Abstract
Investigator disturbance while monitoring seabirds can result in lower survival rates and breeding success, leaving lasting negative impacts on the population and biasing observations. For example, monitoring rhinoceros auklets (Cerorhinca monocerata) and other burrowing alcids can reduce breeding success or even survival through handling stress and damage to nesting habitat. For this reason, researchers must seek to decrease colony disturbance. Automated radio-frequency identification (RFID) via passive integrated transponder (PIT) tags is an inexpensive and reliable way to identify individual presence and record attendance behaviour, avoiding the need to recapture seabirds or visit the colony frequently. PIT tags either can be implanted subcutaneously or attached externally to leg bands, but it is unclear which method causes lower disturbance. To examine the impact of PIT tagging on rhinoceros auklets nesting in artificial burrows on Middleton Island, Alaska, we monitored burrow entrances with automated recording RFID readers to collect presence and nest attendance data. PIT-tagged (either band attachment or subcutaneous implant) and control birds had similar breeding success and chick growth rates. Breeding success was similar between nests with one or two parents marked. Birds tagged externally were detected less often than birds marked with a subcutaneous implant. We conclude that PIT tagging of rhinoceros auklets is a relatively non-invasive method for seabird monitoring, and that subcutaneous implants do not cause more disturbance than external attachment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The uncertainty principle in field ecology states that an investigator will always disturb the behavior of the biological model they are studying (Lenington 1979). Investigator disturbance and its consequences are widely studied in field ecology (Hockin et al. 1992; De Jong and Hoback 2006; Carey 2009). For example, monitoring avian species can induce higher mortality and nest predation (Ibáñez-Álamo et al. 2012), reduced breeding success (Sandvik and Barrett 2001; Blackmer et al. 2004) or elicit abnormal behaviors (Brown and Morris 1995; Burger 1998). Thus, investigator disturbance can bias the life of history traits and behaviours of interest, and innovative techniques may reduce these biases.
Radio Frequency Identification (RFID) presents a potential solution to reduce disturbance of monitored seabirds while measuring patterns of nest attendance behavior (Tyson 2021). This can be achieved by marking individuals with passive integrated transponders (PIT) tags. A PIT tag is a small, lightweight bio-logging device that is typically an electronic chip encapsulated in glass. These can be quickly attached to an individual either externally via a leg band or subcutaneously. As a PIT tag passes through a RFID detector, its unique ID is recorded along with the date and time of detection. This non-visual identification method can be incorporated into automated networks, allowing for data collection without recaptures for band-reading (Andrews 2009; Bonter and Bridge 2011). The technology is already used on a variety of taxa and is contributing knowledge on population dynamics by providing strong estimates of individual survival probability and recruitment rates (Rebke et al. 2010; Sutherland and Dann 2012; Horswill et al. 2014). These demographic parameters can inform predictive models to fine-tune conservation plans depending on the different constraints a population is facing (Weller et al. 2014). While the size, weight and attachment position of bio-logging devices can have adverse effects even over short-term deployments (Whidden et al. 2007; Sun et al. 2020), the small size and light weight of PIT tags likely minimizes such effects. However, PIT tags tend to have longer duration deployment which may have effects over an individual’s lifetime.
Puffins (Fratercula and Cerorhinca spp.), show strong responses to their environment through breeding success and foraging behaviour (Bost and Le Maho 1993; Gjerdrum et al. 2003; Sydeman et al. 2017). However, puffins and other burrow-nesting auks are notably difficult to study because of their high sensitivity to human disturbance (Rodway et al. 1996; Whidden et al. 2007; Elliott et al. 2010; Harris and Wanless 2011; Sun et al. 2020; but see Kelly et al. 2015). Rhinoceros auklets (Cerorhinca monocerata) are burrow nesting alcids closely related to puffins, and are mostly active at their colonies at night. Their nocturnal colony behaviour makes the collection of observational data challenging, as color bands cannot be easily read from a distance, resulting in knowledge gaps for the species ecology. Rhinoceros auklets exhibit breeding site fidelity (Kubo et al. 2018), making them a good subject for long-term monitoring (e.g. annual survival, lifetime reproductive success, divorce rate, recruitment). However, executing such studies might require accessing burrows regularly, accelerating habitat degradation (Priddel and Carlile 1995). Excavating burrows regularly can have negative and long-term effects (Wilson 1986), increasing the risk of burrows collapsing which can ultimately result in the failure of a breeding attempt or even the death of one or both parents.
Artificial burrows have been proposed as a solution to mitigate this habitat degradation for different species of burrowing seabirds (Wilson 1986; Priddel and Carlile 1995; Bedolla et al. 2016). They reduce burrow collapse and design can allow researchers to easily access the nest chamber, reducing disturbance and increasing data collection efficiency (Wilson 1986). Ultimately, using artificial nests allows researchers to decrease habitat loss and impacts on breeding success (Wilson 1986; León and Mínguez 2003).
With the objective of developing a minimally invasive protocol for the long-term monitoring of rhinoceros auklets breeding in artificial burrows on Middleton Island, Alaska, we used an experimental approach to test the effects of different PIT tagging protocols on breeding success of rhinoceros auklets. We tagged breeding adult individuals (i) at different times of the breeding season (late incubation vs early chick rearing), (ii) internally (subcutaneous implantation in the neck) or externally (attachment to a 3D-printed leg band) and (iii) at different intensities (single vs both adults in burrow tagged). After each individual was marked with a PIT tag, their burrow was equipped with an automatic RFID reader. The number of detections per night was tested in relation to the position of the tag (leg or neck). We tested for effects of these tagging protocols on breeding success via chick growth rates (wing length and mass gain) and nest abandonment. We were particularly interested in whether handling for PIT-tagging during different breeding stages impacted abandonment rates, as handling during incubation previously increased abandonment in this population (Sun et al. 2020). We were also interested in whether leg-mounted or implanted PIT tags would cause less abandonment, as leg-mounted devices can alter flying and diving behavior due to imbalanced distribution of weight and buoyance, but implanted tags may cause infection (Hatch et al. 2000; Elliott et al. 2007; Vandenabeele et al. 2014). Handling is an essential part of PIT-tagging, and we wished to test the impact of the entire PIT-tagging process, therefore we did not have a separate set of birds that were handled but not PIT-tagged. In addition to answering these questions, this study provided an opportunity to learn more about nest attendance behavior of burrow nesting rhinoceros auklets due to constant monitoring via RFID.
