Abstract
Speciation processes in the marine environment are often directly associated with vicariant events. In the case of loliginid squids (Cephalopoda: Loliginidae), these processes have been increasingly elucidated in recent years with the development of molecular technologies and increased sampling in poorly studied geographical regions, revealing a high incidence of cryptic speciation. Doryteuthis pealeii is a commercially important squid species for North Atlantic fisheries and has the second broadest geographic distribution in this genus. This study aimed to investigate the evolutionary history of this species and which biogeographic events may have influenced its diversification by assessing mitochondrial and nuclear markers. Our findings indicate that two previously detected lineages diverged from one another ~ 8 million years, compatible with the formation of the Caribbean and the establishment of the Amazon plume. Furthermore, separation between a North Atlantic and a Gulf of Mexico lineage during the Pleistocene period was noted. The inadequate classification of this cryptic diversity may have negative implications for the development of effective conservation and fisheries measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main goals of studies of evolutionary research is to identify which forces were responsible for shaping the evolutionary history of extant taxa (Cowman and Bellwood 2013). Evolutionary mechanisms that act in marine environments and on marine species differ from those affecting terrestrial environments and species (Mayr 1954). The relative importance of population isolation versus interconnectivity is a central point in this discussion, alongside the premise that allopatric barriers comprise the primary speciation process initiation point and, in many cases, the promoting factor (Bowen et al. 2013). Connectivity between populations plays a key role in the dynamics of species that inhabit marine environments, where population genetic diversity and differentiation are directly affected by three fundamental evolutionary forces: natural selection, genetic drift, and gene flow (Slatkin 1987).
It has long been thought that marine species typically exhibit broad geographic distributions with continuously interconnected populations (Cox and Moore 2005). This view, however, has changed in recent years, with the observation of spatially disjunct populations resulting from speciation events associated with past vicariant events (Cowman and Bellwood 2013; Sales et al. 2017; Bowen et al. 2020). Thus, oceanographic regions presenting high environmental heterogeneity (e.g., in currents and temperature and salinity gradients) can represent substantial barriers to population connectivity and exert strong speciation pressure, most notably on species with wide distributions across large latitudinal ranges (Hare et al. 2005; Galarza et al. 2009). The role of oceanographic heterogeneity as a speciation-promoting factor has not, however, been clearly investigated for some marine biogeographic regions (Cowman et al. 2017; Delic et al. 2020). In this sense, species with the ability to migrate or move throughout the water column would not be directly affected by soft barriers (Floeter et al. 2008).
Loliginidae (Lesueur 1821) comprises several commercially important squid species present in neritic and coastal regions worldwide, inhabiting both tropical and subtropical waters and present on the continental shelf, seagrasses, and coral reefs (Vecchione et al. 2005; Jereb and Roper 2010). Due to the high environmental heterogeneity of coastal and shelf habitats under distinct hydrological and oceanographic regimes, which directly influence the dispersal capacity of both adults and the paralarva stage (Boyle and Rodhouse 2005), loliginids have played a significant role as organisms capable of indicating past biogeographic events. For example, the absence of hard marine substrates for egg mass deposition has been shown to be a determinant in promoting speciation within some species (Sales et al. 2017; Costa et al. 2021; Jesus et al. 2021) as well as biological traits (Ibañez et al. 2023).
In recent years, molecular revisions have aided in the molecular delimitation and phylogenetic positioning of rare species or those with questionable taxonomic status (Sales et al. 2019; Anderson and Marian 2020; Avendaño et al. 2020; Pardo-Gandarillas et al. 2021). Molecular techniques have also demonstrated the presence of a high number of cryptic lineages within cephalopod species previously indicated as distributed across wide regions. For example, in the East Atlantic, the Strait of Gibraltar was found to separate lineages within Sepiolidae Leach, 1817 (Fernández-Álvarez et al. 2021), while in the southwestern Atlantic, ecological speciation linked to the utilization of new habitats has been proposed for Pickfordiateuthis G. L. Voss, 1953 (Anderson and Marian 2020), and the formation of the Caribbean and associated changes in suitable areas for reproduction has been proposed for Lolliguncula brevis (Blainville 1823) (Sales et al. 2014; Costa et al. 2021) and Doryteuthis pleii (Blainville 1823) (Sales et al. 2013; 2017). The latter genus comprises species of high economic importance, especially Doryteuthis pleii, Doryteuthis gahi (d’Orbigny, 1835), Doryteuthis opalescens (S. S. Berry, 1911), and Doryteuthis pealeii (Lesueur 1821) (Jereb and Roper 2010).
D. pealeii is widely distributed across shelf regions of the northwestern Atlantic Ocean and Caribbean Sea, from Newfoundland (47°N) to the Orinoco Delta (10°N), and is usually found far from the coast in colder regions (Jereb and Roper 2010). The species is similar in appearance, sympatric throughout the majority of the northwestern Atlantic Ocean, Gulf of Mexico, and the Caribbean Sea, with D. pleii (Sánchez et al. 1996; Jereb and Roper 2010). However, there is some evidence of distinct subpopulations within D. pealeii (Herke and Foltz 2002), and recent studies have indicated that the distribution of this species extends to the southwestern Atlantic, possibly representing a cryptic lineage (Sales et al. 2013). The main aims of the present study are to use a broader sampling to verify the presence and determine the degree of divergence of cryptic lineages within D. pealeii utilizing mitochondrial and nuclear markers, and discuss the biogeographic events that could have been responsible for causing diversification in these lineages.
Materials and methods
Sampling and molecular procedures
D. pealeii samples were collected in the Western Atlantic from 2009 to 2014, totaling 39 individuals from the southwestern Atlantic (33), the Gulf of Mexico (4), and the Western North Atlantic (2) (Fig. 1A, Table S1). All samples were obtained under a full license for biological material collection (Brazilian biodiversity collection authorization license: Permanent License SISBIO 5857–1) and SisGen research registration (AD53401). Two datasets were created, the first a population analysis database composed only of cytochrome C oxidase subunit I (cox1) sequences and the second, a divergence time inference database composed of sequences of two mitochondrial fragments, the mitochondrial large ribosomal subunit (rrnL, also known as 16S, and cox1) and one nuclear fragment (Rhodopsin). All molecular procedures (DNA extraction, PCR and sequencing of rrnL, cox1 and Rhodopsin gene fragments) were based on the protocols described by Sales et al. (2013, 2017).
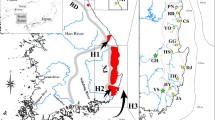
A Sampling places and sequence origin utilized in the present study. Numbers on the shorelines of Northern Brazil, Gulf of Mexico, and the US represent the number of sequences sampled from each point. Colors represent marine ecoregions according to Spalding et al. (2007). Dark blue: Virginian; Green: Northern Gulf of Mexico; light blue: Southern Gulf of Mexico and cream: Amazonia; B species tree generated in *BEAST for Doryteuthis based on three genes (rrnL, coxI, and Rhod); the ML and BI trees have a similar topology. Only support values greater than 80% (0.8) are presented. The support values correspond to ML and BI, respectively. Bars on the right represent the species delimitation methods results for each species/lineage; C haplotype network of coxI sequence data using the maximum-likelihood method (GTR + I + G model). Map projection on WGS 84
Sequence alignment, phylogenetic methods, and species delimitation
Sequences from Sales et al. (2013) and Costa et al. (2021) (rrnL, cox1, and Rhod) were implemented in addition to these in our concatenated dataset employed for divergence estimation, maximum likelihood and species tree inference, and reconstruction of ancestral states. For the population inference dataset (haplotype networks, species delimitation methods, and populational demography analyses), cox1 sequences from Herke and Foltz (2002) and Díaz-Santana-Iturrios et al. (2019) were added to the dataset generated herein, generating a final total of 85 sequences (Table S1).
