Abstract
Cleaning behavior is a well-known example of trophic specialization that is widespread in marine organisms from both tropical and temperate ecosystems. Cleaner species can be more or less specialized in different aspects of cleaning interactions, and it is often assumed that the more specialized cleaners are, the more they rely on clients for food. However, cleaners can consume different items in different sites, and the factors influencing such variation are still poorly understood. Here, we investigated the diet and distribution of the barber goby Elacatinus figaro across marginal reefs of the Brazilian coast. We evaluated E. figaro’s reliance on cleaning interactions for food and asked whether its diet and abundance corresponded to the availability of ectoparasites and/or clients. The diet of E. figaro varied across sites, but ectoparasite reliance was similar and did not correspond to client’s infestation loads. Moreover, the density of E. figaro did not correlate with the density or richness of potential clients. These support the hypothesis that E. figaro is less reliant on cleaning interactions for food than other cleaning goby species and suggest a high feeding and behavioral plasticity in marginal reefs. This study also highlights that the current dichotomous classification scheme of dedicated versus facultative cleaners fails to capture the subtle nuances of cleaning behavior and should therefore be used with caution in future comparative studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine animals have evolved a great diversity of trophic specializations and strategies, some involving complex behaviors and communication, like in the fascinating cleaning interactions. During these interactions, smaller organisms, known as cleaners, consume ectoparasites from the body surface of larger organisms, known as clients (Côté 2000; Vaughan et al. 2016). Cleaners are often classified into dedicated (more specialized) or facultative (less specialized) species according to their level of specialization in cleaning interactions (Côté 2000; Vaughan et al. 2016). One important criterion for determining a cleaner species’ specialization is how much they rely on cleaning interactions for food (Côté 2000), but this has only been investigated in a limited number of cleaner species (Grutter 1997, 1999; Arnal 2000; Oates et al. 2012; Narvaez et al. 2015; Morado et al. 2019). Moreover, the reliance of cleaners on cleaning interactions varies within and between species (Grutter 1997, 2000; Whiteman and Côté 2002; White et al. 2007; Dunkley et al. 2020) making it difficult to classify cleaners based on their levels of specialization and to identify their functional relevance throughout their distributions.
One of the main factors influencing how much cleaners rely on cleaning interactions for food is the level of ectoparasite infestation in clients. Clients with high ectoparasite load visit cleaning stations more frequently (Sikkel et al. 2000, 2004; Arnal et al. 2001; Soares et al. 2007), increasing the availability of client-gleaned materials as potential food for cleaners (Grutter 1997; Cheney and Côté 2005). In contrast, when ectoparasite loads are low, the costs of searching for cleaning stations can exceed the benefits of ectoparasite removal (Cheney and Côté 2001), leading to fewer visits from clients (Arnal and Morand 2001; Bansemer et al. 2002) and less client-gleaned materials available for cleaners. In such cases, cleaners must use different strategies to meet their nutritional requirements. For example, in sites with low ectoparasite infestation, some cleaners increase the consumption of mucus and scales (Grutter 1997; Arnal et al. 2001; Bansemer et al. 2002; Cheney and Côté 2005) while others diversify their diet and feeding strategies ingesting food items not derived from cleaning interactions (Whiteman and Côté 2002; White et al. 2007; Dunkley et al. 2020).
Cleaning gobies (family Gobiidae) from the genus Elacatinus are ideal models for investigating how ectoparasite infestation variation affects the feeding strategy of cleaners. These gobies exhibit intra- and interspecific variability in how much they rely on ectoparasites for food (Whiteman and Côté 2002; Cheney and Côté 2005), and some species adopt a noncleaning habit as adults, usually associated with the use of barrel sponges (Whiteman and Côté 2004; White et al. 2007). Although there is evidence that ectoparasite infestation is the main factor determining the feeding strategy of cleaning gobies (Cheney and Côté 2005), only a few studies have directly measured the relationship among ectoparasite ingestion, ectoparasite infestation and the abundance of cleaners and clients in the same location. Moreover, most studies focused on cleaning gobies in the Caribbean, whereas not much is known about cleaning gobies distributed in other regions (but see Francini-Filho and Sazima 2008; Campos and Sá-Oliveira 2011; Quimbayo et al. 2017, 2018; Quimbayo and Zapata 2018). In particular, studies with cleaning gobies inhabiting marginal reefs, where environmental conditions, like temperature, salinity, and nutrient levels fluctuate more than in typical coral reefs (Kleypas et al. 1999; Perry and Larcombe 2003), could clarify how cleaners adjust their feeding behavior to variable environmental conditions.
