reported that even though montelukast is considered as a safe drug, its use is sometimes associated with Churg-Strauss syndrome, depression, suicidal thinking, anxiety, and sleep disturbances [115–118].
In vivo animal studies on the role of montelukast on the cardiovascular disease
Considering the emerging role of cysteinyl-LTs in the pathophysiology of CV disease (CVDs), several studies have been conducted to assess the role of LTRAs in animal models of CVDs.
LTRAs such as montelukast or pranlukast have been shown to have a protective role after cerebral ischemia [15] and reduce the blood-brain barrier permeability as well as brain injuries in several experimental models, in vivo [119–125]. Saad MA. et al.’s findings demonstrated that montelukast had a neuroprotective effect mediated through its antioxidant, antiapoptotic, anti-inflammatory and mechanism, in cerebral ischemia/reperfusion (I/R) injury in rats [126]. In line with that, another study has shown a neuroprotective effect in spinal cord I/R injury in rats treated with montelukast [127]. Indeed, a number of articles have reported the involvement of also CysLT2 in the inflammatory process subsequent to a brain vascular insult [59, 128–130]. In accordance with the previously mentioned results, evidences have been provided of the protective role of HAMI 3379, a selective CysLT2 antagonist, against brain injury after focal cerebral ischemia in rats [131, 132] or in ischemia-like neuronal injury [133].
In addition, in vivo studies in the mouse revealed that montelukast can reduce the production of vascular reactive oxygen species (ROS), improving EC function, inhibiting atherosclerotic damaged area and intimal hyperplasia [134, 135] as well as decreasing atherosclerotic plaque generation [136]. In line with these data, other studies in rabbits indicate that LTRAs can prevent atherosclerosis progression, and that it inhibits neointimal hyperplasia, increases SMC, and decreases macrophage accumulation and the expression of matrix metalloproteinases (MMP-2 and MMP-9), which have an essential role in the development of atherosclerotic plaque [137]. Interestingly, in a rabbit carotid balloon injury model conducted to investigate the effect of montelukast on atherosclerosis compared to atorvastatin, the results indicate no significant differences between the two drugs in reducing neointima and MCP-1 expression. However, atorvastatin reduces also the trigycerides and LDL levels, unlike montelukast, suggesting that montelukast exerts its anti-atherogenic effect through the MCP-1 down regulation [138]. Becher et al. found that the inhibition of LTC4 activity in mice by montelukast reduces oxidative stress and apoptosis in cardiomyocytes having a beneficial effect on myocardium remodeling after left ventricular injury [139]. In this context, in apoE−/− mice, an attenuated myocardial necrosis area was found after acute hypoxia, suggesting that montelukast exerts a cardio-protective role during myocardial injury likely improving the oxygen supply to areas of myocardial ischemia [140].
Other in vivo animal studies have shown that montelukast can prevent the liver and intestine injury by reducing apoptosis and oxidative stress in a hepatic I/R model [141], can protect against I/R-induced intestinal damage [142, 143] and liver injury in rats improving hepatic structure and function [144], and can prevent damage in a model of colonic anastomotic wound healing [145]. In addition, montelukast has been demonstrated to prevent I/R-induced damage in rat ovary [146, 147] and testis [148], to protect against renal and bladder I/R injury by its anti-inflammatory and antioxidant properties [149, 150], and has been found effective in ameliorating I/R-induced vasculitic neuropathic pain [151], as well as, in association with iloprost, in reducing I/R damage in transient spinal cord ischemia [152].
Complementary studies also conducted in animals making use of other LTRAs such as zafirlukast or pranlukast mostly confirmed the montelukast data. Haga et al. demonstrated that zafirlukast reduces the severity of ischemic acute renal failure, through the anti-inflammatory action [153], while other studies suggested that zafirlukast can be added to combined therapy of necrotic, hypoxic, and ischemic injuries of the myocardium [154]. In addition, De Clue et al. confirmed that zafirlukast ameliorated the acute hypotensive response to endotoxin in cats, but without significant effect in the heart rate and TNF or IL-6 production [155]. These data were also confirmed for another LTRA, pranlukast, which reduced infarct size [156], attenuated hydrogen peroxide-induced necrosis [157] and ischemia-like injury in ECs [158], suggesting that pranlukast can be a potential therapeutic alternative for the treatment of ischemic stroke.
However, some contradictory results have also been published, likely due to the use of old, non-selective, and reduced potency CysLT receptor antagonists (see [45] for a recent review).
Clinical trials on the role of montelukast in the prevention of CV diseases
Taken together, all these in vivo animal data strongly indicate a potential role of montelukast in reducing the CV risk. Regarding the inflammatory response, present not only in pathological situation such as asthma but also in carotid and coronary atherosclerosis, it can be hypothesized that montelukast might be useful to prevent the MI or stroke in humans. However, a very limited number of studies have been conducted in humans to further confirm the results obtained in the animal models.