For the rrnL sequences generated herein, the hypervariable regions containing indels were excluded by eye from the final pool to avoid any homologous alignment errors. The sequences were then aligned using the Clustal W tool (Thompson et al. 1997) available in the BioEdit v.7.2.6.1 program (Hall 1999). The most appropriate evolutionary models for each of the datasets for each marker were estimated using jModelTest 2 (Darriba et al. 2012) where the AIC criterion was used to determine the evolutionary model to be used for phylogenetic inferences employing the maximum-likelihood (ML) technique. The ML tree with the concatenated dataset of three markers (rrnL + cox1 + Rhod) was built using PhyML 3.0 (Guidon et al. 2010), employing 1000 bootstrap pseudo-replicates (Felsenstein 1985) for node support values under HKY + G for rrnL, GTR + I + G for cox1, and GTR + I for Rhod evolutionary models selected by jModelTest 2.
The StarBEAST3 procedure (Douglas et al. 2022) was run in BEAST 2.7 (Bouckaert et al. 2019) where the definition of each species was made based on the results obtained in the previously inferred ML tree, where only lineages with more than 80% branch support values in the ML tree were selected as separate species a priori. The substitution models of each data partition were then unlinked in BEAUTi and the models previously selected by jModelTest 2 (TN93 + I for rrnL, GTR + I + G for cox1, and TN93 for Rhod) were implemented. We then applied a Strict clock model and a Yule Process as species tree prior (Heled et al. 2011) to perform four simultaneous analyses using Markov Chain Monte Carlo (MCMC) simulations for 80 million generations, with one sample taken every 40,000 generations. Tracer 1.5 (Rambaut and Drummond 2009) was used to check ESS values; only runs with ESS values greater than 200 for all marginal parameters were used. The first 10% of the trees were discarded as burn-in during the production of a maximum clade credibility (MCC) tree using TreeAnnotator v. 2.5 (Bouckaert et al. 2019).
We performed three different species delimitation methods using the cox1 dataset: single- and multiple-threshold Generalized Mixed Yule Coalescent (s-GMYC and m-GMYC, Fujisawa and Barraclough 2013; Pons et al. 2006), Bayesian Poisson tree process (bPTP) (Zhang et al. 2013), and Automatic Barcode Gap Discovery (ABGD) (Puillandre et al. 2012). For the delimitation of mtDNA lineages using GMYC, we built an ultrametric tree. For this, the outgroups were removed and only one sequence per haplotype was maintained to avoid the accumulation of short branches on the tree. For the selection of unique haplotypes, we used the ElimDupes portal (https://www.hiv.lanl.gov/content/sequence/elimdupesv2/elimdupes.html). With this haplotype dataset, we estimated an ultrameric tree in BEAST v. 2.5 (Bouckaert et al. 2019) using the Yule model as a tree prior and a strict molecular clock. The analysis was performed with two independent runs of 2 × 107 MCMC generations each, sampling every 1,000 generations. Convergence of the chains and ESS values were verified in Tracer 1.5 (Rambaut and Drummond 2009). An MCC tree was obtained using TreeAnnotator (Bouckaert et al. 2019) to be used as an input tree for GMYC analyses. In the case of bPTP, an ML tree was constructed in PhyML 3.0 (Guidon et al. 2010) under the TrN + I model (-InL = 1233.6799) estimated in jModelTest 2 for the unique haplotype dataset. This tree was used as a guide tree. Both analyses (GMYC and bPTP) were then performed using the Species delimitation web server [Species delimitation server (h-its.org)], and for bPTP, the tree was reconstructed based on the GTRGAMMA model and 1,000 bootstrap iterations.
Finally, for ABGD, analyses were performed through the ABGD web server (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html) using three distinct distance options (Jukes-Cantor, Kimura 2-parameter and Simple), intraspecific prior ranges from 0.001 to 0.25 in ten steps and a relative gap width of 1.5 following the default parameters of ABGD (Puillandre et al. 2012).
Divergence time estimation and historical biogeographic analyses
The divergence times between D. pealeii lineages were estimated using BEAST 2.5 (Bouckaert et al. 2019) employing the partitions of the three genes. The HKY + I + G (rrnL, cox1) and TrNef + I + G (Rhod) models were indicated as optimal, but the model used for the Rhodopsin dataset was GTR + I + G; TrNef is not available in BEAUti. The Yule speciation prior was used for the tree prior, modeled with a relaxed log-normal clock (Drummond et al. 2006). Relaxed log-normal distributions were employed for all calibration priors. We used the same calibration points as Sales et al (2017) and included the ones estimated for Lolliguncula in Costa et al. (2021), as follows: (a) origin of Vampyromorpha (~ 162 million years ago) (Fischer and Riou 2002; Strugnell et al. 2006), (b) estimated origin of Doryteuthis (41.2–47.8 mya) (Neige et al. 2016), (c) probable origin of Loligo Lamarck, 1798 (~ 44 mya) based on loliginid statoliths found from mid-Eocene (Clarke and Maddock 1988; Strugnell et al. 2006), (d) the split between Pacific and Atlantic Lolliguncula lineages (~ 18.4 mya), (e) the middle Miocene orogeny of the Caribbean plate (~ 15.8 mya), (f) the separation of the northern and southern L. brevis lineages (~ 13.7 mya), and (g) a ~ 3 mya statolith fossil identified as belonging to D. opalescens (Clarke and Fitch 1979). Four independent MCMC runs with 200 million interactions were used, with samples taken every 1000 generations. The MCMC log files were combined in Tracer 1.5 (Rambaut and Drummond 2009) to summarize posterior divergences times with 95% highest posterior density limits; only values equal to or higher than 200 for all marginal parameters were used after discarding 10% of the first trees as burn in.
RASP 4 (Yu et al. 2020) was utilized for biogeographic ancestral area reconstruction employing the Bayesian Binary Method (BBM). The TMRCA tree generated previously for the concatenated data set was used as a guide tree along with the sampling locations of the analyzed species summarized in Spalding et al (2007), employing ecoregion levels for reconstructions. The BBM analysis was run for seven million cycles, using ten chains, and one sampling every 100 cycles. The temperature was set at 0.1 and a fixed Jukes–Cantor model was used. The maximum number of areas for all nodes was set to four. All information was then summarized and plotted as a pie chart.
Demographic history
We used the cox1 dataset to estimate the genetic diversity indices of haplotype number (Hap), haplotype diversity (H), and nucleotide diversity (π) using Arlequin 3.01 (Excoffier et al. 2005), because it was more extensive. A neutrality test was performed to detect population expansion signals, using Tajima’s D (Tajima 1989) and Fu’s Fs (Fu 1997) with their respective P values based on 1000 coalescent simulations. The pairwise FST fixation index (Weir and Cockerham 1984) and an Analysis of Molecular Variance (AMOVA; Excoffier et al. 1992) were also applied to test for significant genetic differentiation among sampling areas. Genealogical haplotype relationships were obtained by creating a haplotype network using the maximum-likelihood algorithm estimated for the cox1 dataset in jModelTest 2 (Darriba et al. 2012) using the Haploviewer software (Salzburger et al. 2011).