Methods
Study system
We collected data from rhinoceros auklets breeding in a colony on Middleton Island, Alaska (59.42° N, 146.32° W). The colony has been monitored since 1977 and diet and population metrics are collected annually since 1993. During the breeding season (April–August), this population of rhinoceros auklets nest in burrows dug in soil slopes dominated by salmonberry bushes (Rubea spectabilis). Breeding pairs lay a single egg in late April to early May and incubate for approximately 45 days. During incubation (and the first days of chick rearing), the parents typically take turns incubating the egg (or hatchling) for two days shifts. Parents will then cease daytime burrow attendance and switch to spending every day foraging at sea, returning to the colony once each night to feed the chick by delivering a “bill load”. These deliveries tend to include multiple prey items (usually fish) carried between the mandibles and dropped inside the nest chamber of the burrow (Gaston and Dechesne 1996; Davoren and Burger 1999; Kato et al. 2003; Cunningham et al. 2018). This chick-rearing phase lasts until the chick fledges (≈ 50 days, Harfenist and Ydenberg 1995).
Sensitivity to investigator disturbance (handling and GPS deployment) differ between incubation and chick-rearing where parents are more likely to abandon the nest after disturbance in the early stages of breeding (Sun et al. 2020).
We conducted this study using artificial nest boxes. Between 2017 and 2021, 121 artificial nest boxes were installed in a limited area within a breeding colony of rhinoceros auklets on Middleton Island. The artificial burrows consist of two parts: an L-shaped wooden box made with ½-inch-thick plywood treated with wood preservative and a corrugated plastic tubing as an entrance tunnel (Fig. 1). The top of the box is equipped with an access lid to the nesting chamber and the floor is made of wire mesh. Nest boxes are buried about 15 cm underground, partially filled with soil, and then marked with a uniquely numbered stake.
Reproductive success monitoring
We first checked experimental nest boxes for contents on 20 May 2022 during daytime. This corresponded to early incubation for most individuals, however, the latest-breeding individuals laid their egg in late May. We examined the boxes for the presence of an incubating adult and floated eggs to estimate laying date. When exact dates are not observed, floating height and angle can be used to estimate laying date (Liebezeit et al. 2007). We used the flotation technique described in Sun et al. (2020) for rhinoceros auklets at our study site by measuring either the angle that eggs rested (sinking eggs) or the height of exposed egg above water (floating eggs).
For a sinking egg, we used the equation:
For a floating egg:
Given the flotation technique is only accurate within about 10 days of hatching, we used the following estimate for hatching date:
However, we kept the egg flotation estimate (1) or (2) when the egg did not hatch.
We visited occupied nest boxes on 30 May and every 5 days thereafter until hatching to monitor breeding parameters (hatching success, hatching date). At each visit, we recorded the presence or absence of an adult and we checked manually for the presence and temperature of an egg (cold or warm, indicative of whether an adult was recently incubating). When a nest box was checked, the entrance was plugged to prevent the adult from escaping and potentially abandoning the nest. We measured chick weight (g) and wing chord (mm) 5 and 20 days after they were first found. Chicks were not disturbed between these checks.
A breeding attempt was considered successful (breeding success = 1) if the chick fledged. We checked for the presence of a live chick every 5 days, 45 days after the chick was first recorded as hatched. A chick was considered a successful fledgling if it was absent from the burrow during one of those post-45 day checks. The breeding attempt was considered unsuccessful (breeding success = 0) if the egg was found cold on three consecutive checks, or the chick was found dead or missing before the second measurement (no chick was found dead after 45 days). During the 2023 season, we monitored the nest boxes used for the 2022 experiment to determine occupancy (eggs laid, presence of tagged individuals).
PIT tagging
We found 63 occupied nest boxes on the first check (20 May). Nest boxes were then assigned a temporal treatment (Control, Late-incubation, or Chick-rearing), for the PIT-tagging that would occur later in the breeding season. We assigned 20 nest boxes each to the Late-incubation and Chick-rearing groups: 10 boxes with a single adult tagged and 10 boxes with both adults of the couple tagged (using same internal/external treatment) for a total of 60 birds to be marked with a PIT tag. Half of the birds were PIT-tagged subcutaneously in the neck and the other half were banded on one leg with a custom 3D-printed plastic band to hold a PIT tag. We used a random number generator to generate the temporal treatment (Control, Incubation, Chick-rearing) while ensuring the treatments were distributed evenly across breeding phenology and spatial location (Appendix C). Then, the tag position (neck or band) and number of adults tagged per nest box (single or both) were assigned via a random number generator.
The remaining 23 nest boxes were assigned to the Control group. We monitored reproductive parameters for these nest boxes, but Control individuals were not further manipulated. Burrows assigned to the Late-incubation treatment were PIT-tagged 10 days before the estimated date of hatching. Chick-rearing birds were PIT-tagged as soon as they were found with a chick. If a chick was found without a parent during a productivity check the nest box would be checked every night for 1 week until the parent was found. If an individual was marked less than 3 days before the next productivity check, its burrow was skipped for that check to reduce disturbance.
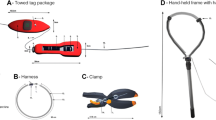
Birds were marked with 12-mm EM4102 PIT tags (frequency = 125 kHz, mass ≈ 0.1 g). Individuals tagged on the leg were equipped with a 1.25 mm thick 3D-printed nylon leg band (≈ 1 g; 0.2% body mass) designed to hold the PIT tag on the left leg. The band was secured with super glue and Tesa tape (tesa tape inc. 5825 Carnegie Boulevard, Charlotte, North Carolina 28209). Individuals marked in the neck had a PIT tag inserted in the loose neck skin between the scapulae. A single-use needle was used for each bird. The PIT tag was sterilized in a chlorhexidine solution overnight and then stored in PBS solution until injection. The injection site was disinfected with a cotton ball soaked in 70% isopropyl solution to part the feathers. We then pinched the insertion point and used the syringe to manually pierce the skin before pushing the PIT tag under it. The same cotton ball was applied on the wound for several seconds after the injection to prevent the tag from falling out during the closing of the skin around the puncture wound.
