Abstract
Purpose
We aimed to assess the influence of CYP2C19*17 on R-warfarin clearance as well as the effect of CYP2C19, CYP2C8, CYP2C9, and VKORC1 polymorphisms together with non-genetic factors on warfarin international normalized ratio (INR)/daily dose.
Methods
One hundred fifty Caucasian Italian outpatients with data on steady-state plasma concentrations of S- and R-warfarin were genotyped for CYP2C19 (*2, *3, *4, *17), CYP2C9 (*2, *3), CYP2C8*3, and VKORC1*2. The statistical analysis was performed on the effect of genotypes/haplotypes, age, sex, and body weight on the clearance of warfarin enantiomers and dose-normalized INR.
Results
R-warfarin clearance was 32 % higher in carriers of CYP2C19*17 than in carriers of CYP2C19*2 (mean 2.5 mL/min, 95 % confidence interval (CI) 2.3–2.8 vs. 1.9 mL/min, 95 % CI 1.7–2.2; P post hoc = 0.01). Patients with CYP2C19*1/*1 genotype had an intermediate clearance (mean 2.1 mL/min, 95 % CI 1.8–2.4). The genotypes of VKORC1, CYP2C9, and CYP2C19, together with non-genetic factors (age, sex, and body weight) explained 52 % of the variability in warfarin INR/daily dose, of which CYP2C19 genotypes accounted for 7 %.
Conclusions
This is the first study to include the gain-of-function CYP2C19*17 allele when assessing the impact of CYP2C19 polymorphisms on the clearance of warfarin enantiomers. CYP2C19 genotypes influenced the clearance of R-warfarin and contributed significantly to the variability in INR/daily dose, indirectly indicating a clinical relevance of R-warfarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oral anticoagulant warfarin, commonly used for the treatment and prevention of thromboembolic disorders, exerts its effect by inhibition of the vitamin K epoxide reductase (VKOR) [1]. Due to its narrow therapeutic index, large inter-individual variability in kinetics and dose requirement, and multiple drug interactions, dose titration, and follow-up of warfarin is guided by repeated measurements of the prothrombin time, expressed as the international normalized ratio (INR or PT-INR), with a target therapeutic range of 2.0–3.0 [2].
Warfarin is administered as a racemic (1:1) mixture of its S- and R-enantiomers. The two enantiomers differ in plasma clearance, elimination half-life, and the degree of protein-binding. S-warfarin is considered to be 3–5 times more potent than R-warfarin as an inhibitor of VKOR [3], and is almost exclusively metabolized by the polymorphic cytochrome P450 CYP2C9 [4]. The metabolism of R-warfarin is, on the other hand, catalyzed by multiple CYP enzymes including CYP2C19, CYP3A4, CYP1A2, and possibly CYP2C8 [4, 5]. There is, by now, a large body of evidence confirming the impact of functional CYP2C9 polymorphisms on S- (but not R-) warfarin clearance as well as on warfarin dose requirement [6, 7]. Polymorphisms in CYP2C9 and the gene coding for vitamin K epoxide reductase complex subunit 1 (VKORC1) together with sex, age, and body weight explain about 50 % of the variability in warfarin dose requirement [8, 9]. Several dosing algorithms with VKORC1 and CYP2C9 genotypes included have been developed [10, 11] and compared to clinical algorithms in prospective randomized trials [12, 13].
Compared to S-warfarin, the pharmacokinetics (PK) and pharmacodynamics (PD) of R-warfarin are much less studied, possibly due to the widely held view that R-warfarin is unlikely to be of relevance for the clinical effects of the drug [3]. This has, however, been challenged recently by Maddison et al. [14] with a single-dose study in healthy subjects, where S- and R-warfarin were administered separately and in combination. Their data, together with the longer half-life, lower clearance, and higher steady-state concentration, indicated the contribution of R-warfarin to the hypoprothrombinemic effects of racemic warfarin. Thus, factors influencing the activity of enzymes involved in the metabolism of R-warfarin could potentially affect the dose–effect relationship of the racemic drug. In a single-dose study in Japanese healthy volunteers, CYP2C19 poor metabolizers had higher area under the plasma concentration-time curve (AUC) and longer elimination half-life of R-warfarin as compared to extensive metabolizers [15]. However, no significant effect of CYP2C19*2 (the most common loss-of-function allele of CYP2C19 in Caucasians) on R-warfarin clearance was observed by us in an Italian patient cohort [6].
