Abstract
Along European coasts, the rapid expansion of marine renewable energy devices and their buried power cables, raises major societal concerns regarding the potential effects of their magnetic field emissions (MFs) on marine species and ecosystem functioning. MFs occur at a local spatial scale, which makes sessile species the primary target of chronic and high-intensity exposures. Some of them, as ecosystem engineers, have critical functions in coastal habitats whose behavioral alteration may drive profound consequences at the ecosystem level. In this context, the present experimental study explored the effects of short exposure to direct current MFs, on the feeding behavior of a widespread ecosystem engineer, the blue mussel (Mytilus edulis). A repeated measure design was carried out with adult mussels successively exposed to control treatment (ambient magnetic field of 47 µT) and artificial MF treatment (direct current of 300 µT produced by Helmholtz coils), as measured around power cables. The filtration activity was assessed through valve gap monitoring using an automated image analysis system. The clearance rate was estimated simultaneously by measuring the decrease in algal concentration using flow cytometry. Our findings revealed that mussels placed in MF treatment did not exhibit observable differences in valve activity and filtration rate, thus suggesting that, at such an intensity, artificial MFs do not significantly impair their feeding behavior. However, additional research is required to investigate the sensitivity of other life stages, the effects of mid to long-term exposure to alternative and direct current fields and to test various MF intensities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adoption of renewable energy has become a priority for several developing countries in recent decades (Sen and Ganguly 2017). After a first phase of on-land projects, the development of the offshore renewable energy sector (i.e. wind, currents, waves) is currently prevailing and is generally considered a promising option to meet global energy demands (Gill 2005; Rinaldi 2020; Soares-Ramos et al. 2020). Moreover, the electrical interconnections between adjacent countries are intensifying to promote the production and widespread adoption of renewable energies (European commission 2019). Accordingly, the spread of submarine power cables to support electricity transfer in rich and sensitive coastal environments is becoming a major concern, particularly regarding the introduction of artificial magnetic fields (MFs) (Petersen and Malm 2006; Taormina et al. 2018).
MFs are defined as spaces that both influence and are influenced by electrical particles flowing through a power cable. Their strength or magnetic induction, which is measured in microteslas (µT), is proportional to the current intensity and sharply declines with distance from the cable (Normandeau et al. 2011; Otremba et al. 2019; Albert et al. 2020). MFs can also be influenced by other power line characteristics such as the number of conductors, the distance between them, the internal arrangement of copper wires (i.e. twisting) and the type of current: alternating or direct (AC or DC) (Meißner et al. 2006; Worzyk 2009; CSA Ocean Sciences Inc. and Exponent 2019). For example, DC cables produce static MFs that modify the ambient geomagnetic field (Otremba et al. 2019), whereas AC cables emit independent 50–60 Hz fields that vary over time (Kavet et al. 2016). Long-distance electricity transfer (> 50 km) is mainly achieved with high voltage direct current (HVDC) technology (Wei et al. 2017), whereas AC cables are preferred for shorter distances and often have lower transmission capacities (e.g. offshore wind farms) (Meißner et al. 2006).
Several marine species orient and navigate over short- or long-range migrations (e.g. for feeding, spawning, or reproduction), using the Earth’s magnetic field (Wiltschko 1995; Walker et al. 2003; Vacha 2017). In the literature, such sensory modality is referred to as magneto-reception, magneto-sensitivity or magneto-sensation (Wiltschko 1995; Gill et al. 2014; Putman et al. 2022). By causing local geomagnetic field alterations, artificial MFs may thus impair the capacity of species to geolocate (Fischer and Slater 2010; Copping et al. 2016). Moreover, artificial MFs can be detrimental for biological structures, as for instance, they can delay embryonic development and increase the proportion of abnormalities in sea urchins and fish (e.g. Zimmerman et al. 1990; Levin and Ernst 1997; Li et al. 2014; Fey et al. 2019a, b). They can also magnify genotoxic and cytotoxic effects in polychaetes and bivalve mollusks (Stankevičiūtė et al. 2019). However, artificial MFs seem not to constitute an immediate threat to the survival of adult stages (Bochert and Zettler 2006; Jakubowska et al. 2019).
Current knowledge on the potential effects of artificial MFs remains highly inconclusive and heterogeneous across taxonomic groups (Petersen and Malm 2006). Particularly, invertebrates are largely under-represented in impact studies and data regarding their magneto-sensitivity is severely lacking (Isaacman and Lee 2009; Emma 2016; Albert et al. 2020). Yet, the likelihood of encountering MFs is highly dependent on the lifestyle of a given species, which in turn determines the duration and intensity of its exposure. Given that power cables are typically buried in the sediment (0.3 to 3 m depth), benthic, sessile and endogenous organisms such as bivalves, echinoderms, worms, and decapods would experience the highest values of magnetic induction, as opposed to pelagic species (Michel et al. 2007). For example, based on theoretical calculations, a 1000 A DC single-phase cable (15 cm diameter) would create 533 and 65 µT MFs at a 0.3 m and 3 m distance, respectively (the formula to calculate these estimates was obtained from Salinas et al. 2009).
So far, the effects of artificial MFs were mainly investigated in motile crustaceans. A few studies thus reported positive (Scott et al. 2018, 2021) or negative (Ernst and Lohmann 2018) taxis, changes in exploratory behaviors (Hutchison et al. 2020), disruption of orientation abilities (Tomanova and Vacha 2016), and alteration of physiological mechanisms (i.e. d-lactate, d-glucose and THC) controlling the circadian rhythm (Scott et al. 2018, 2021). Despite the risk of chronic exposure to artificial MFs, the sessile epifauna and sedentary endofauna have received far less attention. To our knowledge, only two species of sessile bivalves, the blue mussel (Mytilus edulis) and the Mediterranean mussel (Mytilus galloprovincialis), were used as biological models to highlight the effects of 50 Hz MFs on the immune system functionality (Ottaviani et al. 2002; Malagoli et al. 2003, 2004).
The filter-feeding bivalves are keystone species that control the benthic-pelagic coupling (Prins et al. 1997). These latter form a direct link between primary production and higher trophic levels by performing top-down control on phytoplankton biomass and composition through filtration (Cloern 1982; Stein et al. 1995; Bastviken et al. 1998). Moreover, they profoundly influence benthic remineralization processes and nutrient cycling through biodeposition (i.e. biogenic organic matter flux from the water column to the sediment surface) (Jansen et al. 2019). Up to now, the potential ecological consequences of MFs have not been evaluated on bivalve species at the organism, population or community level. To address this issue, organism-level functional traits and behavior can be used as markers of physiological alteration due to their sensitivity to sublethal effects (Hasenbein et al. 2015). For instance, in bivalve filter-feeders, the feeding behavior is critical for the functioning of both individual energy metabolism and ecosystem functioning. This endpoint thus constitutes a biomarker with strong ecological value, whose impairment may lead to marked population-level consequences (Amiard-Triquet 2009; Hartmann et al. 2016). Mussel gaping/feeding behavior, typically occurs at a high and constant rate under an optimal range of algal concentrations (Riisgård et al. 2011). Conversely, at upper or lower critical thresholds, food availability leads to a reduction in valve gap and exhalant siphon area, as well as a retraction of mantle edges up to complete closure, which constitutes an adaptation to balance energetic gains and losses (Dolmer 2000; Riisgård et al. 2003; Maire et al. 2007). Bivalve gaping behavior is known to vary in response to several environmental parameters including temperature (Kittner and Riisgård 2005), salinity (Riisgård et al. 2013a, b), turbidity (Cranford 2019), flow velocity (Wildish and Miyares 1990), and food quality and quantity (Newell et al. 2001; Saurel et al. 2007). Moreover, valve gaping patterns, including valve-closure, are linked to the metabolic state of the mussel and can be used as bio-indicators of environmental stress (Hartmann et al. 2016; Redmond et al. 2017).