In support to this hypothesis, Allayee et al. group conducted a clinical trial investigating the role of montelukast and low-dose theophylline on CVD risk factors, such as inflammatory biomarkers and lipid levels. The result demonstrate a reduction of C-reactive protein levels, as well as reduced levels of all lipids such as total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density cholesterol (HDL-C) in patients taking montelukast compared to that in placebo. Despite the reduction of HDL-C levels, the result suggests that the asthmatic patient group taking montelukast has lower levels of CVD-associated inflammatory biomarkers and lipid levels [159]. More recently, Ingelsson et al. conducted a retrospective study in a nationwide population-based cohort of approximately 7 million Swedish hypothesizing that there may be a potential role of montelukast for secondary prevention of CVDs [160]. The cohort consisted of all Swedish residents older than 18 years with a follow-up period from July 1, 2005 to December 31, 2008. Patients were considered exposed when they utilized montelukast for at least 3 continual months. Other dispensed medications, gender, age, comorbidity, and socioeconomic status were also assessed. Montelukast use was analyzed as a time-dependent variable, and the data demonstrated a reduction of the risk for recurrent stroke in patients exposed to the drug (HR, 0.62; 95% CI, 0.38–0.99), accompanied with a lower risk for recurrent MI in male subjects (HR, 0.65; 95% CI, 0.43–0.99). Interestingly, the results demonstrated that only patients not taking angiotensin-converting enzyme (ACE) inhibitors had a decreased risk for recurrent stroke (HR, 0.34; 95% CI, 0.14–0.82) associated to montelukast [160].
Despite that these findings are in line with the protective role of LTRAs obtained in in vivo animal models, additional clinical trials should be performed to confirm this hypothesis and to evaluate the mechanism of action behind this additional role of montelukast. In particular, as the assumption of anti-asthmatic drug is a chronic condition that might continue for the entire life, the time of exposure to LTRAs should be extended.
Final considerations
Inflammation is a key process for the cerebro-vascular diseases, and LTs, important mediators of inflammation, are involved in a variety of inflammatory diseases beyond asthma [5, 11]. In particular, cysteinyl-LTs have been postulated to contribute to CVDs such as atherosclerosis and ischemia [44, 45], inducing the proliferation of endothelial [161] and SMCs [19, 134, 162], thus provoking vasoconstriction and reduction of coronary blood flow. These observations strongly suggest that the anti-inflammatory strategy, and particularly LTRAs, could be effective in treating and preventing atherosclerosis. At present, however, LTRAs in general and montelukast in particular have been approved only for the treatment of asthma and both seasonal and perennial AR [163].
The results obtained in in vivo animal models and in yet limited human clinical trial, however, suggest that montelukast, the most prescribed LTRA, can reduce the risk of CV events. Moreover, these results are in line with the findings from other animal studies using different LTRAs such as pranlukast or zafirlukast. Despite using different animal models such as mice, rats, rabbits, or cats and measuring different parameters, all the studies give evidence of a potential protective role of montelukast with respect to CV and cerebral events through its antiapoptotic and anti-inflammatory activities. Neurological deficit score, infarct volume, brain edema, neuron density, cytokine production, lesion volume, blood pressure, lipid levels, macrophage content, intima thickness, lactate dehydrogenase activity, and oxidative stress biomarkers are some of the parameters measured in these animal studies to further evaluate the protective effect of LTRAs [164].
Although there are a limited number of studies in humans, Ingelsson’s contribution is an important piece of information for further confirmation of this speculation [160]. This, hopefully, can open the way towards other uses of montelukast, beyond its traditional use, which can be especially important for patients with high CV risk factors, considering also that asthmatic patient can have a higher risk for developing CVDs [165]. Montelukast, despite being a highly selective CysLT1 antagonist [59, 62], has a broader spectrum of pharmacological effect in vivo beyond its recognized efficacy in airway diseases [118, 166], while the mechanism behind its cardio-protective effect is not yet known, it can probably can be attributed to its anti-inflammatory activity.
In summary, the results of the current work seem to provide evidences that montelukast can be used to prevent CVDs in humans and inhibit the atherosclerosis development in in vivo animal models. We believe that this postulated beneficial effect of montelukast in the CV system can lead to alternate strategies to treat asthma, COPD, AR, urticaria, or other inflammatory diseases in patients with a high CV risk by using a single drug that will provide an anti-inflammatory and preventing effect also for the CV system.
References
Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA (1999) Arachidonic acid oxigenation by COX-1 and COX-2. J Biol Chem 274:22903–22906
Funk CD, Funk LB, Kennedy ME, Pong AS, Fitzgerald GA (1991) Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J 5(9):2304–2312
Takahashi Y, Ueda N, Yoshimoto T, Yamamoto S, Yokoyama C, Miyata A, Tanabe T, Fuse I, Hattori A, Shibata A (1992) Immunoaffinity purification and cDNA cloning of human platelet prostaglandin endoperoxide synthase (cyclooxygenase). Biochem Biophys Res Commun 182(2):433–438
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Haeggstrom JZ, Funk CD (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev 111(10):5866–5898
Yokomizo T, Izumi T, Shimizu T (2001) Leukotriene B4: metabolism and signal transduction. Arch Biochem Biophys 385(2):231–241
Back M, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE (2011) International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol Rev 63(3):539–584
Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE (2014) International Union of Basic and Clinical Pharmacology. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Br J Pharmacol 171(15):3551–3574
Capra V (2004) Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacol Res 50(1):1–11
Kanaoka Y, Boyce JA (2004) Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol 173(3):1503–1510
Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE (2007) Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev 27(4):469–527
Nicosia S, Capra V, Rovati GE (2001) Leukotrienes as mediators of asthma. Pulm Pharmacol Ther 14(1):3–19
Lotzer K, Funk CD, Habenicht AJ (2005) The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta 1736(1):30–37
Riccioni G, Capra V, D'Orazio N, Bucciarelli T, Bazzano LA (2008) Leukotriene modifiers in the treatment of cardiovascular diseases. J Leukoc Biol 84(6):1374–1378
Bäck M (2009) Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther 23(1):41–48
Riccioni G, Bäck M, Capra V (2010) Leukotrienes and atherosclerosis. Curr Drug Targets 11(7):882–887
Bäck M (2007) Leukotriene receptors: crucial components in vascular inflammation. ScientificWorldJournal 7:1422–1439
Michelassi F, Landa L, Hill RD, Lowenstein E, Watkins WD, Petkau AJ, Zapol WM (1982) Leukotriene D4: a potent coronary artery vasoconstrictor associated with impaired ventricular contraction. Science 217(4562):841–843
Porreca E, Di Febbo C, Di Sciullo A, Angelucci D, Nasuti M, Vitullo P, Reale M, Conti P, Cuccurullo F, Poggi A (1996) Cysteinyl leukotriene D4 induced vascular smooth muscle cell proliferation: a possible role in myointimal hyperplasia. Thromb Haemost 76(1):99–104
McIntyre TM, Zimmerman GA, Prescott SM (1986) Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A 83(7):2204–2208
Datta YH, Romano M, Jacobson BC, Golan DE, Serhan CN, Ewenstein BM (1995) Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-selectin surface expression in human umbilical vein endothelial cells. Circulation 92(11):3304–3311
Pedersen KE, Bochner BS, Undem BJ (1997) Cysteinyl leukotrienes induce P-selectin expression in human endothelial cells via a non-CysLT1 receptor-mediated mechanism. Journal of Pharmacology & Experimental Therapeutics 281(2):655–662
Carry M, Korley V, Willerson JT, Weigelt L, Ford-Hutchinson AW, Tagari P (1992) Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo evidence for 5-lipoxygenase activation. Circulation 85(1):230–236
De Caterina R, Giannessi D, Lazzerini G, Bernini W, Sicari R, Cupelli F, Lenzi S, Rugolotto MM, Madonna R, Maclouf J (2010) Sulfido-peptide leukotrienes in coronary heart disease—relationship with disease instability and myocardial ischaemia. Eur J Clin Investig 40(3):258–272
Allen SP, Sampson AP, Piper PJ, Chester AH, Ohri SK, Yacoub MH (1993) Enhanced excretion of urinary leukotriene E4 in coronary artery disease and after coronary artery bypass surgery. Coron Artery Dis 4(10):899–904
Sala A, Rossoni G, Berti F, Buccellati C, Bonazzi A, Maclouf J, Folco G (2000) Monoclonal anti-CD18 antibody prevents transcellular biosynthesis of cysteinyl leukotrienes in vitro and in vivo and protects against leukotriene-dependent increase in coronary vascular resistance and myocardial stiffness. Circulation 101(12):1436–1440
Sala A, Rossoni G, Buccellati C, Berti F, Folco G, Maclouf J (1993) Formation of sulphidopeptide-leukotrienes by cell-cell interaction causes coronary vasoconstriction in isolated, cell-perfused heart of rabbit. Br J Pharmacol 110(3):1206–1212
Rossoni G, Sala A, Berti F, Testa T, Buccellati C, Molta C, Muller-Peddinghaus R, Maclouf J, Folco GC (1996) Myocardial protection by the leukotriene synthesis inhibitor BAY X1005: importance of transcellular biosynthesis of cysteinyl-leukotrienes. J Pharmacol Exp Ther 276(1):335–341
Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M (2004) Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med 350(1):29–37
Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K (2004) The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet 36(3):233–239
Maekawa A, Austen KF, Kanaoka Y (2002) Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem 277(23):20820–20824
Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ (2003) Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A 100(3):1238–1243
Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, Hansson GK, Back M (2011) Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation 123(12):1316–1325
Eaton A, Nagy E, Pacault M, Fauconnier J, Back M (2012) Cysteinyl leukotriene signaling through perinuclear CysLT(1) receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J Mol Med (Berl) 90(10):1223–1231
Minamisawa H, Terashi A, Katayama Y, Kanda Y, Shimizu J, Shiratori T, Inamura K, Kaseki H, Yoshino Y (1988) Brain eicosanoid levels in spontaneously hypertensive rats after ischemia with reperfusion: leukotriene C4 as a possible cause of cerebral edema. Stroke 19(3):372–377
Ciceri P, Rabuffetti M, Monopoli A, Nicosia S (2001) Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol 133(8):1323–1329
Di Gennaro A, Carnini C, Buccellati C, Ballerio R, Zarini S, Fumagalli F, Viappiani S, Librizzi L, Hernandez A, Murphy RC, Constantin G, De Curtis M, Folco G, Sala A (2004) Cysteinyl-leukotrienes receptor activation in brain inflammatory reactions and cerebral edema formation: a role for transcellular biosynthesis of cysteinyl-leukotrienes. FASEB J 18(7):842–844
Sheng WW, Li CT, Zhang WP, Yuan YM, Hu H, Fang SH, Zhang L, Wei EQ (2006) Distinct roles of CysLT1 and CysLT2 receptors in oxygen glucose deprivation-induced PC12 cell death. Biochem Biophys Res Commun 346(1):19–25
Wang ML, Huang XJ, Fang SH, Yuan YM, Zhang WP, Lu YB, Ding Q, Wei EQ (2006) Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem Biophys Res Commun 350(2):399–404
Huang XJ, Zhang WP, Li CT, Shi WZ, Fang SH, Lu YB, Chen Z, Wei EQ (2008) Activation of CysLT receptors induces astrocyte proliferation and death after oxygen-glucose deprivation. Glia 56(1):27–37
Letts LG (1987) Leukotrienes: role in cardiovascular physiology. Cardiovasc Clin 18(1):101–113
Folco G, Rossoni G, Buccellati C, Berti F, Maclouf J, Sala A (2000) Leukotrienes in cardiovascular diseases. Am J Respir Crit Care Med 161(2 Pt 2):S112–S116
Back M (2009) Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr Pharm Des 15(27):3116–3132
Poeckel D, Funk CD (2010) The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res 86(2):243–253
Capra V, Back M, Barbieri SS, Camera M, Tremoli E, Rovati GE (2013) Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev 33(2):364–438
Griffin M, Weiss JW, Leitch AG, McFadden ER Jr, Corey EJ, Austen KF, Drazen JM (1983) Effects of leukotriene D on the airways in asthma. N Engl J Med 308(8):436–439
Dahlen SE, Hansson G, Hedqvist P, Björck T, Granström E, Dahlen B (1983) Allergen challenge of lung tissue from asthmatics elicits bronchial contraction that correlates with the release of leukotrienes C4, D4 and E4. Proc Natl Acad Sci U S A 80:1712–1716
Lewis RA, Robin JL (1985) Arachidonic acid derivatives as mediators of asthma. J Allergy Clin Immunol 76:259–264
Adelroth E, Morris MM, Hargreave FE, O'Byrne PM (1986) Airway responsiveness to leukotrienes C4 and D4 and to methacholine in patients with asthma and normal controls. N Engl J Med 315(8):480–484
Davidson AB, Lee TH, Scanlon PD, Solway J, McFadden ER Jr, Ingram RH Jr, Corey EJ, Austen KF, Drazen JM (1987) Bronchoconstrictor effects of leukotriene E4 in normal and asthmatic subjects. Am Rev Respir Dis 135(2):333–337
Israel E, Dermarkarian R, Rosenberg M, Sperling R, Taylor G, Rubin P, Drazen JM (1990) The effects of a 5-lipoxygenase inhibitor on asthma induced by cold, dry air. N Engl J Med 323(25):1740–1744
O'Byrne PM, Israel E, Drazen JM (1997) Antileukotrienes in the treatment of asthma. Ann Intern Med 127(6):472–480
Claesson HE, Dahlen SE (1999) Asthma and leukotrienes: antileukotrienes as novel anti-asthmatic drugs. J Intern Med 245(3):205–227
Lipworth BJ (1999) Leukotriene-receptor antagonists. Lancet 353(9146):57–62
Salvi SS, Krishna MT, Sampson AP, Holgate ST (2001) The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest 119(5):1533–1546
Currie GP, Lipworth BJ (2002) Bronchoprotective effects of leukotriene receptor antagonists in asthma: a meta-analysis. Chest 122(1):146–150
Capra V, Rovati GE (2004) Leukotriene modifiers in asthma management. IDrugs 7(7):659–666
Capra V, Ambrosio M, Riccioni G, Rovati GE (2006) Cysteinyl-leukotriene receptor antagonists: present situation and future opportunities. Curr Med Chem 13(26):3213–3226
Capra V, Carnini C, Accomazzo MR, Di Gennaro A, Fiumicelli M, Borroni E, Brivio I, Buccellati C, Mangano P, Carnevali S, Rovati G, Sala A (2015) Autocrine activity of cysteinyl leukotrienes in human vascular endothelial cells: signaling through the CysLT receptor. Prostaglandins Other Lipid Mediat 120:115–125
Labat C, Ortiz JL, Norel X, Gorenne I, Verley J, Abram TS, Cuthbert NJ, Tudhope SR, Norman P, Gardiner P et al (1992) A second cysteinyl leukotriene receptor in human lung. J Pharmacol Exp Ther 263(2):800–805
Tudhope SR, Cuthbert NJ, Abram TS, Jennings MA, Maxey RJ, Thompson AM, Norman P, Gardiner PJ (1994) BAY u9773, a novel antagonist of cysteinil-leukotrienes with activity against two receptor subtypes. Eur J Pharmacol 264:317–323
Carnini C, Accomazzo MR, Borroni E, Vitellaro-Zuccarello L, Durand T, Folco G, Rovati GE, Capra V, Sala A (2011) Synthesis of cysteinyl leukotrienes in human endothelial cells: subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J 25(10):3519–3528