Here, we aimed to fill this gap by investigating the diet and abundance of the Brazilian endemic barber goby Elacatinus figaro Sazima, Moura and Rosa 1997 in marginal reefs along the Brazilian coast. Some studies have classified this cleaner as dedicated, due to its committed mode of cleaning lifestyle throughout ontogeny (Vaughan et al. 2016), while others have classified it as facultative, based on its ability to occasionally adopt a noncleaning lifestyle (Côté and Soares 2011; Baliga and Mehta 2019; Huie et al. 2020). The noncleaning lifestyle only seems to occur in very particular circumstances (when occupying barrel and tubular sponges in deeper reefs, Rocha et al. 2000) and for the largest part of the shallow distribution of E. figaro, we are still unaware of how much they rely on cleaning for food. Previous studies have shown that the daily number of interactions between E. figaro and its clients varies across different sites on the Brazilian coast (Sazima et al. 2000; Campos and Sá-Oliveira 2011). This indicates that E. figaro may adjust its feeding strategy to the availability of ectoparasites and/or clients at different sites. Therefore, we asked the following: (i) what does E. figaro eat at different sites on the Brazilian coast, (ii) do their diet and abundance correspond to the availability of ectoparasites and/or clients in each reef site, and (iii) how specialized in cleaning behavior is this species? We predict that the reliance of E. figaro on cleaning interactions for food will be closely linked to the availability of ectoparasites or clients along the Brazilian coast and that its cleaning specialization level should be similar to that of other cleaning goby species.
Material and methods
Study area
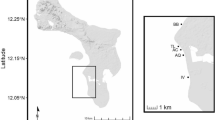
The Brazilian marginal reefs are overall more variable in terms of abiotic and biotic factors (e.g., sedimentation, Castro et al. (2012); visibility, Maida and Ferreira (1997); productivity, Ferreira et al. (1998) and Coelho-Souza et al. (2012); and temperature, Ferreira et al. 2004; Morais et al. 2017) than the typical coral reefs in the Caribbean and the Indo-Pacific (Spalding et al. 2001). We selected three Brazilian marginal reef sites, separated by a minimum of 600 up to 1,700 km from each other, to best represent the diversity of habitats and conditions occupied by Elacatinus figaro along the Brazilian coast (Fig. 1). Maragogi (9° 01′ S; 35° 11′ W) and Salvador’s (12° 48′ S; 38° 34′ W) reefs are of biogenic or sandstone origins (Leão and Dominguez 2000), have a higher relative coral cover (Aued et al. 2018), and warmer waters (Palmeira et al. 2015), being more similar to typical tropical coral reefs. In contrast, Arraial do Cabo’s (22°58′S; 42°00′W) rocky reefs have a lower relative coral cover (Aued et al. 2018) and are seasonally enriched by colder upwelling waters (Ekau and Knoppers 1999), being thus a typical subtropical reef. We sampled three similar reefs at each site and divers simultaneously collected data and specimens for the analyses of E. figaro diet, availability of ectoparasite in clients, and density of E. figaro and its potential clients. We collected the data in March 2012 for Salvador and Maragogi and June 2012 for Arraial do Cabo.
Diet composition
We collected seventy specimens of E. figaro for diet analysis (23 in Maragogi, 25 in Salvador, and 22 in Arraial do Cabo, mean depth = 5 ± 2 m). We collected each fish using hand nets and a clove oil and ethanol solution (1:4) and preserved the specimens in 75% alcohol up to 1 h after collection. We measured the total length (LT) and standard length (LS) of each fish to the nearest millimeter. We dissected fish guts under a binocular microscope and examined the full gut content of each specimen on a Petri dish placed above mesh grids. We sexed the individuals by visually inspecting the gonads. As individual food items had similar size and area, and relative cover can underestimate food items’ abundance (Baker et al. 2014), we counted each identifiable item found in the stomachs to calculate the percentage of contribution of food items to diet. Food items were identified to the lowest taxonomic level possible and classified into the following categories: gnathiids (parasitic isopods larvae), caligids (parasitic copepods), cyprid larvae (free-living cyprid larvae), copepods (mainly free-living harpacticoid), scales, gastropods, diatoms, sand, and nonidentified (nonidentified crustaceans).