CYP2C19*17 is a gain-of-function allele associated with increased CYP2C19 activity as compared to CYP2C19*1, contributing to the variability in the metabolic capacity among extensive metabolizers of CYP2C19 [16]. It is prevalent among Caucasians with population allele frequencies of 18–32 % [16]. CYP2C19*17 is also reported to co-appear almost exclusively with wild-type alleles (*1) of CYP2C9 and CYP2C8 in Northern European populations [17]. This pattern of linkage disequilibrium (LD) has not yet been assessed in Italian population. Moreover, to the best of our knowledge, no study has so far addressed the potential impact of CYP2C19*17 on the clearance of warfarin enantiomers or warfarin dose requirement. Similarly, the effect of CYP2C8 genotype on the PK of warfarin enantiomers is unknown. Overall, the influence of genetic factors on the clearance of R-warfarin and the pharmacodynamic role of the R-enantiomer are not well understood.
The primary aims of this study were to assess the potential influence of CYP2C19*17 and extended CYP2C haplotypes on R-warfarin clearance. The combined effect of CYP2C19, CYP2C8, CYP2C9, and VKORC1 polymorphisms together with non-genetic factors on INR response in relation to dose was also studied.
Materials and methods
Patients and study design
This study was based on 150 Italian warfarin-treated patients included in two previously published studies, study I (93 patients) by Scordo et al. 2002 [6] and study II (57 patients) by Takahashi et al. 2006 [18]. All patients were of Caucasian origin and none was included in both studies I and II. Parts of the data have also been used in the pharmacometric NONMEM modeling described by Hamberg et al. [7]. All patients were on stable maintenance dose of warfarin, aimed to reach a target INR value between 2.0 and 3.0. The main demographic data of the two study cohorts and the total material of 150 patients are given in the Supplementary table. Patients who were taking drugs known to interfere with warfarin metabolism (such as nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, rifampin [INN, rifampicin], and amiodarone) were not included in the studies. Plasma samples were taken at steady state, 12–14 h after the last drug administration, for analysis of S- and R-warfarin concentrations and INR. Full details of study design and patient characteristics have been described elsewhere [6, 18]. Total plasma concentrations of S- and R-warfarin were analyzed by HPLC as described in [6]. Both clinical studies were approved by the Ethics Committee at the Azienda Ospedaliera di Padova, Padova, Italy, and all patients gave informed consent to participate. The genetic analysis linked to anonymous phenotype and genotype characteristics from previous studies was approved by the Regional Ethical Review Board in Stockholm 2014.
Warfarin plasma clearance and dose-normalized INR
Assuming complete oral bioavailability of warfarin, the oral plasma clearance (CL) of each enantiomer was calculated based on the equation: CL = [(D/τ)]/Css where D is the average daily dose of each enantiomer (half the weekly racemic dose divided by 7), τ is the dose interval (i.e., 24 h), and Css the total concentration of S- or R-warfarin, respectively, at steady state.
As a measure of the dose–effect relationship, the INR values were normalized by the average daily dose of the racemic drug (warfarin INR/daily dose).
Genotyping
DNA was extracted from peripheral leukocytes using the Cell Culture DNA kit (QIAGEN, Hilden, Germany). Five single-nucleotide polymorphisms (SNPs) were genotyped using validated TaqMan genotyping assays from Applied Biosystems according to the manufacturer’s guidelines (for rs12248560C>T, CYP2C19*17, assay ID C__469857_10; for rs4244285G>A, CYP2C19*2, assay ID C_25986767_70; for rs4986893G>A, CYP2C19*3, assay ID C__27861809_10; for rs28399504A>G, CYP2C19*4, assay ID C__30634136_10; for rs10509681A>G, CYP2C8*3, assay ID C__25625782_20; for rs992323G>A, VKORC1*2, assay ID C__30403261_20). The analyses were carried out using an ABI Prism 7500 real-time PCR system or an Applied Biosystems StepOnePlus Real-Time PCR system. CYP2C19*17 and *4 were genotyped successfully in all 150 patients and CYP2C8*3 in 148 patients (DNA depleted in two patients). CYP2C19*2 and*3 were analyzed in the 57 patients from study II only as these genotypes were already available from study I [6]. Similarly, VKORC1*2 genotypes for patients from study II were already available [18]. Of the 93 patients in study I, 92 were successfully genotyped for VKORC1*2 (DNA depleted in one patient). The genotypes rs1799853 (CYP2C9*2) and rs1057910 (CYP2C9*3) of all 150 patients were available since before as described [6, 18].