The genetic structure of D. pealeii was inferred using GENELAND v4.0.3 (Guillot et al. 2005a, 2005b) implemented in R software (v.4.0.2), using Rstudio (R Core Team 2020). For these simulations, we used two datasets: cox1 and concatenated (rrnL + cox1 + Rhod) for estimating the similarity of populations across the sampling areas. Five independent runs were performed with 5,000,000 Monte Carlo Markov Chain (MCMC) iterations, sampling every 100 iterations, and discard of the first 10% of samples as a ‘‘burn-in” phase. The best run was selected using the greatest mean posterior probability using the post-processing function, and the ‘‘Map of population membership” and ‘‘Map of probability of population membership” produced based on populations identified under Hardy–Weinberg equilibrium with linkage equilibrium between loci (HWLE). Nucleotide distance matrix, based on uncorrected p distances, was generated in MEGA 11 (Tamura et al. 2021) for cox1 dataset.
Results
Phylogeographical inferences
Both phylogenetic reconstructions supported the separation of the South Atlantic D. pealeii lineage from the North Atlantic/Gulf of Mexico lineages, with high statistical support values (100/1 for ML/BI, respectively, Fig. 1B) and with 22 mutations separating South Atlantic populations in relation to the North Atlantic/Gulf of Mexico haplotype clusters in cox1 (Fig. 1C). A second structuring, indicated by high Bayesian posterior probability and moderate ML support was detected within the North Atlantic/the Gulf of Mexico lineage, indicating the possible presence of another cryptic D. pealeii lineage in the region (Fig. 1B). The s-GMYC and m-GMYC species delimitation analyses separated D. pealeii from Gulf of Mexico, Gulf of Mexico (North Atlantic) and North Atlantic, but there was no genetic separation between these sampling locations under the bPTP and ABGD analyses (Fig. 1B).
According to the TMRCA analysis, the time of separation between Lolliguncula and Doryteuthis was estimated at ~ 49.80 mya (Fig. 2, node I, Table 1). Separation of the clade containing the ancestors of most Atlantic lineages from the D. gahi/D. sanpaulensis/D. opalescens ancestor occurred ~ 40.22 mya (Fig. 2, node II, Table 1) followed by separation between D. gahi and the D. sanpaulensis/D. opalescens ancestor, at ~ 35.92 mya (Fig. 2, node III, Table 1). The separation between the ancestors of the D. pleii + D. surinamensis/D. pealeii species complex occurred ~ 34 mya (Fig. 2, node IV, Table 1), followed by the separation between D. sanpaulensis and D. opalescens (~ 30.76 mya, Fig. 2, node V, Table 1). The South Atlantic lineage of D. pleii (including samples from around the type locality from Isla Margarita) split from D. surinamensis at ~ 12 mya (Fig. 2, node VI, Table 1). The separation between the southern Atlantic D. pealeii lineages and the northern D. pealeii lineage and Gulf of Mexico D. pealeii lineage ancestor occurred ~ 8.5 mya (Fig. 2, node VII, Table 1), finally followed by the separation of these latter two D. pealeii lineages at approximately 2.11 mya (Fig. 2, node VIII, Table 1).
Estimate of TMRCA of Doryteuthis inferred from BEAST 2, using a relaxed log-normal clock and the concatenated three-gene dataset. Pie charts on the nodes represent the ancestral area reconstruction estimated with RASP4 (BBM). Red numbers represent nodes detailed in Table 1
The RASP 4 analysis indicated that the ancestral area for Doryteuthis was the Tropical Northwestern Atlantic region, with a high association with the Southern Gulf of Mexico ecoregion (42.77%) (Fig. 2, node I, Table 1). The Tropical Northwestern Atlantic region remains the primary ancestral area for the Lolliguncula + Doryteuthis ancestor, and the most recent common ancestor of Doryteuthis probably also lived in the Tropical Northwestern Atlantic (55.88%) (Fig. 2, node II, Table 1). The Malvinas/Falkland ecoregion was inferred as the ancestral area for the most common ancestor of D. gahi, D. sanpaulensis, and D. opalescens (62.63%) (Fig. 2, node III, Table 1). The ancestor of the remaining Doryteuthis lineages (D. pealeii, D. plei, and D. surinamensis) appears to have originated in the Southern Gulf of Mexico (82.90%) (Fig. 2, node IV, Table 1). This was followed by later dispersal of D. sanpaulensis to the Warm Temperate Southwestern Atlantic region, occupying the Southeastern Brazil ecoregion (43.27%), while D. opalescens became restricted to the Warm Temperate Northeast Pacific region, in the Southern California Bight ecoregion (27.50%) (Fig. 2, node V, Table 1). Later speciation processes within the genus resulted in distributions of the remaining lineages associated with the Guianan (D. pleii Southwestern + D. surinamensis) (26.68%) and Amazonian (D. pealeii South Atlantic) ecoregions (VI and VII nodes, respectively), but always with the predominant origins associated with the Southern Gulf of Mexico ecoregion (51.05% and 78.35%, respectively), especially for the two D. pealeii lineages present in the North Atlantic and Gulf of Mexico (Fig. 2, node VIII, Table 1).
Overall, the non-corrected nucleotide distances (p) estimated for the cox1 dataset show that the intraspecific average level of divergence estimated between D. pealeii from North Atlantic/Caribbean in relation to the South Atlantic D. pealeii lineage was 0.051 (~ 5.1%) corresponding to the lowest nucleotide diversity value of Doryteuthis (Table 2), but very close to other p distances associated with cryptic species in other loliginid genera.
Population and demographic analyses
Two distinct grouping schemes for D. pealeii, one based simply on geography and the other based on geography and phylogenetic history, were simulated. For the first one, AMOVA shows no structuring detected between the North Atlantic, Gulf of Mexico (USA) and the Gulf of Mexico. In the second, the North Atlantic/Gulf of Mexico localities formed one group and the South Atlantic another group (Table 3).
The second AMOVA results reinforced differentiation between these groups, with the highest differentiation index noted between groups (North Atlantic/Gulf of Mexico x South Atlantic, 88.28%) compared to within groups (11.51%) (Фst: 0.88 P > 0.05). The same pattern was noted when comparing the Fst indices, where the South Atlantic populations exhibited high and significant differentiation indices compared to the North Atlantic, Gulf of Mexico (North Atlantic), and Gulf of Mexico populations (Table 4). Following the AMOVA structure recovered in our study, GENELAND analysis supported two genetically distinct groups (K = 2) with high posteriori probability (bars on Fig. 3a and b). All samples from the South Atlantic had isolated from the Gulf of Mexico/Gulf of Mexico (North Atlantic) and North Atlantic groups with a posterior attribution probability of 90%, whereas the other five sampling locations had posterior probabilities of attribution to that group of only 10% (Fig. 3A). The opposite pattern [isolation of Gulf of Mexico/Gulf of Mexico (North Atlantic) and North Atlantic with 90% posterior probabilities in relation to South Atlantic] was recovered (Fig. 3B).
Population structure of Doryteuthis pealeii. The highest probability is indicated in the bar graphs in each figure. A Spatial posterior probability of belonging to northwestern Atlantic D. pealeii populations; B spatial posterior probability of belonging to south Atlantic D. pealeii populations. Map projection on WGS 84. Colors representing each population are as described for Fig. 1
A total of 39 haplotypes were defined by variation in the cox1 fragment sequenced here and showing high haplotypic and low nucleotide diversity (Table 5). Individually, the North Atlantic and Gulf of Mexico populations displayed the highest haplotypic diversity rates, with the latter displaying the highest number of unique haplotypes. In the case of the South Atlantic population, haplotypic diversity was almost half that estimated for the North Atlantic and Gulf of Mexico populations, even though a higher number of sampled individuals was assessed (Table 5). Values of Fu’s Fs for all populations were negative and significant. Values of Tajima’s D were negative (also indicative of population expansions) but not significant for all populations (Fig. 2).