Tagged birds were also banded with metal US Fish and Wildlife bands on the right leg. The first tagged bird in each nest box had a small piece of Tesa tape attached to the metal band. This facilitated rapid identification during productivity checks as well as the monitoring of tag failure rates, for example, if the plastic leg bands were to fall off or PIT tags were to migrate or be ejected from in the body.
When the bird was caught, its head was placed in a bag to reduce stress. The bird was released in the nest box from the tunnel immediately after PIT-tagging and the entrance was kept plugged for at least 5 min to prevent early abandonment. Capture and release time were recorded. Every late incubation bird (n = 30) and 10 chick rearing birds were tagged early in the day between 09:00 and 13:00. The 15-remaining chick rearing birds were tagged at night between 23:00 and 04:00. Implanting a PIT tag in the neck took 6 min 44 s (SD: 2 min 33 s) attaching a leg band equipped with a PIT tag took 4 min 8 s (SD: 1 min 7 s). In total, 55 birds from 37 different artificial burrows were PIT-tagged (Table 1).
During productivity checks, individuals in nest boxes that had potentially been PIT-tagged before were identified using a handheld RFID reader (RT100V8, RealTrace, ATRIA Trading SA, Le Parray en Yvelines, France). If the bird showed signs of stress, we tried to feel its metal band instead to limit disturbance and abandonment risks. Indeed, the detection range of our handheld reader was low (around 2 cm). It was efficient on individuals marked in the neck, especially on calm individuals that were not moving, as it allowed for identification without any manipulation. However, PIT tag reading could increase the time spent for a check when the bird was aggressive or carrying a leg band as we needed to secure the bird with one hand while trying to identify it with the other.
In one burrow, both parents were supposed to be tagged in the neck during chick rearing. However, one parent was not found after 1 week of marking effort. This burrow was subsequently considered in the single parent tagged category. Three nest boxes in the chick rearing treatment group were excluded as the individuals were not tagged since their egg never hatched.
During the 2023 season, the occupancy rate of the burrows we monitored the year before was recorded.
Automatic PIT tag reading
We used automated RFID detectors to detect marked individuals in between and after the productivity checks. Those detectors allowed to record the presence/absence data and measure nest attendance patterns. Loggers used custom-built automated, low-powered PIT tag readers. The device is made of two parts: a custom designed circuit board and a circular-shaped antenna. The antennae were placed inside the tunnel, approximately 15 cm from the entrance (Fig. 2). The system was designed for very low power consumption, and so the antenna-generated magnetic field that powered the PIT tag is weak. Because of this, the PIT tag needed to be very close (within 1 cm) or preferably within the circular antenna ring to be activated and read. The field is strongest near the perimeter of the ring and weakest at the center. For this reason, PIT tags located on a leg band or injected in the back are positioned near the most sensitive part of the antenna as the bird proceeds through the tunnel giving maximum likelihood of being read. When this happens, the unique ID of the PIT tag, the date and time of the event are logged and stored in the circuit board (more information in Appendix B).
Automatic passive integrated transponder (PIT) tag detector installed in a rhinoceros auklet artificial burrow. The antenna (white arrows) is placed inside the tunnel so that individuals entering or exiting the nest must pass through the antenna. Part of the antenna and its cable are buried to reduce disturbance and prevent damage
The power station and the PIT tag readers were connected with 30-m cables allowing sufficient length to reach most of the colony. The readers were then spread in the colony and set up at the nest boxes. All equipment was protected from the rain through waterproof casings. We had 12 working readers. Every burrow was equipped with one detector for a week, then we changed the position of the readers to be able to monitor as many burrows as possible during the season. Every burrow still occupied after we measured all chicks from tagged parents for the first time, as well as 2 burrows where the chick disappeared from the nest were monitored with automatic detectors. This represented 27 boxes and 38 birds (15 tagged in the neck and 23 tagged on the leg).
Statistical analyses
All statistical analyses were completed using R version 4.0.2 (R Core Team 2020). We tested the effect of different aspects of PIT tagging on breeding success with binomial generalized linear models using a chi squared significance test. All interactions were first considered unless specified. If the highest rank interaction term was not significant, it was removed until only significant interactions and/or single terms remained.
Effect of tagging on breeding success
To investigate the effect of PIT tagging on the breeding success of nests with marked parents (n = 37), we modelled the breeding success (no = 0, yes = 1) in response to PIT tag location, number of adults PIT tagged, and number of adults tagged with a 3-way interaction with a binomial generalized linear model.
Since some chicks were still in the burrow at the end of the field session, we modelled the probability of a chick being fledged on our last check (0/1) (n = 60) depending on their treatment group and their age with a binomial generalized linear model.
We also compared the breeding success of late incubation individuals (n = 20) and control individuals who had an egg 10 days before estimated hatching (n = 20). The same was done between control individuals who successfully hatched an egg (n = 19) and chick rearing individuals (n = 17). Those comparisons were done using Fisher’s exact test and were followed by a statistical power analysis with the function power.fisher.test from the R package “statmod”. We also estimated the sample size we would need to achieve a sufficient statistical power level (taken as 0.8). To do that, we iterated the calculation of the power of every test until it reached 0.8, increasing the effective of both groups we compared by 5 each step. As the statistical power of a Fisher test is simulated and not explicitly calculated by the power.fisher.test function, we simulated 100 datasets every single iteration to balance precision and calculation time.
Finally, we modelled the probability of the rhinoceros auklets to successfully hatch an egg (0/1) fledge a chick when they hatched the egg (0/1) and the overall breeding success (0/1) (n = 60) depending on if they are PIT tagged or not and the egg laying date with a 2-way interaction.