This study focuses on the blue mussel Mytilus edulis (Linnaeus, 1758), a widely distributed ecosystem engineer species that plays a central role in the functioning of coastal habitats (Commito et al. 2008). M. edulis is a gregarious intertidal to shallow subtidal organism that occurs in high population densities, forms large aggregated structures (Christensen et al. 2015) and dominates the French mussel farm production (Agreste 2019). In particular, it is a major colonizer of anthropogenic hard substrates of offshore buildings (Krone et al. 2013), such as wave power buoys and the foundations of offshore infrastructures (Joschko et al. 2008). Very few studies had evaluated the in situ biological colonization of submarine power cables (Carlier et al. 2019). However, using test equipment, Paschen et al. (2014) identified M. edulis colonies both on laid and free-flow cables located up to 10–12 m depths. Overall, mussel settlement is expected to be highly plausible over cables (or their protective structures) located in the intertidal and subtidal zones or over the cable sections located in open water.
Therefore, our study aimed to investigate the potential effects of DC MFs on the filtration activity of M. edulis, which may indirectly impair its ecosystem engineering role. To assess potential temporal changes, valve gap was measured over 6 h following a single algal addition using an automated image acquisition and analysis system (Maire et al. 2007). Short-term exposure to MFs was evaluated because mussels are known to instantly adjust their filtration behavior in response to external stimuli. Algal concentrations were also determined via flow cytometry to quantify filtration rates. The same individuals were tested under control (ambient magnetic field, 47 µT) and MF (homogeneous 300 µT) treatments.
Materials and methods
Magnetic field exposure device
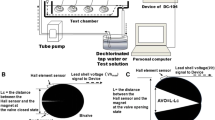
Artificial MFs were produced using two squared Helmholtz coils (1.5 m × 1.5 m), each composed of 200 copper wire turns (2.5 mm2 section) sealed inside a Plexiglas hollow frame. Both coils were placed vertically on Plexiglas racks, spaced 1 m apart, and connected with a branch circuit to a DC power supply (14.8 V; 4.6 A). This system, which will hereinafter be referred to as “Magnotron”, produced a uniform 300 µT magnetic field within a tank equidistant from both coils (Fig. 1). Such value is the estimated magnetic induction at 0.6 m from a DC single-phase cable (15 cm diameter, 1000 A) (Salinas et al. 2009). The tank was used as a buffer to maintain a constant temperature inside two smaller experimental glass tanks filled with 10 L filtered still seawater (35 × 20 × 25 cm) (Fig. 1). The geomagnetic field inside the experimental tanks was measured at approximately 47 µT when the coils were turned off.
The Magnotron system functions in much the same way as other similar devices created by other research teams (e.g. Scott et al. 2018; Jakubowska et al. 2019), with the added benefit of being mobile and specifically designed for regular use across various experimental setups. Moreover, all electrical parameters (voltage, electric intensity, on/off switching, and coil temperature) can be monitored in real time, recorded, and programmed using a purpose-built software developed by MAPPEM Geophysics© (http://www.mappem-geophysics.com/). Magnetic induction is measured every 10 s by a magnetometer (Mag690 Three-axis, Bartington Instruments®) and maintained at a single intensity by an automatic control loop that continuously adjusts the electric intensity.
Mussel sampling and maintenance
Field sampling was conducted in February 2021 in the Bay of Brest (France, North-East Atlantic Ocean, 48°23′32.5"N–°25′59.3"W). Approximately 60 mussels (30–50 mm) were hand-collected from a natural bed located on floating docks. After removing epibionts through gentle brushing, individuals were kept in large tanks (60 × 50 × 40 cm; water depth: 30 cm) for a 9-day period, away from the Magnotron system and the emission of artificial MFs. The acclimation tanks were connected to a semi-closed water recirculating system with a 45% hourly renewal rate (84 L h−1 flux). The inflow was stopped for 3 h each day when bivalves were fed with an Isochrysis galbana monospecific culture (concentration of 3000 cells mL−1). The water was directly pumped from the Bay of Brest and maintained at a natural temperature (approximately 12.6 °C) using a water chiller. The outflow of the tanks was filtered with a mechanical polyethylene filter followed by Biogrog® biological filtering and UV-radiation, before recirculation. Each tank was provided with an air pump to maintain air saturation levels > 98% (oxygen concentration > 8.7 mg L−1). The pH and salinity were maintained at approximately 7.9 and 32.7 PSU, respectively, and other parameters were maintained below threshold values (NH4+ < 0.1 mg L−1; NH3 < 0.01 mg L−1; NO2 < 0.05 mg L−1; NO3 < 10 mg L−1). To avoid a potential alteration of their filter-feeding activity caused by artificial light (Robson et al. 2010; Comeau et al. 2018), mussels were kept in total darkness during both the acclimation period and the experiments.
Experimental procedure
Twenty-four hours before, each experimental procedure 10 mussels were randomly collected and glued on two PVC stands using cyanoacrylate cement in groups of 5 at 6 cm intervals, and thus formed two mussel batches. A small white paint mark was then made on the margin of the free valve of each mussel (Fig. 1). To account for the high inter-individual variability of filtering activity in mussels, behavioral measurements were carried out through a repeated-measure design. For each set of experiment, two batches (5 mussels in each) were sequentially tested under control (CT treatment, 47 µT) on day 1 and magnetic field treatment (MF treatment, 300 µT) on day 2.
Before each treatment, the two mussel batches were starved overnight in two distinct 10 L tanks (i.e. 35 × 20 × 25 cm) filled with filtered (1 µm) seawater. On day 1, one hour before testing, both batches were transferred to the Magnotron’s experimental tanks (Fig. 1). The coils were switched off during CT treatment. In CT or MF treatment, clearance rates and valve activity were then measured for 6 h (i.e. 21 600 s), at the batch (10 L, n = 5) and individual level, respectively, following the addition of an initial algal concentration of approximately 3000 cells mL−1 (I. galbana) (more details will be provided in the next section). Once the CT treatment ended, mussels were transferred back into the starvation tanks. On day 2, for the MF treatment, the same mussel batches were submitted to a 300 µT MF, that was initiated prior to their transfer and the same set of measurements was collected. Apart from the 300 µT MF, all other experimental conditions were strictly similar. In total, filter feeding activity was monitored in six batches of five mussels, i.e. a total of 30 individuals.
Monitoring of valve activity
The distance between the two valves was monitored using an automated image acquisition system consisting of an infrared camera (uEye® camera fitted with a 25 mm Fujinon® objective lens) connected to a laptop computer driven by the Obvious MicroLum software developed at the EPOC Laboratory, University of Bordeaux (Romero-Ramirez et al. 2016). The video sensor was located 110 cm in front of the mussels and infrared lights maximized the contrast between the white mark and the mussel shell. As recommended by Maire et al. (2007), gray-scale images were acquired every 10 s and gathered into an AVI file (5 frames s−1). The AVI explore software was then used to measure the valve gap through the detection of the white mark successive positions, based on pixel color. For each image, the valve gap was expressed as the distance in pixels between y-coordinates (yn) of the white mark barycenter (i.e. center of mass) and a reference point (y0), corresponding to complete closure. Valve gap data were converted into mm and then into angles ϴ (expressed in degrees) using the following equation from (Wilson et al. 2005), where W is the valve gap (mm) and L is the maximum shell length (mm).