Ni NC, Yan D, Ballantyne LL, Barajas-Espinosa A, St Amand T, Pratt DA, Funk CD (2011) A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J Pharmacol Exp Ther 339(3):768–778
Wunder F, Tinel H, Kast R, Geerts A, Becker EM, Kolkhof P, Hutter J, Erguden J, Harter M (2010) Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT(2)) receptor. Br J Pharmacol 160(2):399–409
Drazen JM, Israel E, O'Byrne PM (1999) Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 340(3):197–206
Sasaki K, Ueno A, Kawamura M, Katori M, Shigehiro S, Kikawada R (1987) Reduction of myocardial infarct size in rats by a selective 5-lipoxygenase inhibitor (AA-861). Adv Prostaglandin Thromboxane Leukot Res 17A:381–383
Hashimoto H, Miyazawa K, Hagiwara M, Miyasaka K, Nakashima M (1990) Beneficial effects of a new 5-lipoxygenase inhibitor on occlusion- and occlusion-reperfusion-induced myocardial injury. Arzneimittelforschung 40(2 Pt 1):126–129
Welt K, Fitzl G, Mark B (2000) Lipoxygenase inhibitor FLM 5011, an effective protectant of myocardial microvessels against ischemia-reperfusion injury? An ultrastructural-morphometric study. Exp Toxicol Pathol 52(1):27–36
Jawien J, Gajda M, Rudling M, Mateuszuk L, Olszanecki R, Guzik TJ, Cichocki T, Chlopicki S, Korbut R (2006) Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Investig 36(3):141–146
Bäck M, Sultan A, Ovchinnikova O, Hansson GK (2007) 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res 100(7):946–949
Amsterdam EA, Pan HL, Rendig SV, Symons JD, Fletcher MP, Longhurst JC (1993) Limitation of myocardial infarct size in pigs with a dual lipoxygenase-cyclooxygenase blocking agent by inhibition of neutrophil activity without reduction of neutrophil migration. J Am Coll Cardiol 22(6):1738–1744
Vidal C, Gomez-Hernandez A, Sanchez-Galan E, Gonzalez A, Ortega L, Gomez-Gerique JA, Tunon J, Egido J (2007) Licofelone, a balanced inhibitor of cyclooxygenase and 5-lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis. J Pharmacol Exp Ther 320(1):108–116
Mullane K, Hatala MA, Kraemer R, Sessa W, Westlin W (1987) Myocardial salvage induced by REV-5901: an inhibitor and antagonist of the leukotrienes. J Cardiovasc Pharmacol 10(4):398–406
Hahn RA, MacDonald BR, Simpson PJ, Wang L, Towner RD, Ho PP, Goodwin M, Breau AP, Suarez T, Mihelich ED (1991) Characterization of LY233569 on 5-lipoxygenase and reperfusion injury of ischemic myocardium. J Pharmacol Exp Ther 256(1):94–102
Adamek A, Jung S, Dienesch C, Laser M, Ertl G, Bauersachs J, Frantz S (2007) Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur J Pharmacol 571(1):51–54
Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD (2004) The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med 10(9):966–973
Cao RY, St Amand T, Grabner R, Habenicht AJ, Funk CD (2009) Genetic and pharmacological inhibition of the 5-lipoxygenase/leukotriene pathway in atherosclerotic lesion development in ApoE deficient mice. Atherosclerosis 203(2):395–400
Whatling C, McPheat W, Herslof M (2007) The potential link between atherosclerosis and the 5-lipoxygenase pathway: investigational agents with new implications for the cardiovascular field. Expert Opin Investig Drugs 16(12):1879–1893
Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, Manolescu A, Arnar DO, Andersen K, Sigurdsson A, Thorgeirsson G, Jonsson A, Agnarsson U, Bjornsdottir H, Gottskalksson G, Einarsson A, Gudmundsdottir H, Adalsteinsdottir AE, Gudmundsson K, Kristjansson K, Hardarson T, Kristinsson A, Topol EJ, Gulcher J, Kong A, Gurney M, Thorgeirsson G, Stefansson K (2005) Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA 293(18):2245–2256
Tardif JC, L'Allier PL, Ibrahim R, Gregoire JC, Nozza A, Cossette M, Kouz S, Lavoie MA, Paquin J, Brotz TM, Taub R, Pressacco J (2010) Treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging 3(3):298–307
Matsumoto S , Ibrahim R , Grégoire JC , L'Allier PL , Pressacco J , Tardif J-C and Budoff MJ (2016) Effect of treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) on coronary plaque progression: a serial CT angiography study. Clin Cardiol. doi:10.1002/clc.22646
Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510(7503):92–101
Serhan CN (2017) Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. doi:10.1096/fj.201601222R
Fredman G, Spite M (2017) Specialized pro-resolving mediators in cardiovascular diseases. Mol Aspects Med pii: S0098–2997(17)30017–1. doi:10.1016/j.mam.2017.02.003
Hecht I, Rong J, Sampaio AL, Hermesh C, Rutledge C, Shemesh R, Toporik A, Beiman M, Dassa L, Niv H, Cojocaru G, Zauberman A, Rotman G, Perretti M, Vinten-Johansen J, Cohen Y (2009) A novel peptide agonist of formyl-peptide receptor-like 1 (ALX) displays anti-inflammatory and cardioprotective effects. J Pharmacol Exp Ther 328(2):426–434
Fierro IM, Kutok JL, Serhan CN (2002) Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4). J Pharmacol Exp Ther 300(2):385–392
Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM (2005) Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol 289(3):C557–C563
Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM (2007) Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost 97(1):88–98