Discussion
The cryptic history of Doryteuthis diversity
Sales et al. (2013) performed the first molecular phylogenetic analyses including D. pleii and D. pealeii samples from the South Atlantic. The results clearly indicated that these species each comprise two genetically distinct clades, one occupying the Gulf of Mexico and North Atlantic and the second, the northern portion of the South Atlantic. This pattern was later confirmed for D. pleii, where, even following an extremely broad sampling of the South Atlantic lineage (Venezuela to Southern Brazil), the two lineages (North Atlantic/Gulf of Mexico and South Atlantic) were very well defined (Sales et al. 2017). In this study, we observed three different lineages of D. pealeii in the North Atlantic, the Gulf of Mexico and the South Atlantic by analyzing regions of two mitochondrial genes and one nuclear gene.
The cox1 genetic p distances inferred here are also concordant with previous distances inferred for other cryptic loliginid lineages (Cheng et al. 2014; Sales et al. 2013, 2014, 2017) as well between valid species of Loligo (5.7% between Loligo reynaudii d'Orbigny [in Ferussac & d'Orbigny], 1839–1841 and Loligo. vulgaris Lamarck, 1798) where it reflects a very recent divergence (Costa et al. 2021). The divergence between populations of D. pealeii from the North Atlantic and South Atlantic is therefore comparable to species-level divergence observed in other loliginid squid. The Fs and D values suggest a historical population expansion, and it seems that D. pealeii lineages most likely followed a similar process as that proposed for D. pleii lineages (Sales et al. 2017) as the data show the same patterns (higher haplotype diversity in the northern lineage and lower haplotype diversity in the southern lineage).
A genetic break between D. pealeii populations in the Gulf of Mexico and the North Atlantic has been previously reported (Herke and Foltz 2002), although no sign of cryptic speciation was noted by the authors. Their use of only cox1 may have been responsible for this proposition as no separation between the North Atlantic and Gulf of Mexico lineages was observed in the present study when analyzing the datasets for individual genes, reinforcing the need for phylogenetic investigations employing multiple markers reflecting different evolutionary rates, as well as adequate phylogenetic reconstruction methods that minimize the effect of incomplete lineage sorting (ILS) (Maddison and Knowles 2006), as well as sampling along the entire known area of occurrence of the target species, to increase the phylogenetic signal of the reconstructions (Zwickl and Hillis 2002).
The utility of these approaches has been shown in previous species assessments within Decapodiformes (Anderson et al. 2011; Fernández-Álvarez et al. 2021; Sanchez et al. 2021) and especially for loliginids (Anderson et al. 2008; Sales et al. 2013; 2014; Cheng et al. 2014; Anderson and Marian 2020; Costa et al. 2021). The presence of two additional lineages within D. pealeii raises some additional questions. Cohen (1976) described two species of Doryteuthis (Loligo at the time) from the West Atlantic: one similar to D. pealeii, but apparently limited to the Caribbean Sea, from the edge of the Grand Bahamas Bank near Punta Alegre in Cuba (Doryteuthis ocula Cohen 1976) and another, similar to D. pleii, with a wider distribution, occurring in both the Caribbean Sea and in the Gulf of Mexico, with the type specimen from Bimini Island, Bahamas (Doryteuthis roperi) (Cohen 1976; Jereb and Roper 2010). However, these latter two species are the subject of recent debates about their validity (Sales et al. 2013; Díaz-Santana-Iturrios et al. 2019).
Doryteuthis ocula has been confused with D. pealeii and D. roperi with D. pleii (Cohen 1976; Jereb and Roper 2010; Díaz-Santana-Iturrios et al. 2019). For the latter, Díaz-Santana-Iturrios et al. (2019) performed morphological, morphometric, and genetic revisions of loliginid species present in the Gulf of Mexico, utilizing museum specimens of D. roperi. The authors pointed out several similarities between D. pleii and D. roperi, and noted that it was impossible to separate females of the two species due to overlap of the described morphological characteristics (Blainville 1823; Cohen 1976). For the males, no consistent differences were observed in hectocotylus sucker counts or in the shape of the pedicels of the hectocotylized suckers; however, the authors state that the full sexual maturity of all the examined males was warranted. The authors concluded that although the lack of consistent differences suggests that D. pleii and D. roperi represent the same species, molecular evidence was necessary to test this hypothesis. Based on this, the authors suggest that these characters (sucker counts and pedicel shape) must not be considered for identification for Doryteuthis species (Díaz-Santana-Iturrios et al. 2019), with similar suggestions also made for Lolliguncula (Sales et al. 2014). However, the results of Díaz-Santana-Iturrios et al. (2019) must be interpreted with caution, because the authors do not clearly state whether the individuals analyzed in their study were mature or not (they considered the “size-at-maturity” of these squids and determined that every squid larger than that size was mature). Size at maturity can be very variable for squid species (Juanicó 1983; Forsythe and Van Heukelem 1987; Hatfield and Cadrin 2002).
Only three species of Doryteuthis (D. pleii, D. pealeii, and D. ocula) have been reported for the Gulf of Mexico (Judkins et al. 2009; Jereb and Roper 2010). We speculate that D. ocula and the lineage of D. pealeii reported for the Gulf of Mexico in the present study could be the same species, a question that can only be clarified with further morphological and molecular comparisons. Three Doryteuthis species have been previously listed for the South Atlantic coastal waters: Doryteuthis surinamensis (G. L. Voss, 1974), D. pleii, and Doryteuthis sanpaulensis (Brackoniecki, 1984). The latter was described by Brackoniecki (1984) after he considered Loligo brasiliensis Blainville 1823 a nomen dubium, that was also used for other related species in Blainville's original description (Brakoniecki 1984).
Until recently, the southern limit of the distribution of D. pealeii was the Gulf of Venezuela in the Caribbean Sea (Jereb and Roper 2010). However, Sales et al (2013) identified D pealeii collected from the Northern Coast of Brazil based on their phylogenetic proximity with the North Atlantic lineage, for which the type specimen from South Carolina was described by LeSueur (1821). Further comparative morphological and molecular studies are necessary to establish the taxonomic status of the southern lineage of D. pealeii to clarify the present diversity of Doryteuthis from the Western Atlantic.
Biogeographic implications
Vicariant events in Caribbean biogeography have impacted the evolution of several marine species (Bellwood et al. 2004; Baums et al. 2005; Rocha et al. 2005; Sales et al. 2017; Costa et al. 2021). The separation time between Doryteuthis and Lolliguncula estimated in the present study is within the confidence interval proposed by Costa et al. (2021) for the separation between these genera and possibly the formation of shallower waters associated with the beginning of the formation of the Isthmus of Panama (~ 40 mya) (O’Dea et al. 2007) affecting ancestors belonging to both genera. Although Doryteuthis was not the focus of Costa et al. (2021), some of the biogeographic patterns inferred by those authors were also recovered here (i.e., dispersion routes of Doryteuthis and Lolliguncula) and the timing of cladogenetic events was concordant between both studies. However, there are some differences between our results and the results of Ulloa et al. (2017). After diverging from the Lolliguncula lineage, two major Doryteuthis clades were formed followed by an extinction event in the Southern Gulf of Mexico ecoregion (Table 1, node II). One clade comprised the ancestor of the western Atlantic Doryteuthis species, and another comprised the D. gahi lineage and the ancestor of D. sanpaulensis and D. opalescens. In our study, the D. gahi lineage arose ~ 35.9 mya in the Malvinas/Falkland ecoregion, resulting in reconstruction of the southwestern Atlantic as the ancestral area of Doryteuthis, as proposed by Ulloa et al. (2017).