Measurement of filtration rates
Filtration rates were quantified using the clearance method (Coughlan 1969) defined as the volume of water cleared of algal cells per unit of time. Isochrysis galbana cells (4.5 µm diameter) have an optimal retention efficiency in Mytilus edulis, as their size is above the 100% retention efficiency threshold, and therefore the clearance rate is equal to the filtration rate (Møhlenberg and Riisgård 1978). The filtration rate of M. edulis remains high and constant at concentrations ranging from 2000 to 6000 cells mL−1 (Riisgård 1991), which is consistent with the phytoplankton winter concentrations observed in the Bay of Brest (Delmas et al. 1983; Hafsaoui et al. 1985). Accordingly, all experiments were performed at an initial algal concentration of approximately 3000 cells mL−1 (corresponding to 9.32 10–5 mg dry weight (DW) mL−1 and 2.98 10–5 mg C mL−1, from Maire et al. 2007). During the experiments, water was continuously aerated with air bubbling to maintain a homogeneous concentration of suspended algal cells. Water samples (0.5 mL in triplicates) were collected at the center of each tank 10 min after algal addition and then every 20 min. The samples were kept in cryotubes filled with 10 µL glutaraldehyde solution, for cell fixation, and stored at −80 °C.
Cell counting (cell mL−1) was performed using a Guava® easyCyte™ 5HT flow cytometer (LUMINEX, 12212 Technology Blvd, Austin, Texas USA). The system was equipped with a 488-nm argon laser (50 mW), forward (FSC) and side scatter (SSC) detectors for relative cell size and complexity, respectively, and three fluorescence detectors: Green-B (525/30 nm), Yellow-B (583/26 nm) and Red-B (695/50 nm). Further, the system was also equipped with a direct, absolute cell count system. Samples were distributed in 96-well micro-plates and analyzed in the flow cytometer for 90 s at a high flow rate (1.18 µL s−1). Phytoplankton cells were selected according to their relative size and/or complexity (FSC/SSC) and their red fluorescence after exciting the chlorophyll pigments with a blue laser. Gating of cells of interest was performed using the Guava SOFT 4.0 software.
Water samples without mussels were also collected to measure algal loss due to sedimentation. In all experiments, clearance rates were calculated at the batch level, as previously described (Maire et al. 2007):
where CR is the clearance rate (L h−1) and is equivalent to the filtration rate F; V is the water volume (10 L) and b is the slope of the linear regression line representing the gradual reduction in algae concentration (R2 > 0.72). Algal sedimentation was highly negligible (± 100 cells). At the end of each experiment, individual measurements of maximal shell length (L; in cm) and flesh dry weight (DW; in mg) (24 h, 77 °C) were performed to calculate the condition index (CI) (Supplementary Material A):
Statistical analyses
Before conducting the analysis, valve angles were converted to percentages of the maximum value recorded over the experiment and distributed into 10 ranges in steps of 10%. Using principal component analysis (PCA), ranges (%) were condensed into higher grouping categories that were compared using the Kruskal–Wallis and Wilcoxon tests. PCA was based on a covariance matrix and principal components were chosen based on Kaiser’s criterion (Kaiser 1960) (Supplementary Material B and C). Feeding responses occurred in two periods, P1 and P2. P1 period started with algal addition (T = 0) and was characterized by an increase in valve angle, which then reached maximal values. During the P2 period, valve angle was generally high and rather constant but then declined gradually in response to low algal concentrations (< 500 cell mL−1). Mussel filtration activity was described by a set of 7 response variables: P1 duration (h), batch filtration rate (L h−1 ind−1), mean valve angle over P2, and average duration (s) spent in the four valve angle categories derived from PCA (‘low,’ ‘average,’ ‘high,’ and ‘maximum’). The relationships between the aforementioned response variables and the treatment (CT or MF) was assessed using linear mixed effect models (LMM), as described by (Brown 2021). Magnetic treatment and one of the seven response variables were entered as fixed effects and batch number was nested within the treatment variable for the random structure. Models were selected based on the Akaike information criterion (AIC) and maximum likelihood estimations. The effect of MF was investigated by likelihood-ratio tests and PCA and LMM were respectively conducted using the ‘nlme’ (Pinheiro and Bates 2000), and ‘ez’ packages in R version 4.0.2 (R Core Team 2021). Assumptions of residual normality and homoscedasticity were verified by plotting the residuals versus the fitted values. All statistical analyses were performed at a significance level of 5%.
Results
From the 30 tested individuals (6 batches × 5 mussels), 10 were excluded from valve angle analysis due to the absence of filter-feeding activity in at least one of the two treatments. These mussels remained either closed during both the CT and MF treatments (n = 5) or had no distinct P1 and P2 periods (n = 2 in CT and n = 3 in MF). In total, the valve activity was thus monitored in 20 mussels (the biometrical characteristics of these organisms are provided in Supplementary Material A). All individuals from the two treatments quickly opened between 14 and 97% of the valve angle after algal addition (T = 10 min), with a mean (± standard error) of 60.9% ± 4.3% and 60.2% ± 4.0% in the CT and MF treatment, respectively. The filtration rate was calculated at the batch level for comparisons between treatments, and was estimated a posteriori at the individual level, excluding mussels that remained totally closed during the duration of the experiments (n = 22 and n = 23 in CT and MF treatments, respectively).
P1 duration, P2 mean valve angle, and filtration rates
The duration of P1 (i.e. the initial period of valve gap increase in response to algal addition) varied from 0.44 to 2.90 h, with a mean (± standard error) of 1.15 ± 0.14 h in the CT treatment and 0.93 ± 0.09 h in the MF treatment. The mean valve angle during the P2 period (i.e. the period of high and constant valve angle) was 11.52 ± 0.74° and 9.77 ± 0.64° in the CT and MF treatments, respectively (Table 1). Figure 2 illustrates a typical valve angle temporal variation as a function of algal concentration. During the experiment, the average batch filtration rates were 8.06 ± 1.66 L h−1 in the CT treatment and 7.54 ± 1.20 L h−1 in the MF treatment (Table 2 and Fig. 3). None of these response variables varied significantly as a function of magnetic treatment (P1 duration: L.ratio = 0.89, p = 0.346; P2 valve angle: L.ratio = 2.81, p = 0.094, filtration rate: L.ratio = 0.08, p = 0.782).