Fredman G, Van Dyke TE, Serhan CN (2010) Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol 30(10):2005–2013
Yedgar S, Krimsky M, Cohen Y, Flower RJ (2007) Treatment of inflammatory diseases by selective eicosanoid inhibition: a double-edged sword? Trends Pharmacol Sci 28(9):459–464
Lipworth BJ (2001) Emerging role of antileukotriene therapy in allergic rhinitis. Clin Exp Allergy 31(12):1813–1821
Nayak AS, Philip G, Lu S, Malice MP, Reiss TF (2002) Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol 88(6):592–600
Meltzer EO, Malmstrom K, Lu S, Prenner BM, Wei LX, Weinstein SF, Wolfe JD, Reiss TF (2000) Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J Allergy Clin Immunol 105(5):917–922
Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, Hisada S (2012) The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. J Asthma 49(6):649–656
Reiss TF, Sorkness CA, Stricker W, Botto A, Busse WW, Kundu S, Zhang J (1997) Effects of montelukast (MK-0476); a potent cysteinyl leukotriene receptor antagonist, on bronchodilation in asthmatic subjects treated with and without inhaled corticosteroids. Thorax 52(1):45–48
Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, Reiss TF (1998) Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Montelukast Asthma Study Group. Eur Respir J 11(6):1232–1239
Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W, Weinland DE, Reiss TF (2000) Comparison of the effects of intravenous and oral montelukast on airway function: a double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax 55(4):260–265
Rubinstein I, Kumar B, Schriever C (2004) Long-term montelukast therapy in moderate to severe COPD—a preliminary observation. Respir Med 98(2):134–138
Celik P, Sakar A, Havlucu Y, Yuksel H, Turkdogan P, Yorgancioglu A (2005) Short-term effects of montelukast in stable patients with moderate to severe COPD. Respir Med 99(4):444–450
Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM (2015) Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med 163(10):756–767
Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, Juniper EF, Ayres JG, Kemp L, Blyth A, Wilson EC, Wolfe S, Freeman D, Mugford HM, Murdoch J, Harvey I (2011) Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med 364(18):1695–1707
Chauhan BF, Ducharme FM (2012) Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane database of systematic reviews (Online) 5:CD002314
Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, Becker A (1998) Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast study Group. JAMA 279(15):1181–1186
Bisgaard H, Skoner D, Boza ML, Tozzi CA, Newcomb K, Reiss TF, Knorr B, Noonan G (2009) Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr Pulmonol 44(6):568–579
Berube D, Djandji M, Sampalis JS, Becker A (2014) Effectiveness of montelukast administered as monotherapy or in combination with inhaled corticosteroid in pediatric patients with uncontrolled asthma: a prospective cohort study. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology 10(1):21
Schmitt-Grohe S, Eickmeier O, Schubert R, Bez C, Zielen S (2002) Anti-inflammatory effects of montelukast in mild cystic fibrosis. Ann Allergy Asthma Immunol 89(6):599–605
Stelmach I, Korzeniewska A, Stelmach W, Majak P, Grzelewski T, Jerzynska J (2005) Effects of montelukast treatment on clinical and inflammatory variables in patients with cystic fibrosis. Ann Allergy Asthma Immunol 95(4):372–380
Fitzgerald DA, Mellis CM (2006) Leukotriene receptor antagonists in virus-induced wheezing: evidence to date. Treat Respir Med 5(6):407–417
Sener G, Sehirli O, Cetinel S, Ercan F, Yuksel M, Gedik N, Yegen BC (2005) Amelioration of sepsis-induced hepatic and ileal injury in rats by the leukotriene receptor blocker montelukast. Prostaglandins Leukot Essent Fatty Acids 73(6):453–462
Maeba S, Ichiyama T, Ueno Y, Makata H, Matsubara T, Furukawa S (2005) Effect of montelukast on nuclear factor kappaB activation and proinflammatory molecules. Ann Allergy Asthma Immunol 94(6):670–674
Wu Y, Zhou C, Tao J, Li S (2006) Montelukast prevents the decrease of interleukin-10 and inhibits NF-kappaB activation in inflammatory airway of asthmatic guinea pigs. Can J Physiol Pharmacol 84(5):531–537
Tahan F, Jazrawi E, Moodley T, Rovati GE, Adcock IM (2008) Montelukast inhibits tumour necrosis factor-alpha-mediated interleukin-8 expression through inhibition of nuclear factor-kappaB p65-associated histone acetyltransferase activity. Clin Exp Allergy 38(5):805–811
Chiba M, Xu X, Nishime JA, Balani SK, Lin JH (1997) Hepatic microsomal metabolism of montelukast, a potent leukotriene D4 receptor antagonist, in humans. Drug metabolism and disposition: the biological fate of chemicals 25(9):1022–1031
Bush A (2015) Montelukast in paediatric asthma: where we are now and what still needs to be done? Paediatr Respir Rev 16(2):97–100
Price D (2000) Tolerability of montelukast. Drugs 59(Suppl 1):35–42 discussion 43-35
Garcia-Marcos L, Schuster A, Perez-Yarza EG (2003) Benefit-risk assessment of antileukotrienes in the management of asthma. Drug Saf 26(7):483–518
Virchow JC, Bachert C (2006) Efficacy and safety of montelukast in adults with asthma and allergic rhinitis. Respir Med 100(11):1952–1959