By that time, the Caribbean plate was compressed as it was forced between the westward-drifting North American and South American plates, resulting in uplift of the Aves Ridge (Karig 1972) to form an ostensibly continuous land bridge linking the Puerto Rico region to northern South America (Ali and Hedges 2021) establishing the Greater Antilles and Aves Ridge land bridge (GAARlandia) (MacPhee and Iturralde-Vinent 1994; Iturralde-Vinent and MacPhee 1999; Philippon et al. 2020; Garrocq et al. 2021). However, unlike the Pacific Lolliguncula species, the Tropical Northwestern Atlantic region and the Southern Gulf of Mexico ecoregion were the predominant ancestral areas for Doryteuthis. During this period, the D. gahi/sanpaulensis/opalescens ancestor separated from the ancestor of the other Atlantic lineages and dispersal took place toward colder waters to the North American Pacific, as well toward colder waters of southern South America.
During this period, the planet underwent severe climatic transitions, changing from warm, high-sea-level conditions during the Paleocene and early Eocene to a colder climate in the Oligocene, with rapid formation of the Antarctic glaciation at ~ 34 mya (Zachos et al. 2001; Miller et al. 2005). At this time, ocean cooling and opening of the Drake Passage around the southern tip of South American created a mixed region of cold waters rich in nutrients, which, alongside pulses of warm waters and winds, produced an upwelling region with increasing primary productivity, especially in the Tropical and Subtropical Atlantic (Zachos et al. 1996; Suto et al. 2012). This process may have shaped the distribution of D. gahi, as well as the distribution of D. sanpaulensis in the subtropical Atlantic region, as reflected in the TMRCA estimated here and the routes estimated with RASP 4 (Malvinas/Falkland ecoregion).
Additionally, the decreasing oceanic temperatures of this period generated several sharp primary productivity reductions, leading to changes in the abundance of both nektonic and planktonic organisms, which generated regions containing lower concentrations of marine resources (Shackleton et al. 1984; Rea et al. 1991; Zachos et al. 1996). Thermohaline and atmospheric circulation also intensified, leading to high oceanic turnover rates (Mackensen and Ehrmann 1992; Diester-Haass 1992), thus moving primary productivity zones previously associated with coastal zones to shelf areas (Sarnthein et al. 1987). These changes in primary productivity coupled with decreasing marine temperatures may have been responsible for shaping the dispersal of the ancestor of the D. pleii/D. surinamensis species complex to the southwestern Atlantic, where this environment was more suitable to maintaining populations of these two species and making them more associated with coastal regions, which was different from the D. pealeii species complex ancestor. These temperature and primary productivity changes may have also influenced the vicariance and subsequent dispersal of D. sanpaulensis to the Southeastern Brazil ecoregion and D. opalescens to the Southern California Bight (27.50%) via the South Atlantic/Western Pacific, as indicated by RASP 4. Also, at that time (~ 30 mya), there was no land connection between North and South America, meaning that it is possible that this dispersal occurred directly from the NW Atlantic to the NE Pacific.
The two D. pleii lineages appear to have separated from each other during the formation of the Lesser Antilles during the Middle Miocene (~ 16 mya) as part of the tectonic processes that formed the Caribbean plate (the orogeny of the western portion of the Andes), which modified the plate between the Caribbean and South America in a convergent fault, intensifying local volcanism (James 2005). Additionally, a series of changes to local ocean circulation currents caused by global ocean circulation pattern alterations (De Schepper et al. 2015; Cárcamo-Tejer et al. 2021) resulted in sea floor topography alterations, perhaps affecting substrate availability for egg laying by squids.
The continuity of the Andes uplift, especially during the late Miocene (~ 10 mya), altered the course of the Amazon River (Figueiredo et al. 2009; Hoorn et al. 2017), creating a less saline and more turbid environment in the Western South Atlantic. At this time, significant sea level changes took place globally, decreasing by more than 70 m compared to today, and more than 100 m in relation to the previous sea level (Haq et al. 1988). This probably constituted the combination of events responsible for the vicariance between D. pleii and D. surinamensis in the region, since this phenomenon has been already associated with speciation in other marine taxa in the region (Tourinho el al. 2012; Rodrigues-Filho et al. 2023). Doryteuthis surinamensis became more associated with the Amazon plume-influenced region (Guianan ecoregion, 26.68%) in contrast with D. pleii, which had recently separated from the North Atlantic/Gulf of Mexico lineage and may have been undergoing the same vicariance process as D. pealeii.
However, while D. pleii is distributed in the western South Atlantic (north and south of the Amazon River plume), D. pealeii is not found in the same region (Isla Margarita, Venezuela) where Sales et al (2017) sampled D. pleii (João Braullio Sales, pers. comm.). The Northwestern Atlantic D. pealeii lineage may have dispersed to the Southwestern Atlantic (Amazonia ecoregion), and the divergence time estimated in the present study between these lineages coincides with the beginning of the establishment in the Western South Atlantic Amazon plume (Figueiredo et al. 2009; Hoorn et al. 2017). The significant temperature fluctuations caused by intensification of Northern Hemisphere glaciations (NHG) at ~ 2.75 mya (Ravelo et al. 2004) also affected global sea levels (Pillans et al. 1998; Miller et al. 2005), which play a significant role in marine and coastal taxa (Floeter et al. 2008; Pardo-Gandarillas et al. 2018; Costa et al. 2021).
These oscillations had a major impact on the distribution of marine and coastal species in various regions, such as the Gulf of Mexico and the North Atlantic. Marine cooling may have forced populations southward, which may have influenced contact between Atlantic and Gulf populations in Mexico in the region now known as South Florida (Avise 1992), where Northern Hemisphere Glaciation (NHG) caused sea level drops of over 150 m, exposing tremendous expanses of the Florida and Yucatan peninsulas, where Florida was bordered by some intermediate-salinity estuaries and saltmarshes, favoring coastal species. This glacial advance may have contributed to the separation of some coastal populations from the Atlantic and Gulf through the establishment of isolated estuarine habitats in the western portion of the Gulf of Mexico, thus favoring speciation between the North Atlantic and Gulf of Mexico D. pealeii lineages (Avise 2006) present in these areas at the time, as has been inferred for other marine species in the same region (Gold and Richardson 1998; Anderson 2007; Anderson et al. 2012).
The present study provides new evidence of potential cryptic speciation within Doryteuthis around the American continent, and suggests that historical biogeographic processes were responsible for shaping the current species composition and distributions within the genus, as seen in another Doryteuthis species (such as D. pleii), as well as within Lolliguncula. The separation between D. pealeii lineages between the Gulf of Mexico and the North Atlantic must, however, be analyzed cautiously, as there are previous proposals of multiple populations (Buresch et al. 2006; Gerlach et al. 2012) as a single stock (Shaw et al. 2012). The recent implementation of next-generation sequencing methods has helped resolve several taxonomic problems for cephalopod species (Anderson and Lindgren 2021; Sanchez et al. 2021) and may aid in elucidating the speciation between the North Atlantic and the Caribbean. Additionally, a broad morphological revision must be conducted for D. pleii and D. pealeii from North Atlantic and the Caribbean to clarify the status of D. ocula and D. roperi. The lineage of D. pealeii detected in the Western South Atlantic represents a new species for the region which reinforces the high cryptic diversity of cephalopods in the Atlantic.
Data and code availability
Biological, distributional data, and sequences were taken from open sources (i.e., GenBank, FAO books).