Effect of tagging on chick growth
The effect of the number of parents tagged and laying date on chick daily wing growth and weight gain was tested with an ANOVA using a 2-way interaction on every nests that successfully fledged a chick (n = 43).
Occupancy rate of the nests marked the last year
We compared the occupancy (whether an egg was laid; yes = 1, no = 0) of the burrows marked the last year between single and double tagged burrows with a Fisher’s exact test. We also compared the return rate (probability to come back to the colony the next year) of individuals marked on the leg and individuals marked in the neck with a chi square test.
Attendance patterns
We investigated differences in attendance pattern (number of detection.night−1, timing of the first and last detection) depending on the position of the tag and how advanced the breeding season was with linear models plus a two-way interaction using a random effect on individual ID to control for inter-individual variation. When an individual was recorded only once during a particular day, this record was removed from the colony presence analysis. Indeed, in this case, we could not determine if this was because an individual approached the detector and then left without entering the burrow, or if some detections were missing because of a malfunction.
To determine whether the position of tagging influenced the detection probability, we ran a capture-mark-recapture (CMR) analysis on the subset of marked individuals. We used one week of capture history from 33 birds detected between 15 July and 7 August (example of detection record given in Appendix A). The analysis was completed in MARK version 9.x (White and Burnham 1999). This was done via standard live encounter mark-recapture models (Lebreton et al. 1992), where the probability of an individual being seen is defined by two parameters: the probability the animal survived and remained in the sample area (ϕ), and the probability that the animal was encountered (p), conditional on being alive and in the sample area. Following Lebreton et al. (1992) methods, we began with a general model and examined simpler alternatives. Model selection was based on Akaike’s Information Criterion (AIC). The model with lowest AIC being considered as the most parsimonious one. The parameters estimated from the best model are given with 95% confidence interval (95% CI) computed from the Hessian matrix.
Results
Among the 63 experimental nest boxes assigned to treatments, 52 eggs successfully hatched (82%) and 43 chicks (68%) successfully fledged. Some chicks were still present in the burrow for the last productivity check on 13 August 2022. These chicks (age ± SD = 50 ± 8 d, n = 22) were considered as successful fledglings. This assumption is reasonable since the probability of not being fledged on the last check depended on the age of the chick (Chi-square test, χ21 = 6.59, p = 0.01) and not the treatment group (Chi-square test, χ22 = 4.59, p = 0.10) (Table 2).
Return and occupancy rate of individuals marked the previous year
Among the 37 monitored burrows with PIT tagged individuals, 22 were occupied again in 2023. There was no significant difference between single tagged (14 burrows occupied, n = 19) and double tagged occupancy (8 burrows occupied, n = 18) (Fisher’s exact test, p = 0.1). The return rate of individuals marked in the leg or in the neck was similar (Chi-square test, χ21 = 0.26, p = 0.60). 24 of the 55 individuals initially marked came back to the colony (Table 2), including 5 individuals that failed to fledge chicks in the previous year.
Effect of PIT tagging on chick growth
PIT tagging of adults also had little effect on chick development, daily wing growth (ANOVA, F(2,38) = 2.69 p = 0.08) (Wing length.day−1 ± SD = Control group: 2.4 ± 0.56 cm.day−1, n = 16, one individual tagged: 2.9 ± 0.62 cm.day−1, n = 15, both individuals tagged: 2.4 ± 0.75 cm.day−1, n = 11) or weight gain (ANOVA, F(2,38) = 0.37 p = 0.69) (Mass.day−1 ± SD = Control group: 6.9 ± 1.6 g.day−1, n = 16, one individual tagged: 7.4 ± 2.2 g.day−1, n = 15, both individuals tagged: 7.2 ± 1.6 g.day−1, n = 11). Laying date did not have an impact on either wing growth (ANOVA, F(1,38) = 1.13, p = 0.29) or weight gain (ANOVA, F(1,38) = 0.02, p = 0.88).
Effect of PIT tagging on breeding success
There were no differences in hatching or fledging success between PIT tagged and control individuals (Hatching success: Chi square test, χ21 = 0, p = 0.98, Control: 0.83, Late incubation: 0.8; Fledging success: Chi square test, χ21 = 0.97, p = 0.32, Control: 0.74, Late incubation: 0.65, Chick-rearing: 0.76). PIT tagging did not impact overall breeding success (Chi-square test, χ21 = 0.4, p = 0.52), and the only significant predictor of breeding success was the laying date (Chi-square test, χ21 = 4.16, p = 0.041) (Laying date (Julian date) ± SD: Control group: 128 ± 9, Late incubation: 125 ± 8, Chick rearing: 126 ± 6) (Table 3). Earlier laying birds had a higher chance of breeding successfully than later breeders (Fig. 3) because eggs laid early in the season were more likely to hatch (Chi-square test, χ21 = 12.18, p < 0.001). However, laying date did not have any effect on chick survival after hatching (Chi square test, χ21 = 0.09, p = 0.77).
The position of tagging (leg or neck) (Chi-square test, χ21 = 0.18, p = 0.67), the number of birds tagged in the pair (Chi-square test, χ21 = 1.49, p = 0.22) and breeding stage (Chi-square test, χ21 = 0.56, p = 0.46) did not influence breeding success among tagged individuals (Table 4).
We observed similar breeding success of control birds and birds PIT tagged during incubation or chick rearing (Fisher’s exact test, all p > 0.3). Statistical power tests then revealed we would need N ≈ 230 individuals to see a statistically significant difference in breeding success between controls and individuals PIT tagged during late incubation, N ≈ 250 to see a difference between individuals marked during chick rearing and controls and N ≈ 270 (power = 0.12) to see a difference between individuals tagged during late incubation or chick rearing. In addition, control individuals, as well as individuals tagged on the leg or in the neck had a similar breeding success (Fig. 4).
Colony attendance, detection probability
Out of the 38 birds we monitored with automatic RFID readers, 37 birds were successfully detected 1 week to 1 month after being tagged. One individual was not detected due to a failure of the reader.