Ectoparasite availability
Three species of reef fish were selected for ectoparasite load assessment: Sparisoma axillare (Steindachner 1878) (family Labridae), Acanthurus bahianus Castelnau 1855 (family Acanthuridae), and Stegastes fuscus (Cuvier in Cuvier and Valenciennes 1830) (family Pomacentridae). We chose these species because they are abundant and widely distributed on the Brazilian coast (Floeter et al. 2001; Ferreira et al. 2004) and frequent clients of E. figaro (R. Mazzei, unpubl. data; Sazima et al. 2000). In each site, we collected 10 to 20 individuals (Online resources, Tab.1s) with spear guns and immediately placed them in hermetically sealed plastic bags. We then soaked each specimen for 10 min in containers filled with tap water, and gently brushed their entire body surface, gills, and fins (Sikkel et al. 2004; Soares et al. 2007). We filtered all fluids with a plankton net (50 µm mesh size) and preserved the ectoparasites in 70% alcohol (Sikkel et al. 2004; Soares et al. 2007). We identified the ectoparasites under a binocular microscope to the lowest taxonomic level. Identified parasites were separated into the following taxonomic categories: Subclass Copepoda (families Caligidae and Bomolochidae: genus Orbitacolax), Class Monogenea (genus Neobenedenia), Phylum Mollusca (Class Gastropoda), Order Isopoda (family Gnathiidae and other nonidentified Isopods), Subclass Digenea, Order Amphipoda, and Phylum Acanthocephala.
Elacatinus figaro and client densities
We assessed the distributional patterns of E. figaro and its potential clients by conducting replicated visual census (26 in Maragogi, 55 in Salvador, and 32 in Arraial do Cabo) using 20 × 2 m strip transects while scuba diving (cf. Floeter et al. 2007). All other reef fishes were identified to the species level and counted along the same transects. We considered all species counted in the transects as potential clients, as there is no evidence that E. figaro does not interact with these species. In addition, because visual census methods usually underestimate the abundance of cryptobenthic species (Willis 2001), and E. figaro is often found sheltered in small crevices (personal observation), another diver conducted a separate set of transects, in the same reefs, counting exclusively E. figaro (N = 20 in Maragogi, 20 in Salvador, and 39 in Arraial do Cabo). In these transects, we also counted the number of cleaning stations per transect and the number of cleaners per cleaning station.
Data analysis
Diet composition and reliance on ectoparasites
We tested for differences in the reliance of E. figaro on ectoparasites (gnathiid isopods + caligid copepods) across sites by using a binomial Generalized Linear Model (GLM) performed with the glm function from the package stats (R Core Team 2020). The model included the proportion of ectoparasites in E. figaro’s diet as the dependent variable, and site of capture (Maragogi, Salvador or Arraial do Cabo), sex, size (total body length) and their interactions as independent factors. The significance of the main factors and all interactions were obtained with the function Anova from package car (Fox and Weisberg 2019) and post hoc comparisons were performed with the function lsmeans from the package lsmeans (Lenth 2016). Visual inspection of residuals plotted against fitted values (plot function) indicated little evidence of departure from binomial model assumptions. We also tested whether the whole diet composition varied among sites by using a Permutational Multivariate Analysis of Variance (PERMANOVA). Dissimilarity matrices were calculated using Bray–Curtis distances and permutational tests were performed using the adonis function from the package vegan (Oksanen et al. 2013). To better visualize the diet similarity across sites, we used the function metaMDS, from the package vegan (Oksanen et al. 2013) set with the Bray–Curtis distance method. The polygons overlaid on the nMDS plots were based on convex hulls calculated using the chull function from package grDevices (R Core Team 2020).
Ectoparasite availability
To test for differences in the general availability of ectoparasites across sites and client species, we calculated the prevalence (percentage of hosts infected by any ectoparasite in the sampled population), the intensity of infestation (total number of parasites per infected host), and density of ectoparasites (total number of parasites per gram of host) for each site and client species (Bush et al. 1997). Parasite prevalence was compared across sites and species using the G test for heterogeneity from the package RVAideMemoire (Hervé 2017). We corrected P values for multiple comparisons using Bonferroni correction. The weight of client species was compared among sites using an Analysis of Variance from the package stats (R Core Team 2020). Because the weight of captured clients significantly differed across sites and species (Online resources, Tab.1s) we tested for differences in the density of ectoparasites (number of parasites per gram of host) using a linear model performed with the function lm from package stats (R Core Team 2020). For this model, we used a logarithmic transformation on the dependent variable to meet the model assumptions. We used weight instead of size for calculating the density of ectoparasites because the former better represents the fish body volume and health condition (Froese 2006) and therefore is more likely to be a limiting factor for parasite settlement than the latter. We assessed the validity of the model by visually inspecting the residual's homogeneity and normality. We estimated the significance of independent variables by using the function Anova from package car (Fox and Weisberg 2019) and performed post hoc comparisons using the function lsmeans from the package lsmeans (Lenth 2016). Finally, we used the adonis and metaMDS functions, as above, to test and visualize potential differences in ectoparasites categories across sites and species.