References
Ali JR, Hedges SB (2021) Colonizing the Caribbean: new geological data and an updated land-vertebrate colonization record challenge the GAARlandia land-bridge hypothesis. J Biogeogr 48(11):2699–2707
Anderson JD (2007) Systematics of the North American menhadens: molecular evolutionary reconstructions in the genus Brevoortia (Clupeiformes: Clupeidae). US Natl Mar Fish Serv Fish Bull 205:368–378
Anderson FE, Lindgren AR (2021) Phylogenomic analyses recover a clade of large-bodied decapodiform cephalopods. Mol Phylogenet Evol 156:107038
Anderson FE, Marian JEA (2020) The grass squid Pickfordiateuthis pulchella is a paedomorphic loliginid. Mol Phylogenet Evol 147:106801
Anderson FE, Pilsits A, Clutts S, Laptikhovsky V, Bello G, Balguerías E et al (2008) Systematics of Alloteuthis (Cephalopoda: Loliginidae) based on molecular and morphometric data. J Exp Mar Biol Ecol 364:99–109
Anderson FE, Engelke R, Jarrett K, Valinassab T, Mohamed KS, Aokan PK et al (2011) Phylogeny of the Sepia pharaonis species complex (Cephalopoda: Sepiida) based on analyses of mitochondrial and nuclear DNA sequence data. J Molluscan Stud 77:65–75
Anderson JD, Karel WJ, Mione AC (2012) Population structure and evolutionary history of Southern Flounder in the Gulf of Mexico and western Atlantic Ocean. Trans Am Fish Soc 141(1):46–55
Avendaño O, Roura Á, Cedillo-Robles CE, González ÁF, Rodríguez-Canul R, Velázquez-Abunader I, Guerra Á (2020) Octopus americanus: a cryptic species of the O. vulgaris species complex redescribed from the Caribbean. Aquat Ecol 54(4):909–925
Avise JC (1992) Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos 63:62–76
Avise JC (2006) Evolutionary pathways in nature: a phylogenetic approach. Cambridge University Press
Baums IB, Miller MW, Hellberg ME (2005) Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol 14(5):1377–1390
Bellwood DR, van Herwerden L, Konow N (2004) Evolution and biogeography of marine angelfishes (Pisces: Pomacanthidae). Mol Phylogenet Evol 33(1):140–155
Blainville HD (1823) Memoire sur les especes du genre Calmar (Loligo, Lamarck). J Phys Chim Hist Nat Arts 96:116–135
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A et al (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15:1–28. https://doi.org/10.1371/journal.pcbi.1006650
Bowen BW, Rocha LA, Toonen RJ, Karl SA (2013) The origins of tropical marine biodiversity. Trends Ecol Evol 28(6):359–366
Bowen BW, Forsman ZH, Whitney JL, Faucci A, Hoban M, Canfield SJ et al (2020) Species radiations in the sea: what the flock? J Heredity 111(1):70–83
Boyle PR, Rodhouse PG (2005) Cephalopods: ecology and fisheries. Blackwell Science Ltd., Oxford, p 438
Brakoniecki TF (1984) A full description of Loligo sanpaulensis, Brakoniecki, 1984 and a redescription of Loligo gahi D’Orbigny, 1835, two species of squid (Cephalopoda; Myopsida) from the Southwest Atlantic. Bull Mar Sci 34(3):435–448
Buresch KC, Gerlach G, Hanlon RT (2006) Multiple genetic stocks of longfin squid Loligo pealeii in the NW Atlantic: stocks segregate inshore in summer, but aggregate offshore in winter. Mar Ecol Progr Ser 310:263–270
Cárcamo-Tejer V, Vila I, Llanquín-Rosas F, Sáez-Arteaga A, Guerrero-Jiménez C (2021) Phylogeography of high Andean killifishes Orestias (Teleostei: Cyprinodontidae) in Caquena and Lauca sub-basins of the Altiplano (Chile): mitochondrial and nuclear analysis of an endangered fish. PeerJ 9:e11917. https://doi.org/10.7717/peerj.11917
Cheng SH, Anderson FE, Bergman A, Mahardika GN, Muchilsin ZA, Dang BT et al (2014) Molecular evidence for co-occurring cryptic lineages within the Sepioteuthis cf. lessoniana species complex in the Indian and Indo-West Pacific Oceans. Hydrobiologia 725:165–188
Clarke MR, Maddock L (1988) Statoliths of fossil coleoid cephalopods. In: Clarke MR, Trueman ER (eds) The mollusca. paleontology and neontology of cephalopods, vol 12. Academic Press, San Diego
Clarke MR, Fitch JE (1979) Statoliths of Cenozoic teuthoid cephalopods from North America. Palaeontology 22(2):479–511
Cohen AC (1976) The systematics and distribution of Loligo (Cephalopoda, Myopsida) in the western North Atlantic, with descriptions of two new species. Malacologia 15:229–367
Costa TA, Sales JB, Markaida U, Granados-Amores J, Gales SM, Sampaio I et al (2021) Revisiting the phylogeny of the genus Lolliguncula Steenstrup 1881 improves understanding of their biogeography and proves the validity of Lolliguncula argus Brakoniecki & Roper, 1985. Mol Phylogenet Evol 154:106968
Cowman PF, Bellwood DR (2013) The historical biogeography of coral reef fishes: global patterns of origination and dispersal. J Biogeogr 40(2):209–224
Cowman PF, Parravicini V, Kulbicki M, Floeter SR (2017) The biogeography of tropical reef fishes: endemism and provinciality through time. Biol Rev 92(4):2112–2130
Cox CB, Moore PD (2005) Biogeography: an ecological and evolutionary approach, 7th edn. Blackweel, Oxford
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772–772
De Schepper S, Schreck M, Beck KM, Matthiessen J, Fahl K, Mangerud G (2015) Early Pliocene onset of modern Nordic Seas circulation related to ocean gateway changes. Nat Commun 6:8659
Delić T, Stoch F, Borko Š, Flot JF, Fišer C (2020) How did subterranean amphipods cross the Adriatic Sea? Phylogenetic evidence for dispersal–vicariance interplay mediated by marine regression–transgression cycles. J Biogeogr 47(9):1875–1887
Díaz-Santana-Iturrios MD, Salinas-Zavala CA, Granados-Amores J, de la Cruz-Agüero J, García-Rodríguez FJ (2019) Taxonomic considerations of squids of the family Loliginidae (Cephalopoda: Myopsida) supported by morphological, morphometric, and molecular data. Mar Biodiver 49(5):2401–2409
Diester-Haass L (1992) Late Eocene-Oligocene sedimentation in the Antarctic Ocean, Atlantic sector (Maud Rise, ODP Leg 113, Site 689): development of surface and bottom water circulation. The Antarctic Paleoenvironment: a Perspective on Global Change. Part One 56:185–202
Douglas J, Jimenez-Silva CL, Bouckaert R (2022) Starbeast3: adaptive parallelised bayesian inference under the multispecies coalescent. System Biol 71:901–916. https://doi.org/10.1093/sysbio/syac010
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4(5):e88. https://doi.org/10.1371/journal.pbio.0040088
Excoffier L, Smouse PE, Quattro M (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider F (2005) Arlequin, v. 3.11. An integrated software package for population genetic data analysis. Evol Bioinform 1:47–50
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fernández-Álvarez FÁ, Sánchez P, Villanueva R (2021) Morphological and molecular assessments of bobtail squids (Cephalopoda: Sepiolidae) reveal a hidden history of biodiversity. Front Mar Sci 7:632261
Figueiredo JJJP, Hoorn C, Van der Ven P, Soares E (2009) Late Miocene onset of the Amazon River and the Amazon deep-sea fan: evidence from the Foz do Amazonas Basin. Geology 37(7):619–622