Statistics
Statistical analysis was carried out using STATISTICA 10 (StatSoft Inc., Tulsa, Oklahoma, USA). A two-tailed P value of up to 0.05 was considered to be statistically significant. The two subpopulations were compared regarding patient characteristics (age, sex, and body weight), warfarin dose, S/R warfarin concentration ratio, INR at steady state and genotype frequencies using Mann–Whitney test (for continuous variables) or Pearson’s chi-square test (for categorical variables).
The probability of deviation from Hardy–Weinberg equilibrium (HWE) was calculated for each SNP. Pair-wise LD was characterized by Dʹ values and illustrated by the LD plots in Haploview (4.2). Haplotypes were inferred using Haploview (4.2) and UNPHASED (v3.1.6). The association of the CYP2C haplotypes with the clearance of the enantiomers was assessed using UNPHASED (v3.1.6), implementing maximum-likelihood inference. To reduce non-normality, the clearance values and the warfarin INR/daily dose were log-transformed before statistical assessment. Shapiro–Wilk test was used for assessing normality before vs. after log-transformation (P < 0.0001 vs. P = 0.43 for R-warfarin CL; P < 0.0001 vs. P = 0.07 for S-warfarin CL; P < 0.0001 vs. P = 0.03 for warfarin INR/daily dose). The results of statistical analysis are presented as antilog values. Log-transformation of the S/R concentration ratio did not result in reduction of non-normality (Shapiro–Wilk test before vs. after: P < 0.0001 vs. P < 0.0001). Variability in the S/R concentration ratio in relation to VKORC1*2 was assessed using the non-parametric Kruskal–Wallis test.
Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to assess the effect of genetic polymorphisms on log(R-warfarin CL) and log(S-warfarin CL). The combined effect of genetic and demographic covariates on log(warfarin INR/daily dose) was assessed in linear regression models applying backwards stepwise regression. The adjusted value of R 2 obtained from the final model is considered a measure of the explained variability. Bonferroni test was used as post hoc analysis to test the statistical significance of specific comparisons.
To avoid small sample size effect in analysis (≤5 patients/group), the CYP2C19 genotypes were grouped as follows: (1) subjects homozygous for CYP2C19*1 (CYP2C19 *1/*1), (2) heterozygous and homozygous carriers of the rs4244285 A-allele only (CYP2C19*2 carriers), (3) heterozygous and homozygous carriers of the rs12248560 T-allele only (CYP2C19*17 carriers), and (4) carriers of the variant alleles of both rs4244285 and rs12248560 (CYP2C19*2/*17). No carriers of either CYP2C19*3 or *4 were found among the 150 patients. The CYP2C9 genotypes were grouped as follows: (1) CYP2C9*1/*1, (2) heterozygous carriers of the rs1799853 T-allele only (CYP2C9*1/*2), (3) heterozygous carriers of the rs1057910 C-allele only (CYP2C9*1/*3), and (4) subjects with two variant alleles (CYP2C9*2/*2, *3/*3 or *2/*3).
Results
Characteristics of the 150 patients are summarized in Supplementary Table 1. The study population was between 22 and 87 years of age and all were on stable maintenance doses of warfarin (6.3–78.8 mg/week). All patients were treated for cardiovascular/ thromboembolic diseases with a target INR value between 2 and 3. The patients from study I had lower median body weight than patients from study II (75 vs. 82 kg, P = 0.004), but showed no difference regarding age, warfarin dose, S/R warfarin concentration ratio, and INR at steady-state or genotype frequencies (P > 0.1 in all cases). No deviation from HWE was shown for any of the SNPs analyzed. The frequency of the CYP2C19*17 allele was 17 % in this Italian patient population. Significant pair-wise LD was observed only between rs1799853 (CYP2C9*2) and rs10509681 (CYP2C8*3), at a moderately high level Dʹ = 0.78 (Supplementary Fig. 1).