Elacatinus figaro and client densities
To test whether the density of E. figaro was positively correlated with the density of potential clients, we used a negative binomial GLM, performed with the function glm, using the same procedure as above. We included the density of E. figaro (number of individuals per 40 m2) as dependent variable and site, the density of potential clients (number of all other reef fish individuals per 40 m2), the richness of potential clients (number of all other reef fish species per 40 m2) and their interactions as independent factors. Finally, we used the data from the surveys performed exclusively to count E. figaro and cleaning stations (see above method section) for testing whether the density of E. figaro, density of cleaning stations (cleaning stations per m2), and the number of individuals per cleaning station differed across sites. For these analyses, we used a negative binomial, Poisson, and mixed-effects Poisson GLMs, respectively, using the same procedure as above. In the mixed effect model, transects were included as a random factor. All analyses were performed with the R software (R Core Team, 2020).
Results
We found that the reliance of Elacatinus figaro on ectoparasites (contribution of Gnathiids + Caligids) significantly differed among sites (Fig. 2, LR Chisq = 7.17, p = 0.02), but was not significantly influenced by the sex (LR Chisq = 0.02, p = 0.86) or size (LR Chisq = 1.59, p = 0.20) of individuals. Furthermore, we found no significant interactions across factors (Online Resources: Tab. 2 s). However, differences among sites were diluted in posthoc pairwise comparisons, probably because the effect of site, which indicated a higher consumption of ectoparasites in Maragogi, was not strong enough (Fig. 2, and Online Resources: Fig. 1s). In contrast, the multivariate analysis of the frequency of food items showed that the diet composition of E. figaro significantly differed across sites (Fig. 3, R2 = 0.17, pseudo-F = 7.06, p value = 0.001).
Comparative contribution of ectoparasites (gnathiids and caligids), scales, and other identifiable items (cyprid larvae, copepods, gastropods, diatoms, sand, and nonidentifiable crustaceans) in the diet of Elacatinus figaro across the study sites. The central line in boxes, the boxes, and the whiskers indicate the median, Q1, Q3, and 1.5*QR. Dots represent individual data points
Although feeding reliance of E. figaro on ectoparasite was similar among sites, the ectoparasite availability significantly varied among sites and client species. The prevalence and intensity of ectoparasites were generally higher in Arraial do Cabo, although differences to other sites depended on the client species (Table 1). The density of ectoparasites showed a similar pattern with a significant interaction between site and client species (F = 3.49, p value = 0.01), generally driven by a higher density of ectoparasites in Arraial do Cabo and Salvador for S. axillare (Fig. 4; and see Online Resources: Tab. 3s and Fig. 2s for complete statistics). The multivariate analyses of the general composition of parasitic taxa also revealed significant differences among client species (Fig. 5, pseudo-F 2.89 = 10.4, p < 0.01), sites (Fig. 5, pseudo-F 2.89 = 11.2, p < 0.01), and a significant interaction between these factors (Fig. 5, pseudo-F4.89 = 3.2, p < 0.01).
Finally, the density of E. figaro varied significantly among sites, but it was neither linked to the availability of ectoparasites, nor the density of potential clients. Using the data from the whole-community fish census, we found that the density of E. figaro was significantly different among sites (Fig. 6a, LR Chisq = 19.81, p value < 0.0001), but not significantly correlated to the density (LR Chisq = 1.71, p value = 0.18) or richness of potential clients (LR Chisq = 1.50, p value = 0.21). We also found a significant correlation between site and client density (LR Chisq = 19.72, p value < 0.0001) and site and client richness (LR Chisq = 13.80, p value = 0.001). In contrast, when using the data from E. figaro exclusive surveys, we found that neither the abundance of E. figaro (Fig. 6b, LR Chisq = 2.46, p value = 0.29) nor the abundance of cleaning stations (Fig. 6c, LR Chisq = 0.26, p value = 0.87) significantly varied among sites. However, we found significant differences among sites in the number of individuals per cleaning station, (Fig. 6d, Chisq = 10.80, p value = 0.004).