Fischer JC, Riou B (2002) Vampyronassa rhodanica nov. gen. nov sp., vampyromorphe (Cephalopoda, Coleoidea) du Callovien inférieur de La Voulte-sur-Rhône (Ardèche, France). Annales de Paléontologie 88(1):1–17
Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith-Vaniz WF, Wirtz P et al (2008) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Forsythe JW, Van Heukelem WF (1987) Growth. In: Boyle PR (eds) Cephalopod life cycles, vol. II. Comparative review. Academic Press, London, pp 135–156
Fu YX (1997) Statistical tests of neutrality of mutations against population growth hitchhiking and background selection. Genetics 147:915–925
Fujisawa T, Barraclough TG (2013) Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. System Biol 62(5):707–724
Galarza JA, Carreras-Carbonell J, Macpherson E, Pascual M, Roques S, Turner GF, Rico C (2009) The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc Natl Acad Sci 106(5):1473–1478
Garrocq C, Lallemand S, Marcaillou B, Lebrun J-F, Padron C, Klingelhoefer F et al (2021) Genetic relations between the Aves Ridge and the Grenada back-arc Basin, East Caribbean Sea. J Geophys Res Solid Earth 126:e2020JB020466. https://doi.org/10.1029/2020J B020466
Gerlach G, Buresch KC, Hanlon RT (2012) Population structure of the squid Doryteuthis pealeii on the eastern coast of the USA: Comment on Shaw et al., 2010. Mar Ecol Progr Ser 450:281–283
Gold JR, Richardson LR (1998) Mitochondrial DNA diversification and population structure in fishes from the Gulf of Mexico and western Atlantic. J Heredity 89:404–414
Guidon S, Dufayard JF, Lefort V, Anisimova M, Hordjik W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. System Biol 59(3):307. https://doi.org/10.1093/sysbio/syq010
Guillot G, Estoup A, Mortier F, Cosson JF (2005a) A spatial statistical model for landscape genetics. Genetics 170:1261–1280
Guillot G, Mortier F, Estoup A (2005b) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5:712–715
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sympos Ser 41:95–98
Haq BH, Hardenbol J, Vail PR (1988) Mesozoic and Cenozoic chronostratigraphy and cycles of sea-level change. In: Wilgus C et al (eds) Sea-level changes: an integrated approach. society of economic paleontologists and mineralogists special publication 42, pp 71–108
Hare MP, Guenther C, Fagan WF (2005) Nonrandom larval dispersal can steepen marine clines. Evolution 59(12):2509–2517
Hatfield EMC, Cadrin SX (2002) Geographic and temporal patterns in size and maturity of the longfin inshore squid (Loligo pealeii) off the northeastern United States. Fish Bull 100(2):200–213
Heled J, Drummonf AJ, Xie W (2011) Bayesian inference of Species Trees from multilocus data using ∗BEAST. Mol Biol Evol 27(3):570–580
Herke SW, Foltz DW (2002) Phylogeograohy of two squid (Loligo pealei and L. plei) in the Gulf of Mexico and northwestern Atlantic Ocean. Mar Biol 140:103–115
Hoorn C, Bogotá-A GR, Romero-Baez M, Lammertsma EI, Flantua SGA, Dantas EL et al (2017) The Amazon at sea: onset and stages of the Amazon River from a marine record, with special reference to Neogene plant turnover in the drainage basin. Glob Planet Change 153:51–65
Ibáñez CM, Luna A, Márquez-Gajardo C, Torres FI, Sales JBL (2023) Biological traits as determinants in the macroecological patterns of distribution in loliginid squids. Mar Biol 170(11):133. https://doi.org/10.1007/s00227-023-04286-1
Iturralde-Vinent MA, MacPhee RDE (1999) Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bull Am Museum Nat Hist 238:1–95
James KH (2005) A simple synthesis of Caribbean geology. Caribb J Earth Sci 39:69–82
Jereb P, Roper CFE (2010) Family Loliginidae. In: Jereb P, Roper CFE (eds) Cephalopods of the world. An annotated and illustrated catalogue of species known to date (No. 4, Vol. 2. Myopsid and Oegopsid squids, 38–117 pp). Rome: Ed. by, C. F. E. FAO Species Catalogue for Fisheries Purposes, FAO
Jesus MD, Sales JBL, Martins RS, Ready JS, Costa TAS, Ablett JD, Schiavetti A (2021) Traditional knowledge aids description when resolving the taxonomic status of unsettled species using classical and molecular taxonomy: the case of the shallow-water octopus Callistoctopus furvus (Gould, 1852) from the western Atlantic Ocean. Front Mar Sci 7:595244
Juanicó M (1983) Squid maturity scales for population analysis. In: Caddy JF (eds) Advances in assessment of world cephalopod resource. FAO Fish. Tech. Pap 231:341–378
Judkins HL, Vecchione M, Roper CFE (2009) Cephalopoda (Mollusca) of the Gulf of Mexico. In: Felder DL, Camp DK (eds) Gulf of mexico–origins, waters, and biota. Biodiversity. A and M Press, College Station, pp 701–709
Karig DE (1972) Remnant arcs. Geol Soc Am Bull 83:1057–1068
Lesueur CA (1821) Descriptions of several new species of cuttlefish. J Acad Nat Sci Phila 2:86–101
Mackensen A, Ehrmann WU (1992) Middle Eocene through early Oligocene climate history and paleoceanography in the Southern Ocean: Stable oxygen and carbon isotopes from ODP sites on Maud Rise and Kerguelen Plateau. Mar Geol 108(1):1–27
MacPhee RDE, Iturralde-Vinent MA (1994) First Tertiary land mammal from Greater Antilles: an early Miocene sloth (Xenarthra, Megalonychidae) from Cuba. Am Museum Novitates 3094:1–13
Maddison WP, Knowles LL (2006) Inferring phylogeny despite incomplete lineage sorting. System Biol 55:21–30
Mayr E (1954) Geographic speciation in tropical echinoids. Evolution 8:1–18
Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz M et al (2005) The Phanerozoic record of global sea-level change. Science 310:1293–1298
Neige P, Lapierre H, Merle D (2016) New Eocene Coleoid (Cephalopoda) diversity from Statolith remains: taxonomic assignation, fossil record analysis, and new data for calibrating molecular phylogenies. PLoS ONE 11(5):e0154062
O’Dea A, Rodríguez F, De Gracia C, Coates A (2007) Patrimonio paleontológico. La paleontología marina en el Istmo de Panamá. Canto Rodado 2:149–179
Pardo-Gandarillas MC, Ibáñez CM, Torres FI, Sanhueza V, Fabres A, Escobar-Dodero J, Mardones FO, Méndez MA (2018) Phylogeography and species distribution modelling reveal the effects of the Pleistocene ice ages on an intertidal limpet from the South-Eastern Pacific. J Biogeogr 45(8):1751–1767. https://doi.org/10.1111/jbi.13362
Pardo-Gandarillas MC, Díaz-Santana-Iturrios M, Fenwick M, Villanueva R, Ibáñez CM (2021) Redescription of the flapjack octopod, Opisthoteuthis bruuni (Cephalopoda: Opisthoteuthidae) from the southeastern Pacific Ocean and evolutionary relationships of cirrate octopods. Malacologia 63(2):155–169. https://doi.org/10.4002/040.063.0201
Philippon M, Cornée J-J, Münch P, van Hinsbergen DJJ, BouDagher-Fadel M, Gailler L et al (2020) Eocene intra-plate shortening responsible for the rise of a faunal pathway in the northeastern Caribbean realm. PLoS ONE 15:e0241000
Pillans B, Chappell J, Naish TR (1998) A review of the Milankovich climatic beat: template for Plio-Pleistocene sea-level changes and sequence stratigraphy. Sed Geol 122:5–21
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S et al (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. System Biol 55(4):595–609