Example of the temporal changes in valve angle of one Mytilus edulis individual (solid line, °) in relation with algal concentration (filled circles, cells mL−1), across CT and MF treatments. Algae were added at T = 0 and counted from T = 10 min. The P1 period starts with algal addition and is characterized by an increase in valve angle up to maximal values. The P2 period is characterized by a high and constant valve angle that gradually declined with low algal concentrations. The limit between P1 and P2 is indicated by the dashed vertical line
Time spent in different valve angle categories
Using PCA, valve angle ranges were classified into four groups: ‘low’ (i.e. 0–10%; 10–20%), ‘average’ (i.e. 20–30%; 30–40%; 40–50%; 50–60%), ‘high’ (i.e. 60–70%; 80–90%) and ‘maximal’ (i.e. 90–100%) valve angles (Supplementary Material B and C). The percentage of time (%) spent across the different categories in both treatments is illustrated in Fig. 4. Irrespective of the treatments (CT vs MF), there was a significant difference in the time spent across the four valve angle categories (χ2 (3) = 54.40, p < 0.01, size effect = 0.33). Overall, the mussels spent 5% of the time at low aperture, 20% at average aperture, 45% at high aperture, and 32% at maximum valve aperture. Particularly, pair-wise comparisons demonstrated that the mussels spent significantly less time at low valve aperture relative to all other categories (Wilcoxon test: p < 0.01 in all comparisons), and the time spent in the average category was significantly below that spent in the high category (W. test: p < 0.01). Nevertheless, the MF did not impact the time spent in neither the low (L.ratio = 0.91, p = 0.313), average (L.ratio = 0.01, p = 0.900), high (L.ratio = 0.22, p = 0.720), nor maximal valve angle (L.ratio = 0.8, p = 0.420).
Time proportion (%) spent by mussels (Mytilus edulis) across the four categories of valve angle opening: low (0–20%), average (20–60%), high (60–90%), and maximal (90–100%) in the CT and MF treatments. The same individuals were tested across both treatments. The histograms that do not share common letters are statistically different (p < 0.01)
Discussion
Despite the rapid development of offshore renewable energies and the subsequent expansion of submarine power cables, research on the effects of their emissions on marine fauna and especially sessile invertebrates, which are particularly exposed, is still very scarce. To our knowledge, this study is the first to investigate the effects of 300 µT DC magnetic fields on a key ecological function, the filtration activity of suspension-feeding bivalves. To detect potential behavioral changes caused by the MFs, one critical aspect of the experiments was to be sure that mussels displayed a natural feeding behavior that was not altered by the experimental conditions (e.g. light, algal concentration). To this end, similar and optimal conditions for filtration activity were provided to the mussels across the treatments. The initial cell concentration (3000 cells mL−1) matched the natural seasonal algal concentration at the sampling site (Delmas et al. 1983) and was optimal for the expression of a natural feeding behavior in the blue mussel (Riisgard 1991). In addition, given that M. edulis tends to reduce its feeding behavior below 800–500 cells. mL−1 (see Fig. 2B in Riisgard et al. 2003), the addition of an initial moderate concentration allowed for the observation of both the increase of the filtration rate (i.e. immediately following algal cells addition) and the reduction of the filtering activity (caused by food limitation), within the experiment duration.
The present results demonstrated that a 300 µT DC magnetic field does not disturb the filter-feeding activity of the blue mussel neither in terms of valve activity nor filtration rate. In particular, there were no changes in the valve angle in relation with algal concentration and the filtration rate remained similar compared to the control treatment (47 µT geomagnetic field). Therefore, individual filtration rates, standardized to DW (g), averaged (± standard error) 6.71 ± 1.04 L h−1 g DW−1 and 7.60 ± 1.73 L h−1 g DW−1 under the CT (47 µT) and MF treatment (DC 300 µT), respectively. These values were fully consistent with the range previously reported for this species in the literature (e.g. 5.39–8.08 L h−1 g DW−1) (Riisgård et al. 2011). Our specimens had a mean condition index of 3.9 ± 1.0 mg cm−3, which was at the lower end of the typical species range according to the seasonal maturation cycle (3.6–8.4 mg cm−3, Bayne and Worrall 1980). Temporal changes in valve opening during the whole duration of the experiments were similar to those described in earlier studies (e.g. Dolmer 2000; Newell et al. 2001; Riisgård et al. 2003). Particularly, following algal addition, the valve angle of the mussels reached maximum values, on average, at 1.15 h in the CT treatment and 0.93 h in the MF treatment. By comparison, Riisgård et al. (2003) estimated the duration of this opening phase around 45 min in M. edulis and Maire et al. (2007) around 1.3 h in the Mediterranean mussel M. galloprovincialis. Afterward, bivalves typically maintain filtration rates at their maximal capacity with a constant valve gap until algal concentrations decrease below a critical threshold. Due to a moderate addition of food (3000 cells mL−1) at the beginning of the experiment, the conditions became suboptimal for the mussel’s filtration activity after about 1–2 h, which may explain lower values of filtration rates compared to previous studies (Riisgard et al. 2013a). As reported by Riisgård et al. (2003, 2013b), actively feeding bivalves rapidly reduced their filtration activity when the algal concentration decreased below 1000 cells mL−1. Although the period (P2) of decreasing valve angle could not exceed the 6 h duration of the experiments, only few mussels displayed complete closure of the valves, which can last up to 500 min in M. edulis (Riisgård et al. 2003). However, there were no significant differences between treatments in temporal changes of valve angle during this P2 period.
In the present study, one-third of the individuals were excluded from the analysis because they remained closed or did not exhibit typical filter-feeding signals. Such response patterns were analogous to the avoidance behavior, described as a stress response to chemicals, in which mussels keep their valves closed (Hartmann et al. 2016). Avoidance behavior is identified as a reliable sublethal indicator of mussel short-term disturbance. However, none of the closures were specific to the MF treatment and always occurred in both treatments. Additionally, some individuals displayed filtering activity in only one treatment but independently of CT or MF. Since the experimental sequence (CT before DC) was kept constant across all mussel batches, the absence of filtration may not be due to post-exposure effects. Therefore, we concluded that MF was not the cause of mussel long-term closing and, overall, that filtration activity of adult mussels was not significantly affected by MF short-term exposure. Behavioral differences observed both within and between treatments were likely attributable to the high natural inter-individual variability of acclimation to transfer, captivity, and other experimental conditions.
As previously acknowledged, data regarding the effects of artificial MFs on bivalves are severely lacking and behavioral measurements are still absent from the literature. In that respect, the present findings cannot be compared or discussed with a directly relevant literature. Nonetheless, some studies addressed the issue at the physiological scale in the blue mussel (M. edulis) and the Baltic clam (Limecola balthica) (Bochert and Zettler 2006; Stankevičiūtė et al. 2019). The authors reported that long-term exposure to strong MF intensities (mussel: 3.7 mT DC for 52 days; clam: 0.85–1.05 mT AC for 12 days) was neither a threat to the bivalves’ survival nor to M. edulis reproductive status. Nevertheless, the immune system of the Mediterranean mussel (M. galloprovincialis) was altered after short-term MF exposure (300–1000 µT at 50 Hz AC, for 15–30 min). Further, the authors identified disruptions in the cellular processes and an increase in genotoxic and cytotoxic effects in the gill cells of the Baltic clam (Ottaviani et al. 2002; Malagoli et al. 2003, 2004; Stankevičiūtė et al. 2019). In marine species, apoptosis and DNA damage are common biomarkers of environmental stress (Falfushynska et al. 2021). Based on this broad-spectrum ecotoxicological approach, MFs may activate the physiological pathways commonly associated with organic contaminants in bivalves. Currently, the leading hypothesis is that MFs would trigger oxidative stress mechanisms (Mahmoudinasab et al. 2016). Further, 48-h experiments were also conducted in freshwater invertebrates (e.g. the snail Elimia clavaeformis and the clam Corbicula fluminea) and found no changes in the spatial distribution patterns relative to the location of a magnet (maximum of 36 mT DC) (Cada et al. 2011). Overall, the few results obtained so far suggest that artificial MFs cause alterations of the biological system that do not occur at organism-level life traits.