Discussion
We investigated the diet and abundance of the cleaning goby Elacatinus figaro on three marginal reefs along the Brazilian coast. We found that the diet of E. figaro varied among sites, but in contrast to our expectations, gobies from sites with higher ectoparasite infestation did not ingest a higher proportion of ectoparasites, and the abundance of gobies was not correlated to the abundance or richness of potential clients. Although not expected, this lack of correspondence was also not surprising, as the link between cleaners, clients, and ectoparasites has never been straightforward. Some studies have found that when and where clients and/or ectoparasites were more available, cleaners were more abundant (Arnal et al. 1999, 2002; Cheney and Côté 2003), engaged in more cleaning interactions (Sikkel et al. 2004; Soares et al. 2007), and consumed more ectoparasites (Grutter 1997; Cheney and Côté 2005). Conversely, others have found contrasting results (Arnal et al. 1999; Cheney and Côté 2003; Cote and Molloy 2003) or failed to find any correlation (Arnal et al. 2000, 2001, 2002).
One possible explanation for the lack of correlation between cleaners, ectoparasites, and clients found in our study is that ectoparasite infestation may not be overall high enough to drive clients to visit cleaning stations more often. In the absence of frequent client visits, cleaners may be prevented from accessing their ectoparasites. In fact, the average ectoparasite density that we found here was similar to what was found for closely related species in Barbados (Soares et al. 2007) reefs, which show overall lower ectoparasite loads than other Caribbean reefs (Cheney and Côté 2005). Likewise, both the cleaning rates (9.1 interactions/h, Sazima et al. 2000) and the contribution of ectoparasites to the diet of E. figaro (around 50% of identifiable items, this study) in Brazilian marginal reefs were similar to what was found for the Caribbean Elacatinus evelynae in sites with lower infestation rates (e.g., Tobago: 10.1 interactions/h, (Dunkley et al. 2019b); around 36% of identifiable items, Cheney and Côté 2005). This pattern of lower reliance on ectoparasites was also found for less specialized cleaner species from the Northeastern Atlantic marginal reefs, like Coris julis, Thalassoma pavo, and Centrolabrus exoletus (Narvaez et al. 2015; Morado et al. 2019). Therefore, lower infestation levels might explain not only why we were unable to find differences in ectoparasite consumption among sites, but also why ectoparasite contribution to E. figaro’s diet was overall only around 50%, while for cleaning gobies at Caribbean sites with higher ectoparasite infestation it can reach up to 90% (percentage of identifiable items, Cheney and Côté 2005). As ectoparasite infestation, cleaning activity, and reliance on ectoparasites in marginal reefs seem to be relatively low, it is not surprising that we did not find a strong correlation between the abundance of cleaners and cleaning stations and the abundance or richness of clients. However, it should be noted that we have only sampled ectoparasites from three out of a multitude of client species that E. figaro interacts with, and including different client species could had led to different correlation patterns. Furthermore, although E. figaro is the only cleaning goby occurring in the Brazilian coast, a few facultative species, e.g., Pomacanthus paru and Bodianus spp. co-occur with E. figaro in the studied sites. Although these cleaners only clean as juveniles and were seldom registered in our surveys (P. paru occurred in 4% of the transects from Arraial do Cabo while Bodianus rufus occurred in 7% of the transects from Salvador) they show very specialized cleaning behaviors (Johnson and Ruben 1988; Sazima et al. 1999) and could potentially be competing for clients and influencing the relationship between cleaners, clients and ectoparasites abundance. Thus, we provide important insights on the patterns of cleaning interactions along the Brazilian coast, but direct observations and field experiments are needed to better elucidate the causes and consequences of the lack of correlation between cleaners, clients and ectoparasites found in our study.