CYP2C haplotype inference and association with warfarin clearance
Six CYP2C haplotypes with frequencies ≥2 % were inferred (Table 1). The more rare haplotypes together accounted for 5 % of the haplotypes. The haplotypes inferred by UNPHASED were the same as those inferred by Haploview regarding both structure composition and frequency distribution. Haplotype 1 consists of wild-type variants at all SNP loci (Table 1). Haplotypes 2, 3, 5, and 6 each included one single variant allele. The variant G-allele of rs10509681 (CYP2C8*3) co-appeared always with the variant T-allele of rs1799853 (CYP2C9*2) and formed haplotype 4. None of the six haplotypes was significantly associated with log(R-warfarin CL). Regarding S-warfarin, haplotypes 4, 5, and 6 containing variant alleles of CYP2C9 SNPs showed significant reductions in log(S-warfarin CL) compared to the reference haplotype 1 (P < 0.0001, data not shown).
The influence of CYP2C genotypes on R- and S-warfarin clearance
Because of the known effect of CYP2C9 on S-warfarin CL, the effect of CYP2C19 genotypes was assessed together with the CYP2C9 genotypes using two-way ANOVA. CYP2C8*3 was not included as an independent variable in ANOVA since it was in strong LD with CYP2C9*2. Variability in log(R-warfarin CL) was significantly associated with CYP2C19 genotype (F 3,143 = 3.1, P = 0.03) but not with CYP2C9 genotype (F 3,143 = 1.9, P = 0.1).
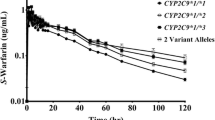
CYP2C19*17 carriers displayed a 1.3-fold higher (P post hoc = 0.01) mean R-warfarin clearance (antilog values: mean 2.5 mL/min, 95 % confidence interval (CI) 2.3–2.8) than CYP2C19*2 carriers (1.9 mL/min, 95 % CI 1.7–2.2) (Fig. 1). These comparisons do not include heterozygous with CYP2C19*2/*17 genotype. Patients with CYP2C19*1/*1 genotype had their mean R-warfarin CL in-between the *17 and *2 carrier groups (2.1 mL/min, 95 % CI 1.8–2.4). Those who carried both CYP2C19*2 and*17 alleles (*2/*17 genotype) showed a mean CL similar to that of the *17 carriers (2.5 mL/min, 95 % CI 1.8–3.3). However, no statistically significant differences in R-warfarin CL compared to the other groups were observed for the *1/*1 or the *2/*17 groups. The influence of CYP2C19 genotype on R-warfarin CL was further assessed with adjustment for age and body weight using ANCOVA. The CYP2C19 genotype remained a significant factor (F 3,118 = 5.4, P = 0.002) with a consistent subgroup difference between carriers of CYP2C19*17 and those of *2 (P post hoc = 0.002). Increase in age was associated with a decrease in log(R-warfarin CL) (Beta = −0.26, F 1,118 = 9.8, P = 0.002) whereas body weight showed no significant influence.
On the other hand, no statistically significant association was observed between CYP2C19 genotypes and the clearance of S-warfarin (F 3,143 = 0.5, P = 0.6). CYP2C9 genotype was a strong predictor of log(S-warfarin CL) (F 3,143 = 38.5, P < 0.001). Mean S-warfarin CLs in the CYP2C9*1/*2 (antilog values: 3.4 mL/min, 95 % CI 2.9–4.0) and *1/*3 genotype groups (3.0 mL/min, 95 % CI 2.6–3.6) were significantly lower than that in the CYP2C9*1/*1 group (5.4 mL/min, 95 % CI 4.9–6.0; P post hoc = <0.0001 in both cases), but higher than in carriers of two defect alleles (1.3 mL/min, 95 % CI 1.0–1.8; P post hoc = <0.0001 in both cases). No significant difference in S-warfarin CL between the CYP2C9*1/*2 and CYP2C9*1/*3 groups was observed.