Paggiaro P, Bacci E (2011) Montelukast in asthma: a review of its efficacy and place in therapy. Ther Adv Chronic Dis 2(1):47–58
Biber N, Toklu HZ, Solakoglu S, Gultomruk M, Hakan T, Berkman Z, Dulger FG (2009) Cysteinyl-leukotriene receptor antagonist montelukast decreases blood-brain barrier permeability but does not prevent oedema formation in traumatic brain injury. Brain Inj 23(6):577–584
Yu GL, Wei EQ, Zhang SH, Xu HM, Chu LS, Zhang WP, Zhang Q, Chen Z, Mei RH, Zhao MH (2005) Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology 73(1):31–40
Yu GL, Wei EQ, Wang ML, Zhang WP, Zhang SH, Weng JQ, Chu LS, Fang SH, Zhou Y, Chen Z, Zhang Q, Zhang LH (2005) Pranlukast, a cysteinyl leukotriene receptor-1 antagonist, protects against chronic ischemic brain injury and inhibits the glial scar formation in mice. Brain Res 1053(1–2):116–125
Qian XD, Wei EQ, Zhang L, Sheng WW, Wang ML, Zhang WP, Chen Z (2006) Pranlukast, a cysteinyl leukotriene receptor 1 antagonist, protects mice against brain cold injury. Eur J Pharmacol 549(1–3):35–40
Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang WP, Yu GL, Chu LS, Chen Z (2006) Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience 140(3):969–979
Zhao R, Shi WZ, Zhang YM, Fang SH, Wei EQ (2011) Montelukast, a cysteinyl leukotriene receptor-1 antagonist, attenuates chronic brain injury after focal cerebral ischaemia in mice and rats. J Pharm Pharmacol 63(4):550–557
Huang XQ, Zhang XY, Wang XR, Yu SY, Fang SH, Lu YB, Zhang WP, Wei EQ (2012) Transforming growth factor beta1-induced astrocyte migration is mediated in part by activating 5-lipoxygenase and cysteinyl leukotriene receptor 1. J Neuroinflammation 9:145
Saad MA, Abdelsalam RM, Kenawy SA, Attia AS (2015) Montelukast, a cysteinyl leukotriene receptor-1 antagonist protects against hippocampal injury induced by transient global cerebral ischemia and reperfusion in rats. Neurochem Res 40(1):139–150
Cavus G, Altas M, Aras M, Ozgur T, Serarslan Y, Yilmaz N, Sefil F, Ulutas KT (2014) Effects of montelukast and methylprednisolone on experimental spinal cord injury in rats. Eur Rev Med Pharmacol Sci 18(12):1770–1777
Fang SH, Zhou Y, Chu LS, Zhang WP, Wang ML, Yu GL, Peng F, Wei EQ (2007) Spatio-temporal expression of cysteinyl leukotriene receptor-2 mRNA in rat brain after focal cerebral ischemia. Neurosci Lett 412(1):78–83
Qi LL, Fang SH, Shi WZ, Huang XQ, Zhang XY, Lu YB, Zhang WP, Wei EQ (2011) CysLT2 receptor-mediated AQP4 up-regulation is involved in ischemic-like injury through activation of ERK and p38 MAPK in rat astrocytes. Life Sci 88(1–2):50–56
Zhao CZ, Zhao B, Zhang XY, Huang XQ, Shi WZ, Liu HL, Fang SH, Lu YB, Zhang WP, Tang FD, Wei EQ (2011) Cysteinyl leukotriene receptor 2 is spatiotemporally involved in neuron injury, astrocytosis and microgliosis after focal cerebral ischemia in rats. Neuroscience 189:1–11
Shi QJ, Xiao L, Zhao B, Zhang XY, Wang XR, Xu DM, Yu SY, Fang SH, Lu YB, Zhang WP, Sa XY, Wei EQ (2012) Intracerebroventricular injection of HAMI 3379, a selective cysteinyl leukotriene receptor 2 antagonist, protects against acute brain injury after focal cerebral ischemia in rats. Brain Res 1484:57–67
Shi QJ, Wang H, Liu ZX, Fang SH, Song XM, Lu YB, Zhang WP, Sa XY, Ying HZ, Wei EQ (2015) HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience 291:53–69
Zhang XY, Wang XR, Xu DM, Yu SY, Shi QJ, Zhang LH, Chen L, Fang SH, Lu YB, Zhang WP, Wei EQ (2013) HAMI 3379, a CysLT2 receptor antagonist, attenuates ischemia-like neuronal injury by inhibiting microglial activation. J Pharmacol Exp Ther 346(2):328–341
Kaetsu Y, Yamamoto Y, Sugihara S, Matsuura T, Igawa G, Matsubara K, Igawa O, Shigemasa C, Hisatome I (2007) Role of cysteinyl leukotrienes in the proliferation and the migration of murine vascular smooth muscle cells in vivo and in vitro. Cardiovasc Res 76(1):160–166
Jawien J, Gajda M, Wolkow P, Zuranska J, Olszanecki R, Korbut R (2008) The effect of montelukast on atherogenesis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 59(3):633–639
Mueller CF, Wassmann K, Widder JD, Wassmann S, Chen CH, Keuler B, Kudin A, Kunz WS, Nickenig G (2008) Multidrug resistance protein-1 affects oxidative stress, endothelial dysfunction, and atherogenesis via leukotriene C4 export. Circulation 117(22):2912–2918
Liu D, Ge S, Zhou G, Xu G, Zhang R, Zhu W, Liu Z, Cheng S, Liu X (2009) Montelukast inhibits matrix metalloproteinases expression in atherosclerotic rabbits. Cardiovasc Drugs Ther 23(6):431–437
Ge S, Zhou G, Cheng S, Liu D, Xu J, Xu G, Liu X (2009) Anti-atherogenic effects of montelukast associated with reduced MCP-1 expression in a rabbit carotid balloon injury model. Atherosclerosis 205(1):74–79
Becher UM, Ghanem A, Tiyerili V, Furst DO, Nickenig G, Mueller CF (2011) Inhibition of leukotriene C4 action reduces oxidative stress and apoptosis in cardiomyocytes and impedes remodeling after myocardial injury. J Mol Cell Cardiol 50(3):570–577
Nobili E, Salvado MD, Folkersen L, Castiglioni L, Kastrup J, Wetterholm A, Tremoli E, Hansson GK, Sironi L, Haeggstrom JZ, Gabrielsen A (2012) Cysteinyl leukotriene signaling aggravates myocardial hypoxia in experimental atherosclerotic heart disease. PLoS One 7(7):e41786