When facing environmental stressors, organisms maintain homeostasis through a suite of adaptive mechanisms at the molecular, biochemical, physiological, and behavioral scales (Goldstein and Kopin 2007). Changes in behavioral patterns may occur as part of a tertiary response level to stress that is modulated by the timing, intensity, persistence, and predictability of the stressor, as well as the genetic traits and conditions of the individual (Barton et al. 1987). Accordingly, in an experimental context, the choice of exposure duration is highly critical as it may interfere with the sensitivity of the behavior assessed (Amiard-Triquet 2009). Until now, neither work, including this one, has conclusively demonstrated that short-term continuous exposure to MFs induces behavioral changes in marine mollusks. This may suggest that tertiary stress responses occur over longer-term continuous exposures. In such case, given that MFs are predictable and of prolonged nature, the outcome might either be habituation with a decrease in response to MF stimuli (Dehaudt et al. 2019) or a maladaptive stress response with potential adverse effects on reproductive success (Suri and Vaidya 2015).
Research conducted on marine bivalves indicated that some effects of MFs would be transitory and that compensatory mechanisms would be implemented at the physiological scale. Studies in M. galloprovincialis indeed demonstrated that immune system alterations (delay in cell adhesion and shape changes) under 300 to 600 µT AC MFs were transitory and reversible (Ottaviani et al. 2002; Malagoli et al. 2003). The authors identified the activation of an alternative stress pathway involved in restoring homeostasis in exposed individuals. Remarkably, MFs have also been found to cause intensity-dependent effects, with no alterations at 200 µT, temporary damages from 300 to 600 µT, and permanent alterations above 600 µT up to 1000 µT. Hence, future dose–response investigations at all stress response levels and with realistic DC and AC MFs intensities are fundamental to assess the environmental impacts of power cables.
Furthermore, the effects of MFs on organisms must also be addressed in the context of their life history and habitat. This would effectively account for the concept of critical windows of exposure (or sensitive periods), over which the individual phenotypic plasticity is higher and largely shaped by environmental or intrinsic factors (Burggren and Mueller 2015). For instance, mussel reproductive metabolism is considered a major confounding factor in monitoring their biological responses (Farcy et al. 2013). As a result, the present work was purposely performed at the end of gonadal development to avoid vulnerable periods of the mussel life cycle. Nevertheless, organisms in poor physiological conditions, such as immediately after a spawning event, might be particularly vulnerable to MF-mediated stress (Berthelin et al. 2000). Similarly, other life stages (e.g. larval fixation stages) must also be considered, as they are generally more sensitive to abiotic factors and might be more responsive to MFs (Gosling 2003). For example, bivalves metamorphosis (pediveliger larvae) is a critical process whose success depends on a wide range of factors (e.g. temperature, food supply, suitable substrate availability, and other biological, physical, and chemical stimuli), which is also vulnerable to exogenous factors (Toupoint 2012). In spite of such context, data about bivalves larval stages are absent from the literature and are strongly needed.
Conclusion
This pioneering study demonstrated that short-term exposure to DC 300 µT magnetic fields had no observable effects on the filtration activity of Mytilus edulis. Feeding behavior has a strong ecological value and our findings provide seminal insights into the potential effects of MFs at the population level. However, additional work is needed to explore the interactions between the intensity and duration of MF exposure, as well as the effects of alternating current and other environmental factors on mussel behavior. Further, the effects of MFs on different life stages and vital functions of marine invertebrates must also be evaluated to determine their population-wide implications.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Agreste (2019) Enquêtes aquaculture 2016–2017 (No. n°2019–8-juillet 2019). Ministère de l’Agriculture et de l’Alimentation
Albert L, Deschamps F, Jolivet A, Olivier F, Chauvaud L, Chauvaud S (2020) A current synthesis on the effects of electric and magnetic fields emitted by submarine power cables on invertebrates. Mar Environ Res 159:104958. https://doi.org/10.1016/j.marenvres.2020.104958
Amiard-Triquet C (2009) Behavioral disturbances: the missing link between sub-organismal and supra-organismal responses to stress? Prospects based on aquatic research. Hum Ecol Risk Assess 15:87–110. https://doi.org/10.1080/10807030802615543
Barton B, Schreck C, Barton L (1986) Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis Aquat Org 2:173–185. https://doi.org/10.3354/DAO002173
Bastviken DTE, Caraco NF, Cole JJ (1998) Experimental measurements of zebra mussel (Dreissena polymorpha) impacts on phytoplankton community composition. Freshw Biol 39:375–386. https://doi.org/10.1046/j.1365-2427.1998.00283.x
Bayne B, Worrall C (1980) Growth and production of Mussels Mytilus edulis from two populations. Mar Ecol Prog Ser 3:317–328. https://doi.org/10.3354/MEPS003317
Berthelin C, Kellner K, Mathieu M (2000) Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France). Comp Biochem Physiol B Biochem Mol Biol 125:359–369. https://doi.org/10.1016/s0305-0491(99)00187-x
Bochert R, Zettler ML (2006) Effect of electromagnetic fields on marine organisms. In: Koller J, Koppel J, Peters W (eds) Offshore wind energy. Springer, Berlin, pp 223–234. https://doi.org/10.1007/978-3-540-34677-7_14
Brown VA (2021) An introduction to linear mixed-effects modeling in r. Adv Methods Pract. Psychol. Sci. 4:1–19
Burggren WW, Mueller CA (2015) Developmental critical windows and sensitive periods as three-dimensional constructs in time and space. Physiol Biochem Zool 88:91–102. https://doi.org/10.1086/679906
Cada GF, Bevelhimer M, Riemer KP, Turner JW (2011) Effects on freshwater organisms of magnetic fields associated with hydrokinetic turbines FY 2010 annual progress report. Oak Ridge Natl Lab (oak Ridge, Tennessee). https://doi.org/10.2172/1025846
Carlier A, Vogel C, Alemany J (2019) Synthèse des connaissances sur les impacts des câbles électriques sous-marins : phases de travaux et d’exploitation. IFREMER, Plouzané. https://doi.org/10.13155/61975
Christensen HT, Dolmer P, Hansen BW, Holmer M, Kristensen LD, Poulsen LK, Stenberg C, Albertsen CM, Støttrup JG (2015) Aggregation and attachment responses of blue mussels, Mytilus edulis—impact of substrate composition, time scale and source of mussel seed. Aquaculture 435:245–251. https://doi.org/10.1016/j.aquaculture.2014.09.043