Attendance patterns were highly variable, and the timings of the first detection were relatively homogeneously distributed during the whole night contrary to the timing of the last detection (Fig. 5). The time between the first and last detection in the same night varied among individuals from 32 s to 6 h 3 min (mean: 2 h 30 min). The number of detections per night varied between 1 and 33 (mean: 6.77), (mean time between two detections ± SD (min) = 22 ± 40 min).
Individuals with neck implants were detected a higher number of times per night than individuals with leg bands (Chi-square test, χ21 = 8.61, p = 0.003) throughout the season (Chi-square test, χ21 = 0.06, p = 0.80). The timing of the first detection every night did not depend on the position of the tag (Chi-square test, χ21 = 2.12, p = 0.14) but first detections tended to occur later at night as the breeding season progressed (Chi-square test, χ21 = 4,49, p = 0.03). The timing of the last detection every night did not depend on the position of the tag (Chi-square test, χ21 = 1.03, p = 0.31) but also tended to occur later at night as the breeding season progressed (Chi-square test, χ21 = 4.21, p = 0.04).
We considered two variables for the CMR analysis: the group g (two different positions of marking) and the time t. We started with the most general model ϕ(g*t)p(g*t) and ended up with the model ϕ(.)p(g) being the one with the lowest AICc (Table 5). There was no significant difference in detection probability between individuals marked on the leg (p = 0.832, 95% CI [0.752, 0.891]) or subcutaneously in the neck (p = 0.942, 95% CI [0.854, 0.979]). Survival chances were close to one (ϕ = 0.993, 95% CI [0.956, 0.999]).
Discussion
We used an experimental approach to determine the effects of PIT tagging on rhinoceros auklet breeding outcomes. Our approach will permit the development of a broader scale rhinoceros auklet automatic detection network on Middleton Island to record occupancy rates, survival, divorce, lifetime reproductive success and recruitment in future years. These basic population parameters will allow us to model the population dynamics. Once set up, the RFID antennae could provide much of this information without needing to regularly visit the burrows.
No aspect of PIT tagging had measurable effect on reproductive success during the 2022 chick rearing season. The absence of effect could be due to our sample size being too small to detect some statistically significant differences between marked individuals and controls. However, our power analysis showed that given our effect size, we would need around 250 individuals to see a statistically significant (p < 0.05) difference with a reasonable power (1 – β = 0.8). In short, the effects would only be measurable statistically with large sample sizes that might not be achievable in a single colony. For our purposes, the effect of the PIT tagging appears negligible.
Parental commitment towards breeding increases as the breeding season progresses (Sun et al. 2020). Thus, we expected that PIT tagging during incubation would cause higher abandonment than during chick rearing. Higher handling-induced nest abandonment probability during the incubation phase has been documented in rhinoceros auklets (Sun et al. 2020), as well as other burrowing seabird species (Carey 2009), although Kelly et al. (2015) found no impact of disturbance during incubation on breeding success of Atlantic puffins. However, we did not detect a difference in abandonment between breeding stages, possibly due to the high effort we exerted in reducing disturbance as much as possible. Artificial nest boxes were particularly useful for that purpose, as the birds were easily accessible. The handling time was only three to five minutes long, a short duration relative to Sun et al. (2020). We did not observe rhinoceros auklets displaying signs of high stress (e.g., breathing heavily, trying to escape) during the marking process.
Our results are encouraging as both tagging and handling have well documented negative effect on seabird reproductive success (Rodway et al. 1996; Whidden et al. 2007), including reduced colony attendance (Söhle et al. 2000), chick growth rate (Ackerman et al. 2010; Villard et al. 2011) and higher nest abandonment (Sun et al. 2020). Considering our results, we can make recommendations for future PIT tagging experiments on rhinoceros auklets, and likely other burrow-nesting birds. Individuals should be marked during the last days of incubation or the very first days of chick rearing when they are accessible during daytime. Tagging individuals at night requires many visits to the same burrows multiple times, which can disturb individuals in the colony. However, this should be considered with precaution as handling seabirds during incubation goes against most recommendations (e.g., Elliott et al. 2010; Elliott 2016; Sun et al. 2020).
As neither the number of adults tagged, nor the position of the tag seem to have strong effects on reproductive success, we cannot yet conclude on what tagging protocol should be preferably used. Tagging individuals either subcutaneously in the neck, or with a leg band, seem to be two successful and unharmful methods allowing to remotely monitor several traits in a rhinoceros auklet colony, such as presence/absence or nest attendance data. Tagging one or both parents of the same nest does not have measurable effects on their breeding success or on chick growth. For this reason, we believe it is better to tag both parents of every nest as it gives an opportunity to study nest attendance and parental care at a finer scale.
We do not have a clear explanation yet on why individuals with neck implants were detected more often each night. PIT tags need to have a particular orientation while going through the antenna to maximize the detection probability which could be better achieved with subcutaneously implanted PIT tags. This could also be caused by a difference in individual behavior. We tried using two antennae per burrow, one at both end of the entrance tunnel to determine the directionality of the detection, and discriminate cases when a bird would simply sit on the detector and the entrance then leave rather than actually visiting its nest. This technique did not work because the distance needed to avoid interference was greater than the length of the entrance tunnel.
Studies report various loss rates for subcutaneously implanted pit tags. The size of the bird and the injection method seem to be important factors (Bonter and Bridge 2011) and most tag loss seems to happen soon after injection. However, the tag loss rate was 0% on our sample (n = 38) after one week to one month of tagging. One effect that should be taken into account is that for diving species, subcutaneous tagging might help decrease the added drag, as internal implantation of tags generally causes reduced impacts (Wilson et al. 1986; White et al. 2013; Evans et al. 2020). For example, positioning tags on the leg or the tail can cause instability (Vandenabeele et al. 2014; Elliott 2016). A study on crested auklets (Aethia cristatella) showed that a tarsus mounted tracking device (1% of the bird body mass) changed the behavior and at sea survival of marked individuals (Robinson and Jones 2014). Similarly, increasing buoyancy or mass via a leg-mounted tag altered the dive behaviour of thick-billed murres (Uria lomvia; Elliott et al. 2007), and attaching a leg-mounted tag for a year increased stress (corticosterone) in the same species (Elliott et al. 2012). The mass and the size of the 3D-printed leg band and PIT tag we used in our study was smaller than in the other studies cited. Yet, it could still have undesirable effects that need to be studied and that we could potentially avoid with subcutaneous implants.