The influence of CYP2C genotypes on warfarin INR/daily dose
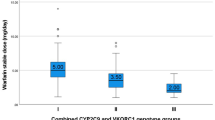
Approximately one third of the patients (47/150) had an INR value outside the target range (2.0–3.0), the minimum and maximum INR being 1.36 and 4.37, respectively. Therefore, warfarin INR/daily dose was used as a measure of the dose–effect relationship. When assessing variability in warfarin INR/daily dose, CYP2C19 genotype was identified as a significant factor (F 3,112 = 2.9, P = 0.04) in addition to the CYP2C9 (F 3,112 = 13.7, P < 0.0001) and VKORC1 genotypes (F 2,112 = 31.7, P < 0.0001, Table 2).
In subsequent subgroup comparison, warfarin INR/dose ratios were decreased by 37 and 42 % in carriers of CYP2C19*17 (mean 0.46, 95 % CI 0.39–0.54) and subjects with the CYP2C19*2/*17 genotype (0.43, 95 % CI 0.31–0.59), respectively, as compared to CYP2C19*2 carriers (0.73, 95 % CI 0.56–0.96; P post hoc = <0.01 in both cases). The CYP2C19*1/*1 group had warfarin INR/dose values in-between the extremes (0.61, 95 % CI 0.52–0.73), with 16 % reduction compared to the CYP2C19*2 carriers.
Compared to patients with CYP2C9*1/*1 genotype (mean 0.46, 95 % CI 0.42–0.52), heterozygous, and homozygous carriers of CYP2C9*2 or *3 displayed 1.4 to 2.4-fold higher warfarin INR/daily dose ratios (the *1/*2 group: 0.64, 95 % CI 0.52–0.78; the *1/*3 group: 0.77, 95 % CI 0.63–0.94; and the *2/*2, *3/*3 or *2/*3 group: 1.12, 95 % CI 0.82–1.56, P post hoc = <0.01 in all cases). Similarly, 1.5- and 2.3-fold higher mean warfarin INR/daily dose ratios were observed in heterozygous and homozygous carriers of VKORC1*2 (0.61, 95 % CI 0.53–0.70 and 0.93, 95 % CI 0.79–1.09, respectively) than in the VKORC1*1/*1 group (0.40, 95 % CI 0.35–0.46; P post hoc < 0.001 in both cases). Of the non-genetic factors included (age, body weight, and sex), only age was observed to be a significant covariate (F 1,112 = 8.6, P = 0.004, Table 2). Increase in age was associated with an increase in warfarin INR/dose (Beta = 0.19). The three genetic factors and age together explained 52 % of the variability in log (warfarin INR/daily dose) (adjusted R 2 = 0.52) with the CYP2C19 genotypes accounting for 7 % of the variability (Table 2).
Discussion
This is, to our knowledge, the first study to include CYP2C19*17 in the analysis of the effect of CYP2C19 genotypes on the enantioselective PK and INR response of warfarin. A significant effect of CYP2C19 genotype on R-warfarin clearance was observed, with carriers of CYP2C19*17 showing on average 32 % higher clearance than carriers of CYP2C19*2. Patients with two functional CYP2C19*1 alleles had clearance values in-between these two groups. On the other hand, no influence of CYP2C19 genotypes on S-warfarin clearance was observed. Our data are in line with published in vitro studies supporting a major role for CYP2C19 in the metabolism of R-warfarin [5].