Daglar G, Karaca T, Yuksek YN, Gozalan U, Akbiyik F, Sokmensuer C, Gurel B, Kama NA (2009) Effect of montelukast and MK-886 on hepatic ischemia-reperfusion injury in rats. J Surg Res 153(1):31–38
Duran A, Otiuk H, Terzi EH, Tosun M, Oziiu H, Ocak T, Kiiuer A (2013) Protective effect of montelukast, a cysteinyl leukotriene receptor-1 antagonist, against intestinal ischemia-reperfusion injury in the rat. Acta Chir Belg 113(6):401–405
Wu S, Zhu X, Jin Z, Tong X, Zhu L, Hong X, Zhu X, Liu P, Shen W (2015) The protective role of montelukast against intestinal ischemia-reperfusion injury in rats. Scientific reports 5:15787
Ozkan E, Yardimci S, Dulundu E, Topaloglu U, Sehirli O, Ercan F, Velioglu-Ogunc A, Sener G (2010) Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res 159(1):588–594
Celik A, Ergun E, Koksal N, Celik AS, Altinli E, Uzun MA, Eroglu E, Kemik A (2013) Effects of montelukast on the healing of ischemic colon anastomoses. Am J Surg 206(4):502–508
Oral A, Odabasoglu F, Halici Z, Keles ON, Unal B, Coskun AK, Kilic C, Surer I, Salman AB (2011) Protective effects of montelukast on ischemia-reperfusion injury in rat ovaries subjected to torsion and detorsion: biochemical and histopathologic evaluation. Fertil Steril 95(4):1360–1366
Akdemir A, Erbas O, Ergenoglu M, Ozgur Yeniel A, Oltulu F, Yavasoglu A, Taskiran D (2014) Montelukast prevents ischaemia/reperfusion-induced ovarian damage in rats. Eur J Obstet Gynecol Reprod Biol 173:71–76
Ozturk H, Ozturk H, Gideroglu K, Terzi H, Bugdayci G (2010) Montelukast protects against testes ischemia/reperfusion injury in rats. Can Urol Assoc J 4(3):174–179
Sener G, Sehirli O, Velioglu-Ogunc A, Cetinel S, Gedik N, Caner M, Sakarcan A, Yegen BC (2006) Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol Res 54(1):65–71
Sener G, Sehirli O, Toklu H, Ercan F, Alican I (2007) Montelukast reduces ischaemia/reperfusion-induced bladder dysfunction and oxidant damage in the rat. J Pharm Pharmacol 59(6):837–842
Muthuraman A, Ramesh M, Sood S (2012) Ameliorative potential of montelukast on ischemia-reperfusion injury induced vasculitic neuropathic pain in rat. Life Sci 90(19–20):755–762
Lafci G, Gedik HS, Korkmaz K, Erdem H, Cicek OF, Nacar OA, Yildirim L, Kaya E, Ankarali H (2013) Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg 8:64
Hagar HH, Abd El Tawab R (2012) Cysteinyl leukotriene receptor antagonism alleviates renal injury induced by ischemia-reperfusion in rats. J Surg Res 178(1):e25–e34
Kezeli T, Gongadze N, Chapichadze Z, Bakuridze K, Chirakadze K (2010) Effect of combination of zafirlukast and quercetin on baroreflex sensitivity and endothelin production in rats with myocardial infarction. Int J Clin Pharmacol Ther 48(5):335–341
DeClue AE, Sharp CR, Cohen RL, Leverenz EF, Reinero CR (2010) Cysteinyl-leukotriene receptor antagonism blunts the acute hypotensive response to endotoxin in cats. J Feline Med Surg 12(10):754–759
Toki Y, Hieda N, Torii T, Hashimoto H, Ito T, Ogawa K, Satake T (1988) The effects of lipoxygenase inhibitor and peptidoleukotriene antagonist on myocardial injury in a canine coronary occlusion-reperfusion model. Prostaglandins 35(4):555–571
Zhao R, Fang SH, Lin KN, Huang XQ, Lu YB, Zhang WP, Wei EQ (2011) Pranlukast attenuates hydrogen peroxide-induced necrosis in endothelial cells by inhibiting oxygen reactive species-mediated collapse of mitochondrial membrane potential. J Cardiovasc Pharmacol 57(4):479–488
Fang SH, Yuan YM, Peng F, Li CT, Zhang LH, Lu YB, Zhang WP, Wei EQ (2009) Pranlukast attenuates ischemia-like injury in endothelial cells via inhibiting reactive oxygen species production and nuclear factor-kappaB activation. J Cardiovasc Pharmacol 53(1):77–85
Allayee H, Hartiala J, Lee W, Mehrabian M, Irvin CG, Conti DV, Lima JJ (2007) The effect of montelukast and low-dose theophylline on cardiovascular disease risk factors in asthmatics. Chest 132(3):868–874
Ingelsson E, Yin L, Bäck M (2012) Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J Allergy Clin Immunol 129(3):702–707
Duah E, Adapala RK, Al-Azzam N, Kondeti V, Gombedza F, Thodeti CK, Paruchuri S (2013) Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Scientific reports 3:3274
Porreca E, Di Febbo C, Reale M, Barbacane R, Mezzetti A, Cuccurullo F, Conti P (1995) Modulation of rat vascular smooth muscle cell (VSMC) proliferation by cysteinyl leukotriene D4: a role for mediation of interleukin 1. Atherosclerosis 113(1):11–18
Bousquet J, Demoly P, Humbert M (2009) Montelukast in guidelines and beyond. Adv Ther 26(6):575–587
Funk CD (2005) Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov 4(8):664–672
Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD (2012) Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol 176(11):1014–1024
Amlani S, Nadarajah T, McIvor RA (2011) Montelukast for the treatment of asthma in the adult population. Expert Opin Pharmacother 12(13):2119–2128
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Hoxha, M., Rovati, G.E. & Cavanillas, A.B. The leukotriene receptor antagonist montelukast and its possible role in the cardiovascular field. Eur J Clin Pharmacol 73, 799–809 (2017). https://doi.org/10.1007/s00228-017-2242-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2242-2