However, this statement has to be taken cautiously as all tags should be considered to have an impact even if they are hard to detect (Elliott 2016). The impact of such small devices might be particularly hard to detect as their mass are negligible. They could still have some long-term negative impact on survival (some marked individuals could suffer lesions from the tag migrating in the body) or may affect reproduction during subsequent years. For this reason, it is important to continue this study for several years to monitor for potential long term PIT tag effects. Nonetheless, PIT tagging reduces disturbance on birds compared to manual burrow checks due to decreased human presence on colonies. Thus, combining PIT tagging and automatic detection devices is a valuable method in studying sensitive seabird species during their breeding season.
During the 2023 season, we only monitored the occupancy rate from burrows already marked the year prior. However, we observed that previously marked pairs could come back to the same colony but in different burrows. In future years, every burrow will be monitored which will bring more knowledge on rhinoceros auklet return and divorce rates. There was no significant difference between the return rate of individuals marked subcutaneously or externally with a leg band during the 2023 season (36% for neck implants and 51% for leg bands). Similar values were found using bands and nest capture (Bertram et al. 2000). However, bands can mechanically wear and become unreadable after several years (Breton et al. 2005) which is not the case for PIT tags if they do not migrate in the body.
Several detectors revealed individuals visiting alternate burrows in our study. This behavior has been observed in Leach’s storm-petrel (Zangmeister et al. 2009) but not in the rhinoceros auklet. This phenomenon is unlikely to be due to a bird seeking for extra pair copulation, as data were collected late in the breeding season. One possibility is that those extra burrow visits could be attempts at kleptoparasitism (Senzaki et al. 2014). Monitoring intra-specific kleptoparasitic behaviors could be a way to monitor ecosystem health via food shortages. Indeed, this behavior is most likely to happen when food availability is scarce (Beintema 1997; Ashbrook et al. 2008).
This experiment also revealed that parents could come back to a burrow even after the chick disappeared. During productivity checks, we found several burrows without a chick that had one before. We considered the absence of a chick as proof of nest abandonment by the parents, yet no dead chick was found in the burrow. In two cases, the parents came back to the nest after we concluded the burrow was abandoned. In one case, both parents had a nest attendance pattern similar to successfully breeding birds even if the chick was considered dead two weeks previously. Clearly, auklets continue visit their burrows even after chicks are dead. Given that some parents continue to visit the nest after the death of their chicks, it will be difficult to automatically detect the loss of a chick without human visits, although a change in the duration or frequency of visits may provide a signature of chick loss.
These observations highlight the potential of PIT tagging to bring more knowledge on cryptic species. There is growing evidence that, even if monitoring seabird species gives us access to helpful data in understanding their biology and the state of their environment, monitoring is done at the expense of their fitness. This problem is concerning, especially during long-term studies that could have negative long-term carryover effects on populations. In extreme cases, researchers could be missing important factors that would be otherwise demonstrated in an undisturbed population. PIT tagging could be used extensively in sensitive seabird species and in the light of our results and previous ones, should be considered for rhinoceros auklets, other puffins and burrow-nesting species.
In conclusion, PIT tagging rhinoceros auklets nesting in artificial burrows is a promising way to monitor their behavior, breeding outcomes and long-term survival while having the least possible disturbance. This technique could be applied to other sensitive seabird species with a similar biology like tufted puffins (Fratercula cirrhata). Combining the use of artificial burrows and automatic nest monitoring with PIT tag detectors for long-term studies can reduce disturbance and the probability of burrow collapse due to less frequent visits.
Data availability
The datasets generated during and/or analyzed during the current study are available as a supplementary file.
References
Ackerman J, Adams J, Takekawa J, Carter H, Whitworth D, Newman S, Golightly R, Orthmeyer D (2010) Effects of radiotransmitters on the reproductive performance of Cassin’s Auklets. Wildl Soc Bull 32:1229–1241. https://doi.org/10.2193/0091-7648(2004)032[1229:EOROTR]2.0.CO;2
Andrews K (2009) PIT tagging: simple technology at its best. Bioscience 54:447–454. https://doi.org/10.1641/0006-3568(2004)054[0447:PTSTAI]2.0.CO;2
Ashbrook K, Wanless S, Harris MP, Hamer CK (2008) Hitting the buffers: conspecific aggression undermines benefits of colonial breeding under adverse conditions. Biol Lett 4:630–633. https://doi.org/10.1098/rsbl.2008.0417
Bedolla-Guzmán Y, Masello J, Aguirre-Muñoz A, Quillfeldt P (2016) A wood-concrete nest box to study burrow-nesting petrels. Mar Ornithol 44:249–252
Beintema AJ (1997) Intra-specific kleptoparasitism in Black Tern Chlidoniasniger triggered by temporary food shortage. Bird Study 44:120–122. https://doi.org/10.1080/00063659709461046