Cloern JE (1982) Does the benthos control phytoplankton biomass in south San Francisco Bay. Mar Ecol Prog Ser 9:191–202. https://pubs.er.usgs.gov/publication/70156387
Comeau L, Babarro JMF, Longa A, Padin XA (2018) Valve-gaping behavior of raft-cultivated mussels in the Ria de Arousa, Spain. Aquac Rep 9:68–73. https://doi.org/10.1016/j.aqrep.2017.12.005
Commito JA, Como S, Grupe BM, Dow WE (2008) Species diversity in the soft-bottom intertidal zone: Biogenic structure, sediment, and macrofauna across mussel bed spatial scales. J Exp Mar Biol Ecol 366:70–81. https://doi.org/10.1016/j.jembe.2008.07.010
Copping A, Sather N, Hanna L, Whiting J, Zydlewska G, Staines G, Gill AB, Hutchison I, O’Hagan A, Simas T, Bald J, Sparling C, Wood J, Masden E (2016) Annex IV 2016 state of the science report. Environmental effect of marine renewable energy development around the world. Pacific Northwest National Laboratory. https://tethys.pnnl.gov/sites/default/files/publications/Annex-IV-2016-State-of-the-Science-Report_LR.pdf
Coughlan J (1969) The estimation of filtering rate from the clearance of suspensions. Mar Biol 2:356–358. https://doi.org/10.1007/BF00355716
Cranford PJ (2019) Magnitude and extent of water clarification services provided by bivalve suspension feeding. In: Smaal AC, Ferreira JG, Grant J, Petersen JK, Strand Ø (eds) Goods and services of marine bivalves. Springer Open. Springer, Cham. https://doi.org/10.1007/978-3-319-96776-9_8
CSA Ocean Sciences Inc. and Exponent (2019) Evaluation of potential EMF Effects on fish species of commercial or recreational fishing importance in Southern New England. U.S. Dept of the Interior, Bureau of Ocean Energy management, Headquarters, Sterling. OCS Study BOEM 2019-049. https://marinecadastre.gov/espis/#/search/study/100263&minYr=NaN&maxYr=NaN&status=undefined®ion=undefined
Dehaudt B, Nguyen M, Vadlamudi A, Blumstein DT (2019) Giant clams discriminate threats along a risk gradient and display varying habituation rates to different stimuli. Ethology 125:392–398. https://doi.org/10.1111/eth.12863
Delmas R, Hafsaoui M, Le jehan S, Quéguiner B, Tréguer (1983) Impact de fertilisations à forte variabilité saisonnière et annuelle sur le phytoplancton d’un écosystème eutrophe. Oceanol Acta (special issue). https://archimer.ifremer.fr/doc/00247/35802/
Dolmer P (2000) Feeding activity of mussels Mytilus edulis related to near-bed currents and phytoplankton biomass. J Sea Res 44:221–231. https://doi.org/10.1016/S1385-1101(00)00052-6
Emma B (2016) A review of the evidence of electromagnetic field (emf) effects on marine organisms. https://www.rroij.com/open-access/a-review-of-the-evidence-of-electromagnetic-field-emf-effects-onmarine-organisms-.php?aid=83798
Ernst D, Lohmann K (2018) Size-dependent avoidance of a strong magnetic anomaly in Caribbean spiny lobsters. J Exp Biol 221:5. https://doi.org/10.1242/jeb.172205
European Commission (2019) Electricity interconnections with neighbouring countries: second report of the Commission expert group on interconnection targets. Publications Office, LU. https://data.europa.eu/doi/https://doi.org/10.2833/924949
Falfushynska H, Sokolov EP, Fisch K, Gazie H, Schulz-Bull DE, Sokolova IM (2021) Biomarker-based assessment of sublethal toxicity of organic UV filters (ensulizole and octocrylene) in a sentinel marine bivalve Mytilus edulis. Sci Total Environ 798:149171. https://doi.org/10.1016/j.scitotenv.2021.149171
Farcy E, Burgeot T, Haberkorn H, Auffret M, Lagadic L, Allenou J-P, Budzinski H, Mazzella N, Pete R, Heydorff M, Menard D, Mondeguer F, Caquet T (2013) An integrated environmental approach to investigate biomarker fluctuations in the blue mussel Mytilus edulis L. in the Vilaine estuary. France Environ Sci Pollut Res 20:630–650. https://doi.org/10.1007/s11356-012-1316-z
Fey D, Jakubowska M, Greszkiewicz M, Andrulewicz E, Otremba Z, Urban-Malinga B (2019a) Are magnetic and electromagnetic fields of anthropogenic origin potential threats to early life stages of fish? Aquat Toxicol 209:150–158. https://doi.org/10.1016/j.aquatox.2019.01.023
Fey DP, Greszkiewicz M, Otremba Z, Andrulewicz E (2019b) Effect of static magnetic field on the hatching success, growth, mortality, and yolk-sac absorption of larval Northern pike Esox lucius. Sci Total Environ 647:1239–1244. https://doi.org/10.1016/j.scitotenv.2018.07.427
Fischer C, Slater M (2010) Electromagnetic field study. Effects of electromagnetic fields on marine species: a literature review. Oregon Wave Energy Trust, Oregon. https://ir.library.oregonstate.edu/concern/technical_reports/9w032369d
Gill AB (2005) Offshore renewable energy: ecological implications of generating electricity in the coastal zone. J Appl Ecol 42:605–615. https://doi.org/10.1111/j.1365-2664.2005.01060.x
Gill AB, Gloyne-Philips I, Sigray P (2014) Marine renewable energy, electromagnetic (EM) fields and EM-sensitive animals. In: Shields MA, Payne AIL (eds) Marine renewable energy technology and environmental interactions. Springer, Dordrecht, pp 61–79. https://doi.org/10.1007/978-94-017-8002-5_6
Goldstein DS, Kopin IJ (2007) Evolution of concepts of stress. Stress 10:109–120. https://doi.org/10.1080/10253890701288935
Gosling EM (2003) Bivalve molluscs: biology, ecology, and culture. Fishing News Books, Oxford
Hafsaoui M, Quéguiner B, Tréguer P (1985) Production primaire et facteurs limitant la croissance du phytoplancton en rade de Brest (1981–1983). Oceanis 11:181–195
Hartmann JT, Beggel S, Auerswald K, Stoeckle BC, Geist J (2016) Establishing mussel behavior as a biomarker in ecotoxicology. Aquat Toxicol 170:279–288. https://doi.org/10.1016/j.aquatox.2015.06.014
Hasenbein S, Lawler SP, Geist J, Connon RE (2015) The use of growth and behavioral endpoints to assess the effects of pesticide mixtures upon aquatic organisms. Ecotoxicology 24:746–759. https://doi.org/10.1007/s10646-015-1420-1
Hutchison Z, Sigray P, He H, Gill A, King J, Gibson C (2020) Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci Rep 10:4219. https://doi.org/10.1038/s41598-020-60793-x
Isaacman L, Lee K (2009) Current state of knowledge on the environmental impacts of tidal and wave energy technology in Canada (report no. 2009/064). Report by Bedford Institute of Oceanography. Report for Fisheries and Oceans Canada. https://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2009/2009_077-eng.htm
Jakubowska M, Urban-Malinga B, Otremba Z, Andrulewicz E (2019) Effect of low frequency electromagnetic field on the behavior and bioenergetics of the polychaete Hediste diversicolor. Mar Environ Res 150:104766. https://doi.org/10.1016/j.marenvres.2019.104766
Jansen HM, Strand Ø, van Broekhoven W, Strohmeier T, Verdegem MC, Smaal AC (2019) Feedbacks from filter feeders: review on the role of mussels in cycling and storage of nutrients in oligo- meso- and eutrophic cultivation areas. In: Smaal AC, Ferreira JG, Grant J, Petersen JK, Strand Ø (eds) Goods and services of marine bivalves. Springer, Cham, pp 143–177. https://doi.org/10.1007/978-3-319-96776-9_9