In our earlier study including part (93 patients) of the current study population, no significant effect of CYP2C19 genotypes on the clearance of unbound R-warfarin was found [6]. At the time of that study, the CYP2C19*17 allele had not yet been described. Carriers of the *17 allele were thus classified as CYP2C19*1 carriers in that study. Analysis of the*17 allele in the current study allowed identification of a subgroup of patients with a higher predicted average CYP2C19 activity as compared to those carrying two CYP2C19*1 alleles. The improved prediction of the phenotype together with a larger patient population is a possible explanation to the observed significant effect of the genotype on R-warfarin clearance. Similar to this study, a significant effect of CYP2C19*17 on the PK of other CYP2C19 substrates such as omeprazole [19] and escitalopram [20] has been shown. It is to be noted that the free fraction of R-warfarin is higher than that of S-warfarin (in average 1.18 and 1.08 %, respectively, for R- and S-warfarin in study I [6]). Calculating total instead of the free unbound clearance of the enantiomers (as in the present study due to the lack of data on the free fraction in study II) would thus be expected to underestimate rather than overestimate the contribution of the CYP2C19 genotype on the clearance and, consequently, plasma exposure of free R-warfarin. This is of interest when discussing the possible contribution of the R-enantiomer for the pharmacological effects of warfarin.
In the present study, R-warfarin clearance was on average 10 % (though not statistically significantly) higher in the CYP2C19*1/*1 genotype group as compared to carriers of CYP2C19*2. In a study in Asian healthy volunteers [15] a 30 % higher clearance of this enantiomer was found in homozygous extensive metabolizers (*1/*1 genotype) of CYP2C19 than in poor metabolizers (i.e., carriers of two loss-of-function alleles). No heterozygous carriers of CYP2C19*2 or *3 were included in that study. As expected, due to the low allele frequency of CYP2C19*2 (and lack of *3) in Caucasians, only four patients in our cohort were homozygous for this allele, and heterozygous and homozygous carriers of *2 were thus grouped together for statistical analysis. A larger patient population with more poor metabolizers would be required for proper analysis of the gene–dose effect of CYP2C19*2 in Caucasians. Another difference between the current study and that by Uno et al. [15] is that the Asian study addressed warfarin kinetics after a fixed single 10-mg dose while our data are from steady-state conditions using individually titrated warfarin doses.
The CYP2C haplotypes with frequencies higher than 10% in our Italian population were the same as those previously reported in Nordic populations [17]. Consistently, CYP2C19*17 was observed with an allele frequency of 17 % and in strong LD with CYP2C9*1 and CYP2C8*1. This result supports the observed correlation between CYP2C19*17 and R-warfarin clearance being independent from the other SNPs assessed in this study. Our analysis also confirmed the moderately strong LD between CYP2C8*3 and CYP2C9*2.
In addition to the effect of CYP2C19 genotypes on R-warfarin clearance, an association with warfarin response was observed, using warfarin INR/daily dose as a marker. CYP2C19 genotypes accounted for 7 % of the variance in warfarin INR/daily dose. Genetic (VKORC1, CYP2C9, and CYP2C19) and non-genetic (age, sex, and body weight) covariates together explained 52 % of the variability. Although the impact of CYP2C19 genotypes was much smaller than that of VKORC1 and CYP2C9, it was nevertheless a significant factor. This is in line with the results of a recent PK/PD study [14] suggesting that the R-enantiomer does indeed contribute to the anticoagulant effect of warfarin, based on both separate and combined administration of pure warfarin enantiomers. Moreover, an uncommon CYP2C19 SNP, rs3814637, has been reported to be significantly associated with elevated INR values during the first week of treatment, stable dose, and warfarin sensitivity [9, 21]. This SNP was subsequently included in a population PK model and correlated to reduced R-warfarin clearance [22]. In that study, the SNP rs3814637 was not in LD with CYP2C19*2 and it is unclear whether this SNP is functional or acts as a marker for additional unknown functional SNPs. This SNP was not analyzed in the present study due to its low frequency (6 %) and high LD with CYP2C9*3 [9, 21].
In conclusion, the clearance of R-warfarin was approximately 30 % higher in Italian warfarin-treated patients carrying CYP2C19*17 compared to CYP2C19*2. CYP2C19 genotypes also contributed significantly to the variability of warfarin INR/daily dose, indirectly indicating a clinical relevance of the R-enantiomer of warfarin. VKORC1 and CYP2C9 genotypes, however, remained the major genetic determinants of INR in relation to daily dose. Further studies are required to assess if adding CYP2C19 in genotype-based dosing algorithms would improve the prediction of warfarin dose requirement.