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21(8):1864–1877
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rambaut A, Drummond AJ (2009) Tracer v.1.5. http://beast.bio.ed.ac.uk/Tr
Ravelo AC, Andreasen DH, Lyle M, Lyle AO, Wara MW (2004) Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature 429:263–267
Rea DK, Lohmann KC, MacLeod ND, House MA, Hovan SA, Martin GD (1991) Oxygen and carbon isotopic records the oozes of ODP sites 752, 754, 756/757, eastern Indian Ocean. Proc Ocean Drill Program Sci Results 121:229–240
Rocha LA, Robertson DR, Roman J, Bowen BW (2005). Ecological speciation in tropical reef fishes. Proc R Soc B Biol Sci 272(1563):573–579
Rodrigues-Filho LFS, Costa NP, Sodré D, da Silva Leal JR, Nunes JLS, Rincon G, Lessa RPT, Sampaio I, Vallinoto M, Ready JS, Sales JBL (2023) Evolutionary history and taxonomic reclassification of the critically endangered daggernose shark, a species endemic to the Western Atlantic. J Zool System Evol Res. https://doi.org/10.1155/2023/4798805
Sales JBL, Shaw PW, Haimovici M, Markaida U, Cunha DB, Ready J et al (2013) New molecular phylogeny of the squids of the family Loliginidae with emphasis on the genus Doryteuthis Naef, 1912: Mitochondrial and nuclear sequences indicate the presence of cryptic species in the southern Atlantic Ocean. Mol Phylogenet Evol 68:293–299
Sales JBL, Markaida U, Shaw PW, Haimovici M, Ready JS, Figueiredo-Ready WMB et al (2014) Molecular phylogeny of the genus Lolliguncula Steenstrup, 1881 based on nuclear and mitochondrial DNA sequences indicates genetic isolation of populations from North and South Atlantic, and the possible presence of further cryptic species. PLoS ONE 9:e88693
Sales JBL, Rodrigues-Filho LFS, Ferreira YS, Carneiro J, Asp NE, Shaw PW et al (2017) Divergence of cryptic species of Doryteuthis plei Blainville, 1823 (Loliginidae, Cephalopoda) in the Western Atlantic Ocean is associated with the formation of the Caribbean Sea. Mol Phylogenet Evol 106:44–54
Sales JBL, Haimovici M, Ready JS, Souza RF, Ferreira Y, Pinon JCS et al (2019) Surveying cephalopod diversity of the Amazon reef system using samples from red snapper stomachs and description of a new genus and species of octopus. Sci Rep 9(1):1–16
Salzburger W, Ewing GB, von Haesler A (2011) The performance of phylogenetic algorithms in estimating haplotype genealogies with migration. Mol Ecol 20:1952–1963
Sánchez G, Perry HM, Trigg CB (1996) Morphometry of juvenile and subadult Loligo pealei and L. plei from the northern Gulf of Mexico. Fish Bull (wash DC) 94:535–550
Sanchez G, Fernández-Álvarez FÁ, Taite M, Sugimoto C, Jolly J, Simakov O, Marlétaz F, Allcock L, Rokhsar DS (2021) Phylogenomics illuminates the evolution of bobtail and bottletail squid (order Sepiolida). Commun Biol 4(1):819. https://doi.org/10.1038/s42003-021-02348-y
Sarnthein M, Winn K, Zahn R (1987) Paleoproductivity of oceanic upwelling and the effect on atmospheric CO2 and climatic change during deglaciation times. In: Berger WH, Labeyrie LD (eds) Abrupt climatic change. Springer, Dordrecht, pp 311–337
Shackleton NJ, Hall MA, Boersma A (1984) Oxygen and carbon isotope data from Leg-74 foraminifers. In: Moore, TC Jr, Rabinowitz PD et al (eds) Initial reports of the deep sea drilling project, 74, pp 599–612
Shaw PW, Hendrickson L, McKeown NJ, Stonier T, Naud MJ, Sauer WH (2012) Population structure of the squid Doryteuthis (Loligo) pealeii on the eastern coast of the USA: Reply to Gerlach et al., 2012. Mar Ecol Progr Ser 450:285–287
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236(4803):787–792
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX et al (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57(7):573–583
Strugnell J, Jackson J, Drummond AJ, Cooper A (2006) Divergence time estimates for major cephalopod groups: evidence from multiple genes. Cladistics 22(1):89–96
Suto I, Kawamura K, Hagimoto S, Teraishi A, Tanaka Y (2012) Changes in upwelling mechanisms drove the evolution of marine organisms. Palaeogeogr Palaeoclimatol Palaeoecolol 339:39–51
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA Polymorphism. Genetics 123:585–595
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Tourinho JL, Solé-Cava AM, Lazoski C (2012) Cryptic species within the commercially most important lobster in the tropical Atlantic, the spiny lobster Panulirus argus. Mar Biol 159:1897–1906. https://doi.org/10.1007/s00227-012-1977-7
Ulloa PM, Hernández CE, Rivera RJ, Ibáñez CM (2017) Biogeografía histórica de los calamares de la familia Loliginidae (Teuthoidea: Myopsida). Lat Am J Aquat Res 45(1):113–129. https://doi.org/10.3856/vol45-issue1-fulltext-11
Vecchione M, Shea E, Bussarawit S, Anderson F, Alexeyev D, Lu CC et al (2005) Systematics of Indo-West Loliginids. Phuket Mar Biol Center Res Bull 66:23–26
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Yu Y, Blair C, He XJ (2020) RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol Biol Evol 37(2):604–606
Zachos JC, Quinn TM, Salamy KA (1996) High-resolution (10(4) years) deep-sea foraminiferal stable isotope records of the Eocene-Oligocene climate transition. Paleoceanography 11:251–266
Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29(22):2869–2876
Zwickl DJ, Hillis DM (2002) Increased taxa sampling greatly reduces phylogenetic error. System Biol 51:588–598
Acknowledgements
The authors would like to thank Rosalia Souza, and the fishermen’s off Bragança for supplying specimens.
Funding
This work has been supported by the National Council for Scientific and Technological Development (CNPq) through scholarships to BLP through orientation by JBLS as part of the Post‐graduate Program in Aquatic Ecology and Fisheries (PPGEAP). Funding for this research was provided by CNPq (Grants 306233/2 009–6 to IS and 3007994/2020–1 to MH), FAPESPA (Grants PET0035/20 10 and APP064/20 11 to IS), and ICB (041/2020/ICB/UFPA to JBLS). This work was also supported in part by the project SAMBA (RCN—INTPART Project No. 322457).
Author information
Authors and Affiliations
Contributions
JBLS, BMLP, and FEA were involved in conceptualization. JBLS, AESR, YTCC, YF, LFSRF, UM, PWS, MH, JSR, and IS compiled data, methodology, formal analysis, writing—original draft, and review and editing. JBLS, FEA, BLP, AESR, and MH were involved in revisions, writing, and editing the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical review and approval were not required for this study, because this work does not contain any experimental studies with live animals.
Additional information
Responsible Editor: R. Villanueva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sales, J.B.L., Anderson, F.E., Paiva, B.L. et al. The vicariant role of Caribbean formation in driving speciation in American loliginid squids: the case of Doryteuthis pealeii (Lesueur 1821). Mar Biol 171, 82 (2024). https://doi.org/10.1007/s00227-024-04391-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04391-9