Bertram D, Jones I, Cooch E, Knechtel H, Cooke F (2000) Survival rates of cassin’s and rhinoceros auklets at Triangle Island, British Columbia. Condor 102:155–162. https://doi.org/10.1093/condor/102.1.155
Blackmer AL, Ackerman JT, Nevitt GA (2004) Effects of investigator disturbance on hatching success and nest-site fidelity in a long-lived seabird, Leach’s storm-petrel. Biol Conserv 116:141–148. https://doi.org/10.1016/S0006-3207(03)00185-X
Bonter DN, Bridge ES (2011) Applications of radio frequency identification (RFID) in ornithological research: a review. J Field Ornithol 82:1–10. https://doi.org/10.1111/j.1557-9263.2010.00302.x
Bost CA, Le Maho Y (1993) Seabirds as bio-indicators of changing marine ecosystems: new perspectives. Acta Oecol 14:463–470
Breton AR, Diamond AW, Kress SW (2005) Adult survival estimates from two Atlantic Puffin (Fratercula Arctica) Colonies in The Gulf of Maine. Auk 122:773–782. https://doi.org/10.1093/auk/122.3.773
Brown KM, Morris RD (1995) Investigator disturbance, chick movement, and aggressive behavior in ring-billed gulls. Wilson Bull 107:140–152
Burger J (1998) Effects of motorboats and personal watercraft on flight behavior over a colony of common terns. Condor 100:528–534. https://doi.org/10.2307/1369719
Carey MJ (2009) The effects of investigator disturbance on procellariiform seabirds: a review. N Z J Zool 36:367–377. https://doi.org/10.1080/03014220909510161
Cunningham J, Elliott K, Cottenie K, Hatch S, Jacobs S (2018) Individual foraging location, but not dietary, specialization: implications for rhinoceros auklets as samplers of forage fish. Mar Ecol Prog Ser 605:225–240. https://doi.org/10.3354/meps12761
Davoren G, Burger A (1999) Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim Behav 58:853–863. https://doi.org/10.1006/anbe.1999.1209
De Jong GD, Hoback WW (2006) Effect of investigator disturbance in experimental forensic entomology: succession and community composition. Med Vet Entomol 20:248–258. https://doi.org/10.1111/j.1365-2915.2006.00618.x
Elliott KH (2016) Measurement of flying and diving metabolic rate in wild animals: review and recommendations. Comp Biochem Physiol Part A Mol Integr Physiol 202:63–77. https://doi.org/10.1016/j.cbpa.2016.05.025
Elliott KH, Davoren GK, Gaston AJ (2007) The influence of buoyancy and drag on the dive behaviour of an Arctic seabird, the thick-billed Murre. Can J Zool 85:352–361. https://doi.org/10.1139/Z07-012
Elliott KH, Shoji A, Campbell KL, Gaston AJ (2010) Oxygen stores and foraging behavior of two sympatric, planktivorous alcids. Aquat Biol 8:221–235. https://doi.org/10.3354/ab00236
Elliott KH, McFarlane Tranquilla L, Burke C, Hedd A, Montevecchi W, Anderson G (2012) Year-long deployments of small geolocators increase corticosterone levels in murres. Mar Ecol Prog Ser 466:1–7. https://doi.org/10.3354/meps09975
Evans T, Young R, Watson H, Olsson O, Akesson S (2020) Effects of back-mounted biologgers on condition, diving and flight performance in a breeding seabird. J Avian Biol. https://doi.org/10.1111/jav.02509
Gaston AJ, Dechesne SB (1996) Rhinoceros Auklet (Cerorhinca monocerata), version 2.0. Birds N Am. https://doi.org/10.2173/bna.212
Gjerdrum C, Vallée AMJ, St-Clair CC, Bertram DF, Ryder JL, Blackburn GS (2003) Tufted puffin reproduction reveals ocean climate variability. Proc Natl Acad Sci USA 100:9377–9382. https://doi.org/10.1073/pnas.1133383100
Harfenist A, Ydenberg RC (1995) Parental provisioning and predation risk in rhinoceros auklets (Cerorhinca monocerata): effects on nestling growth and fledging. Behav Ecol 6:82–86. https://doi.org/10.1093/beheco/6.1.82
Harris MP, Wanless S (2011) The puffin. Bloomsbury Publishing
Hatch SA, Meyers PM, Mulcahy DM, Douglas DC (2000) Seasonal movements and pelagic habitat use of murres and puffins determined by satellite telemetry. Condor 102:145–154. https://doi.org/10.1093/condor/102.1.145
Hockin D, Ounsted M, Gorman M, Hill D, Keller V, Barker MA (1992) Examination of the effects of disturbance on birds with reference to its importance in ecological assessments. J Environ Manag 36:253–286. https://doi.org/10.1016/S0301-4797(08)80002-3
Horswill C, Matthiopoulos J, Green JA, Meredith MP, Forcada J, Peat H, Preston M, Trathan PN, Ratcliffe N (2014) Survival in macaroni penguins and the relative importance of different drivers: individual traits, predation pressure and environmental variability. J Anim Ecol 83:1057–1067. https://doi.org/10.1111/1365-2656.12229
Ibáñez-Álamo JD, Sanllorente O, Soler M (2012) The impact of researcher disturbance on nest predation rates: a meta-analysis. Ibis 154:5–14. https://doi.org/10.1111/j.1474-919X.2011.01186.x
Kato A, Watanuki Y, Naito Y (2003) Foraging behaviour of chick-rearing rhinoceros auklets Cerorhinca monocerata at Teuri Island, Japan, determined by acceleration-depth recording micro data loggers. J Avian Biol 34:282–287. https://doi.org/10.1034/j.1600-048X.2003.03134.x
Kelly K, Diamond T, Holberton R, Bowser A (2015) Researcher handling of incubating Atlantic puffins Fratercula Arctica has no effect on reproductive success. Mar Ornithol 43:77–82
Kubo A, Takahashi A, Thiebot J-B, Watanuki Y (2018) Rhinoceros Auklet pair-mates migrate independently but synchronize their foraging activity during the pre-laying period. Ibis 160:832–845. https://doi.org/10.1111/ibi.12583
Lebreton J-D, Burnham K, Clobert J, Anderson D (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118. https://doi.org/10.2307/2937171
Lenington S (1979) Predators and blackbirds: the “uncertainty principle” in field biology. Auk 96:190–192
León A, Mínguez E (2003) Occupancy rates and nesting success of European storm-petrels breeding inside artificial nest-boxes. Sci Mar 67:109–112