References
Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L et al (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106:2329–2333
Lind M, Fahlen M, Kosiborod M, Eliasson B, Oden A (2012) Variability of INR and its relationship with mortality, stroke, bleeding and hospitalisations in patients with atrial fibrillation. Thromb Res 129:32–35
O’Reilly RA (1974) Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther 16:348–354
Kaminsky LS, de Morais SM, Faletto MB, Dunbar DA, Goldstein JA (1993) Correlation of human cytochrome P4502C substrate specificities with primary structure: warfarin as a probe. Mol Pharmacol 43:234–239
Kim SY, Kang JY, Hartman JH, Park SH, Jones DR et al (2012) Metabolism of R- and S-warfarin by CYP2C19 into four hydroxywarfarins. Drug Metab Lett 6:157–164
Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M et al (2002) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther 72:702–710
Hamberg AK, Wadelius M, Lindh JD, Dahl ML, Padrini R et al (2010) A pharmacometric model describing the relationship between warfarin dose and INR response with respect to variations in CYP2C9, VKORC1, and age. Clin Pharmacol Ther 87:727–734
Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ et al (2009) The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 113:784–792
Jorgensen AL, Al-Zubiedi S, Zhang JE, Keniry A, Hanson A et al (2009) Genetic and environmental factors determining clinical outcomes and cost of warfarin therapy: a prospective study. Pharmacogenet Genomics 19:800–812
Lenzini P, Wadelius M, Kimmel S, Anderson JL, Jorgensen AL et al (2010) Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther 87:572–578
Horne BD, Lenzini PA, Wadelius M, Jorgensen AL, Kimmel SE et al (2012) Pharmacogenetic warfarin dose refinements remain significantly influenced by genetic factors after one week of therapy. Thromb Haemost 107:232–240
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL et al (2013) A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 369:2283–2293
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH et al (2013) A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 369:2294–2303
Maddison J, Somogyi AA, Jensen BP, James HM, Gentgall M et al (2013) The pharmacokinetics and pharmacodynamics of single dose (R)- and (S)-warfarin administered separately and together: relationship to VKORC1 genotype. Br J Clin Pharmacol 75:208–216
Uno T, Sugimoto K, Sugawara K, Tateishi T (2008) The effect of CYP2C19 genotypes on the pharmacokinetics of warfarin enantiomers. J Clin Pharm Ther 33:67–73
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Pedersen RS, Brasch-Andersen C, Sim SC, Bergmann TK, Halling J et al (2010) Linkage disequilibrium between the CYP2C19*17 allele and wildtype CYP2C8 and CYP2C9 alleles: identification of CYP2C haplotypes in healthy Nordic populations. Eur J Clin Pharmacol 66:1199–1205
Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD et al (2006) Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 16:101–110
Baldwin RM, Ohlsson S, Pedersen RS, Mwinyi J, Ingelman-Sundberg M et al (2008) Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol 65:767–774
Ohlsson Rosenborg S, Mwinyi J, Andersson M, Baldwin RM, Pedersen RS et al (2008) Kinetics of omeprazole and escitalopram in relation to the CYP2C19*17 allele in healthy subjects. Eur J Clin Pharmacol 64:1175–1179
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J et al (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121:23–34
Lane S, Al-Zubiedi S, Hatch E, Matthews I, Jorgensen AL et al (2012) The population pharmacokinetics of R- and S-warfarin: effect of genetic and clinical factors. Br J Clin Pharmacol 73:66–76
Acknowledgments
We gratefully acknowledge our collaborators Prof. Roberto Padrini and Dr Vittorio Pengo at the Department of Clinical and Experimental Medicine, University of Padova, Padova, Italy, for providing the clinical data. We thank Larissa Koukel (Department of Clinical Pharmacology, Karolinska University Hospital) and Hugo Kohnke (Department of Clinical Chemistry and Pharmacology, Uppsala University Hospital) for SNP analysis. This work was financially supported by the Swedish Research Council (521-2012-2592).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, M., Söderberg, M.M., Scordo, M.G. et al. CYP2C19*17 affects R-warfarin plasma clearance and warfarin INR/dose ratio in patients on stable warfarin maintenance therapy. Eur J Clin Pharmacol 71, 433–439 (2015). https://doi.org/10.1007/s00228-015-1812-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1812-4