Joschko TJ, Buck BH, Gutow L, Schröder A (2008) Colonization of an artificial hard substrate by Mytilus edulis in the German Bight. Mar Biol Res 4:350–360. https://doi.org/10.1080/17451000801947043
Kaiser HE (1960) The application of electronic computers to factor analysis. Educ Psychol Meas 20:141–151
Kavet R, Wyman MT, Klimley AP (2016) Modeling magnetic fields from a DC power cable buried beneath San Francisco Bay based on empirical measurements. PLoS ONE 11:e0148543. https://doi.org/10.1371/journal.pone.0148543
Kittner C, Riisgård H (2005) Effect of temperature on filtration rate in the mussel Mytilus edulis: no evidence for temperature compensation. Mar Ecol Prog Ser 305:147–152. https://doi.org/10.3354/meps305147
Krone R, Gutow L, Joschko TJ, Schröder A (2013) Epifauna dynamics at an offshore foundation—implications of future wind power farming in the North Sea. Mar Environ Res 85:1–12. https://doi.org/10.1016/j.marenvres.2012.12.004Getrightsandcontent
Levin M, Ernst S (1997) Applied DC magnetic fields cause alterations in the time of cell divisions and developmental abnormalities in early sea-urchin embryos. Bioelectromagnetics 18:255–263. https://doi.org/10.1002/(SICI)1521-186X(1997)18:3%3c255::AID-BEM9%3e3.0.CO;2-1
Li Y, Liu X, Liu K, Miao W, Zhou C, Li Y, Wu H (2014) Extremely low-frequency magnetic fields induce developmental toxicity and apoptosis in Zebrafish (Danio rerio) embryos. Biol Trace Elem Res 162:324–332. https://doi.org/10.1007/s12011-014-0130-5
Mahmoudinasab H, Sanie-Jahromi F, Saadat M (2016) Effects of extremely low-frequency electromagnetic field on expression levels of some antioxidant genes in MCF-7 cells. Mol Biol Res Commun 5:77–85
Maire O, Amouroux J-M, Duchêne J-C, Grémare A (2007) Relationship between filtration activity and food availability in the Mediterranean mussel Mytilus galloprovincialis. Mar Biol 152:1293–1307. https://doi.org/10.1007/s00227-007-0778-x
Malagoli D, Gobba F, Ottaviani E (2003) Effects of 50-Hz magnetic fields on the signalling pathways of fMLP-induced shape changes in invertebrate immunocytes: the activation of an alternative “stress pathway”. Biochim Biophys Acta 1620:185–190. https://doi.org/10.1016/S0304-4165(02)00531-7
Malagoli D, Gobba F, Ottaviani E (2004) 50 Hz magnetic fields activate mussel immunocyte p38 MAP kinase and induce HSP70 and 90. Comp Biochem Physiol C Toxicol Pharmacol 137:75–79. https://doi.org/10.1016/j.cca.2003.11.007
Meißner K, Schabelon H, Bellebaum J, Sordyl H (2006) Impacts of submarine cables on the marine environment. A literature review. Report by Institute of Applied Ecology (IfAO). Report for German Federal Agency for Nature Conservation (BfN). https://tethys.pnnl.gov/sites/default/files/publications/Meissner-et-al-2006.pdf
Michel J, Dunagan H, Boring C, Healy E, Evans W, Dean JM, McGillis A, Hain J (2007) Worldwide synthesis and analysis of existing information regarding environmental effects of alternative energy uses on the outer continental shelf (report No. MMS 2007-038). Report by ICF International. Report for US Department of the Interior (DOI). https://offshorewindhub.org/resource/1093
Møhlenberg F, Riisgård HU (1978) Efficiency of particle retention in 13 species of suspension feeding bivalves. Ophelia 17:239–246. https://doi.org/10.1080/00785326.1978.10425487
Newell CR, Wildish DJ, MacDonald BA (2001) The effects of velocity and seston concentration on the exhalant siphon area, valve gape and filtration rate of the mussel Mytilus edulis. J Exp Mar Biol Ecol 262:91–111. https://doi.org/10.1016/S0022-0981(01)00285-4
Normandeau, Exponent, Tricas T, Gill A (2011) Effects of EMFs from undersea power cables on elasmobranchs and other marine species. U.S. Dept. of the Interior, Bureau of Ocean Energy Management, Pacific OCS Region, Camarillo. OCS Study BOEMRE 2011-09. http://www.gomr.boemre.gov/homepg/espis/espisfront.asp
Otremba Z, Jakubowska M, Urban-Malinga B, Andrulewicz E (2019) Potential effects of electrical energy transmission—the case study from the Polish Marine Areas (southern Baltic Sea). Oceanol Hydrobiol Stud 48:196–208. https://doi.org/10.1515/ohs-2019-0018
Ottaviani E, Malagoli D, Ferrari A, Tagliazucchi D, Conte A, Gobba F (2002) 50 Hz magnetic fields of varying flux intensity affect cell shape changes in invertebrate immunocytes: the role of potassium ion channels. Bioelectromagnetics 23:292–297. https://doi.org/10.1002/bem.10021
Paschen M, Rudorf U, Dally C (2014) Bio-fouling on underwater cables—results of long-term storage tests of different cable sheathing materials in the Baltic Sea. Eur J Sci Tech 3:52–62
Petersen JK, Malm T (2006) Offshore windmill farms: threats to or possibilities for the marine environment. Ambio 35:75–80. https://doi.org/10.1579/0044-7447(2006)35[75:OWFTTO]2.0.CO;2
Pinheiro J, Bates D (2000) Mixed-effects models in S and S-PLUS. Springer, New York. https://doi.org/10.1007/b98882
Prins TC, Smaal AC, Dame RF (1997) A review of the feedbacks between bivalve grazing and ecosystem processes. Aquat Ecol 31:349–359. https://doi.org/10.1023/A:1009924624259
Putman NF (2022) Magnetosensation. J Comp Physiol A. https://doi.org/10.1007/s00359-021-01538-7
R Core Team (2021) R: a language and environment for statistical. R Foundation for Statistical Computing, Vienna
Redmond KJ, Berry M, Pampanin DM, Andersen OK (2017) Valve gape behaviour of mussels (Mytilus edulis) exposed to dispersed crude oil as an environmental monitoring endpoint. Mar Pollut Bull 117:330–339. https://doi.org/10.1016/j.marpolbul.2017.02.005
Riisgård H (1991) Filtration rate and growth in the blue mussel, Mytilus edulis Linneaus, 1758: dependence on algal concentration. J Shellfish Res 10:29–35
Riisgård HU, Kittner C, Seerup DF (2003) Regulation of opening state and filtration rate in filter-feeding bivalves (Cardium edule, Mytilus edulis, Mya arenaria) in response to low algal concentration. J Exp Mar Biol Ecol 284:105–127. https://doi.org/10.1016/S0022-0981(02)00496-3
Riisgård HU, Egede PP, Barreiro Saavedra I (2011) Feeding behaviour of the Mussel Mytilus edulis: new observations, with a minireview of current knowledge. J Mar Sci 2011:1–13. https://doi.org/10.1155/2011/312459
Riisgård HU, Lüskow F, Pleissner D, Lundgreen K, López MÁP (2013a) Effect of salinity on filtration rates of mussels Mytilus edulis with special emphasis on dwarfed mussels from the low-saline Central Baltic Sea. Helgol Mar Res 67:591–598. https://doi.org/10.1007/s10152-013-0347-2
Riisgård HU, Pleissner D, Lundgreen K, Larsen PS (2013b) Growth of mussels Mytilus edulis at algal (Rhodomonas salina) concentrations below and above saturation levels for reduced filtration rate. Mar Biol Res 9:1005–1017. https://doi.org/10.1080/17451000.2012.742549