Liebezeit J, Smith P, Lanctot R, Schekkerman H, Tulp I, Kendall S, Tracy D, Rodrigues R, Meltofte H, Robinson J, Gratto-Trevor C, Mccaffery B, Morse J, Zack S (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47. https://doi.org/10.1093/condor/109.1.32
Priddel D, Carlile N (1995) An artificial nest box for burrow-nesting seabirds. Emu 95:290–294. https://doi.org/10.1071/MU9950290
Rebke M, Coulson T, Becker PH, Vaupel JW (2010) Reproductive improvement and senescence in a long-lived bird. Proc Natl Acad Sci USA 107:7841–7846. https://doi.org/10.1073/pnas.1002645107
Robinson JL, Jones IL (2014) An experimental study measuring the effects of a tarsus-mounted tracking device on the behaviour of a small pursuit-diving seabird. Behaviour 151:1799–1826. https://doi.org/10.1163/1568539X-00003217
Rodway MS, Montevecchi WA, Chardine JW (1996) Effects of investigator disturbance on breeding success of Atlantic puffins Fratercula arctica. Biol Conserv 76:311–319. https://doi.org/10.1016/0006-3207(94)00118-9
Sandvik H, Barrett RT (2001) Effect of investigator disturbance on the breeding success of the Black-Legged Kittiwake. J Field Ornithol 72:30–42. https://doi.org/10.1648/0273-8570-72.1.30
Senzaki M, Suzuki Y, Watanuki Y (2014) Foraging tactics and success of inter- and intra-specific kleptoparasites on Rhinoceros Auklets Cerorhinca monocerata. Ornithol Sci 13:1–8. https://doi.org/10.2326/osj.13.1
Söhle IS, Moller H, Fletcher D, Robertson CJR (2000) Telemetry reduces colony attendance by sooty shearwaters (Puffinus griseus). N Z J Zool 27:357–365. https://doi.org/10.1080/03014223.2000.9518245
Sun A, Whelan S, Hatch S, Elliott K (2020) Tags below three percent of body mass increase nest abandonment by rhinoceros auklets, but handling impacts decline as breeding progresses. Mar Ecol Prog Ser 643:173–181. https://doi.org/10.3354/meps13341
Sutherland D, Dann P (2012) Improving the accuracy of population size estimates for burrowing seabirds. Ibis 154:488–498. https://doi.org/10.1111/j.1474-919X.2012.01234.x
Sydeman WJ, Piatt JF, Thompson SA, García-Reyes M, Hatch SA, Arimitsu ML, Slater L, Williams JC, Rojek NA, Zador SG, Renner HM (2017) Puffins reveal contrasting relationships between forage fish and ocean climate in the North Pacific. Fish Oceanogr 26:379–395. https://doi.org/10.1111/fog.12204
Tyson CW (2021) Aspects of coordinated parental care in several seabird species. Dissertation, University of California, Davis
Vandenabeele SP, Grundy E, Friswell MI, Grogan A, Votier SC, Wilson RP (2014) Excess baggage for birds: inappropriate placement of tags on gannets changes flight patterns. PLoS ONE 9:e92657. https://doi.org/10.1371/journal.pone.0092657
Villard P, Bonenfant C, Bretagnolle V (2011) Effects of satellite transmitters fitted to breeding Cory’s shearwaters. J Wildl Manag 75:709–714. https://doi.org/10.1002/jwmg.90
Weller F, Cecchini L-A, Shannon L, Sherley R, Crawford R, Altwegg R, Scott L, Stewart T, Jarre A (2014) A system dynamics approach to modelling multiple drivers of the African Penguin population on Robben Island, South Africa. Ecol Modell 277:38–56. https://doi.org/10.1016/j.ecolmodel.2014.01.013
Whidden SE, Williams CT, Breton AR, Buck CL (2007) Effects of transmitters on the reproductive success of Tufted Puffins. J Field Ornithol 78:206–212. https://doi.org/10.1111/j.1557-9263.2007.00103.x
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–S139. https://doi.org/10.1080/00063659909477239
White CR, Cassey P, Schimpf NG, Halsey LG, Green JA, Portugal SJ (2013) Implantation reduces the negative effects of bio-logging devices on birds. J Exp Biol 216:537–542. https://doi.org/10.1242/jeb.076554
Wilson UW (1986) Artificial rhinoceros auklet burrows: a useful tool for management and research. J Field Ornithol 57:295–299
Wilson R, Grant W, Duffy D (1986) Recording devices on free-ranging marine animals: does measurement affect foraging performance? Ecology 67:1091–1093. https://doi.org/10.2307/1939832
Zangmeister J, Haussmann M, Jack CP, Mauck R (2009) Incubation failure and nest abandonment by Leach’s Storm-Petrels detected using PIT tags and temperature loggers. J Field Ornithol 80:373–379. https://doi.org/10.1111/j.1557-9263.2009.00243.x
Acknowledgements
We thank Morgan Benowitz-Fredericks for her substantial contribution to the different drafts of this study. We also thank Jennifer Figeroa for the time she spent helping Léo Marcouillier arrange Alaska field work. Hannes Schraft and Josh Cunningham built and installed the nest boxes, with the help of Alice Sun, Alyssa Piauwasdy, Luis Ramos, Catherine Lee-Zuck, Aidan Colligan, Kelly Bacile, Fred Tremblay and Don-Jean Leandri-Breton. Finally, we thank David Jadhon, Stella Solasz, Gabrielle Denis and Haley Gee for their assistance with the field work.
Funding
The research was funded by the Natural Sciences & Engineering Research Council of Canada, GulfWatch Alaska and Environment & Climate Change Canada.
Author information
Authors and Affiliations
Contributions
LM, SW, KHE contributed to the study conception and design. The detectors that made this study possible were built by DF. Material preparation and data collection were performed by LM, EM, SW and CN. Analysis were performed by LM. The first draft of this manuscript was written by LM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All activities were approved by the McGill Animal Use Protocol 2016-7814 and via permits from the US Fish & Wildlife Service and the Alaska Department of Fish & Game.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marcouillier, L., Miranda, E., Whelan, S. et al. PIT tagging does not measurably reduce reproductive success in sensitive burrow-nesting seabirds. Mar Biol 171, 77 (2024). https://doi.org/10.1007/s00227-023-04387-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04387-x