Rinaldi G (2020) Offshore renewable energy. In: Qubeissi MA, El-kharouf A, Soyhan HS (eds) Renewable energy—resources, challenges and applications. IntechOpen. https://doi.org/10.5772/intechopen.91662
Robson AA, Garcia de Leaniz C, Wilson RP, Halsey LG (2010) Effect of anthropogenic feeding regimes on activity rhythms of laboratory mussels exposed to natural light. Hydrobiologia 655:197–204. https://doi.org/10.1007/s10750-010-0449-7
Romero-Ramirez A, Grémare A, Bernard G, Pascal L, Maire O, Duchêne JC (2016) Development and validation of a video analysis software for marine benthic applications. J Mar Syst 162:4–17. https://doi.org/10.1016/j.jmarsys.2016.03.003
Salinas E, Bottauscio O, Chiampi M, Conti R, Cruz Romero P, Dovan T, Dular P, Hoeffelman J, Lindgren R, Maiolo P, Melik M, Tartaglia M (2009) Guidelines for mitigation techniques of power-frequency magnetic fields originated from electric power systems—Task Force C4.204. CIGRE, Paris
Saurel C, Gascoigne JC, Palmer MR, Kaiser MJ (2007) In situ mussel feeding behavior in relation to multiple environmental factors: regulation through food concentration and tidal conditions. Limnol Oceanogr 52:1919–1929. https://doi.org/10.4319/lo.2007.52.5.1919
Scott K, Harsanyi P, Lyndon AR (2018) Understanding the effects of electromagnetic field emissions from marine renewable energy devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Mar Pollut Bull 131:580–588. https://doi.org/10.1016/j.marpolbul.2018.04.062
Scott K, Harsanyi P, Easton BA, Piper AJ, Rochas C, Lyndon AR (2021) Exposure to electromagnetic fields (EMF) from submarine power cables can trigger strength-dependant behavioural and physiological responses in edible Crab, Cancer pagurus (L.). J Mar Sci Eng 9:776. https://doi.org/10.3390/jmse9070776
Sen S, Ganguly S (2017) Opportunities, barriers and issues with renewable energy development—a discussion. Renew Sust Energ Rev 69:1170–1181. https://doi.org/10.1016/j.rser.2016.09.137
Soares-Ramos EPP, de Oliveira-Assis L, Sarrias-Mena R, Fernández-Ramírez LM (2020) Current status and future trends of offshore wind power in Europe. Energy 202:117787. https://doi.org/10.1016/j.energy.2020.117787
Stankevičiūtė M, Jakubowska M, Pažusienė J, Makaras T, Otremba Z, Urban-Malinga B, Fey DP, Greszkiewicz M, Sauliutė G, Baršienė J, Andrulewicz E (2019) Genotoxic and cytotoxic effects of 50 Hz 1 mT electromagnetic field on larval rainbow trout (Oncorhynchus mykiss), Baltic clam (Limecola balthica) and common ragworm (Hediste diversicolor). Aquat Toxicol 208:109–117. https://doi.org/10.1016/j.aquatox.2018.12.023
Stein RA, DeVries DR, Dettmers JM (1995) Food-web regulation by a planktivore: exploring the generality of the trophic cascade hypothesis. Can J Fish Aquat Sci 52:2518–2526. https://doi.org/10.1139/f95-842
Suri D, Vaidya VA (2015) The adaptive and maladaptive continuum of stress responses—a hippocampal perspective. Rev Neurosci 26:415–442. https://doi.org/10.1515/revneuro-2014-0083
Taormina B, Bald J, Want A, Thouzeau G, Lejart M, Desroy N, Carlier A (2018) A review of potential impacts of submarine power cables on the marine environment: knowledge gaps, recommendations and future directions. Renew Sust Energ Rev 96:380–391. https://doi.org/10.1016/j.rser.2018.07.026
Tomanova K, Vacha M (2016) The magnetic orientation of the Antarctic amphipod Gondonia antartica is cancelled by very weak radiofrequency fields. J Exp Biol 1:1717–1724. https://doi.org/10.1242/jeb.132878
Toupoint N (2012) Le succès de recrutement de la moule bleue: influence de la qualité de la ressource trophique. Université du Québec
Vacha M (2017) Magnetoreception of invertebrates. In: Byrne JH (ed) The Oxford handbook of invertebrate neurobiology. Oxford University Press, Oxford, pp 1–40. https://doi.org/10.1093/oxfordhb/9780190456757.013.16
Walker MM, Diebel CE, Kirschvink JL (2003) Detection and use of the Earth’s magnetic field by aquatic vertebrates. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer, New York, pp 53–74
Wei Q, Xu B, Zargari NR (2017) Overview of offshore wind farm configurations. IOP Conf Ser Earth Environ Sci 93:012009. https://doi.org/10.1088/1755-1315/93/1/012009
Wildish DJ, Miyares MP (1990) Filtration rate of blue mussels as a function of flow velocity: preliminary experiments. J Exp Mar Biol and Ecol 142:213–219. https://doi.org/10.1016/0022-0981(90)90092-Q
Wilson R, Reuter P, Wahl M (2005) Muscling in on mussels: new insights into bivalve behaviour using vertebrate remote-sensing technology. Mar Biol 147:1165–1172. https://doi.org/10.1007/s00227-005-0021-6
Wiltschko R (1995) Magnetic orientation in animals. Springer, Berlin
Worzyk T (2009) Submarine power cables: design, installation, repair, environmental aspects. Springer, Berlin, p 296
Zimmerman S, Zimmerman A, Winters W, Cameron I (1990) Influence of 60 Hz magnetic fields on sea urchin development. Bioelectromagnetics 11:37–45. https://doi.org/10.1002/bem.2250110106
Acknowledgements
We are grateful to the University of Bretagne Occidentale, LEMAR and LIA BeBEST laboratories for their scientific support and contribution to this work. We are grateful to the CIFRE grant from the Association Nationale de la Recherche et de la Technologie and the TBM Environnement firm for co-funding the PhD thesis of L. Albert. In addition, many thanks to Oceanopolis Brest for provisioning the experimental facilities.
Funding
This research was supported by the OASICE project which is a collaboration between the French transmission system operator, Rte, the TBM Environnement firm and the LEMAR laboratory. A PhD fellowship was provided by the ANRT (Association Nationale de la Recherche et de la Technologie).
Author information
Authors and Affiliations
Contributions
LA: conceptualization, methodology, validation, formal analysis, investigation, writing-original draft, writing-review and editing, visualization. OM: conceptualization, methodology, validation, writing-review and editing, supervision, resources. FO: conceptualization, methodology, writing-review and editing, supervision. CL: writing-review and editing, methodology, resources, investigation. AR-R: methodology, formal analysis, validation. AJ: conceptualization, methodology, writing-review and editing, supervision. LC: conceptualization, methodology, project administration, supervision, funding acquisition. SC: conceptualization, methodology, project administration, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
National guidelines for mussel collection have been followed.
Additional information
Responsible Editor: C. Pansch-Hattich.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albert, L., Maire, O., Olivier, F. et al. Can artificial magnetic fields alter the functional role of the blue mussel, Mytilus edulis?. Mar Biol 169, 75 (2022). https://doi.org/10.1007/s00227-022-04065-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04065-4