Both client and nonclient materials consumed by E. figaro varied among sites, indicating its ability to change diet according to the local availability of food items. The ingestion of scales by E. figaro was relatively low, while the consumption of nonclient materials relatively high, sometimes representing the majority of items found in an individual stomach. These findings provide support for the hypothesis that mouth position and skull morphology in cleaning gobies vary according to their cleaning specialization (Huie et al. 2020). More specialized cleaning gobies (e.g., E. evelynae) have inferiorly-positioned mouths and shorter jaws, supposedly to favor the removal of scales and parasites when lying on the surface of clients body, while less specialized cleaning gobies (e.g., E. figaro and E. prochilos) maintain the ancestral traits of terminal mouths and longer jaws, which are less efficient during cleaning interactions (Huie et al. 2020). Interestingly, within each site, some individuals had 100% ectoparasites in their stomachs while others had 100% nonclient material. Although this would indicate a split into cleaning and noncleaning habits, this was not the case here, since noncleaning gobies are usually associated with tubular or barrel sponges, and we never registered E. figaro using these substrates in this study (and barrel sponges are more common in deeper reefs of the Brazilian coast). Instead, within-site variation in ectoparasite consumption is more likely to reflect (i) small-scale spatial and temporal variations in cleaning activity (Sazima et al. 2000) and ectoparasite availability (Cote and Molloy 2003), and/or (ii) consistent inter-individual variation in activity, boldness, and exploratory behaviors, as reported for other cleaning gobies (Dunkley et al. 2019a).
The lower reliance of E. figaro on cleaning interactions for food might be strongly linked to its successful colonization of the marginal reefs of the Brazilian coast. The variable environmental conditions of these reefs may have favored the higher behavioral plasticity and the adoption of less specialized feeding strategies of E. figaro. Moreover, E. figaro seems to use a higher diversity of substrates than Caribbean conspecifics (pers. observation), which indicates that this species is also flexible in substrate use, potentially increasing its range of available food items. This plasticity is found in other reef species with wide geographic distribution, like the butterflyfish Chaetodon striatus (Liedke et al. 2016), and might explain the success of other species, including less specialized cleaners in marginal and extreme reef environments (Fulton et al. 2017; Quimbayo et al. 2018). It is still unclear how cleaning compares to other feeding strategies in terms of fitness gains, but there is some evidence that cleaning can be less profitable (slower growth, White et al. 2007), more risky (lower survival, White et al. 2007) and increase exposure to pathogens (Xavier et al. 2019). Therefore, it is possible that cleaning has evolved only in very competitive environments and, when released from the competition (like in marginal reefs), cleaners can return to a more generalist diet.
Our results reinforce that the classification of E. figaro as either dedicated or facultative is a complicated matter. Although all E. figaro registered in our study adopted a cleaning life style, they only partially relied on cleaning interactions for food. According to the ontogenetic and commitment criteria (Vaughan et al. 2016), E. figaro would be classified as a dedicated cleaner, as it cleans throughout their nonlarval ontogeny and its noncleaning life style seems to be restricted to deeper reefs (Rocha et al. 2000). However, the lower reliance on cleaning for food, as well as the less specialized mouth morphology (terminal mouth, Huie et al. 2020), body coloration (black and yellow; see other color specializations in Baliga and Mehta 2019; Huie et al. 2020) and cleaning behavior (no tactile dances or stimulation, Soares et al. 2008), also support its classification as facultative. These inconsistencies apply to other cleaner species. The two most specialized cleaner species (considering color, mouth morphology, and behavior; Baliga and Law 2016; Huie et al. 2020), Elacatinus evelynae and Labroides dimidiatus, mostly regarded as dedicated, could also be classified as facultative species according to the commitment criteria, as they can adopt a noncleaning strategy both when adults and juveniles (sponge dwelling in E. evelynae, White et al. 2007; coral pecking in L. dimidiatus, Dunkley et al. 2020). Likewise, some species classified as facultative have “dedicated-like” specialized colors and behaviors during their juvenile cleaning phase (e.g., P. paru, Sazima et al. 1999). Therefore, it becomes clear that the dichotomous classification of dedicated and facultative cleaners fails to capture the different and subtle aspects of cleaning specialization in E. figaro and other species. Future studies should be more specific in respect to the criteria and specialization aspects chosen to classify cleaner species in comparative studies.
In conclusion, we found that E. figaro has a more flexible diet and is less reliant on client materials for food than the Caribbean cleaning gobies (reviewed in Côté and Soares 2011). This higher flexibility may be related to the low ectoparasite infestation loads in the Brazilian reefs (our study), the lack of advanced morphological specializations for eating from clients in E. figaro (Huie et al. 2020), and/or potential advantages of keeping less specialized feeding morphology in variable marginal reefs. Together, the lower reliance on cleaning for food (our study), the ability to switch to a noncleaning habit (Rocha et al. 2000), the more generalist mouth morphology (Huie et al. 2020) and the absence of very specialized cleaning signaling or behaviors (Sazima et al. 2000) indicate that E. figaro has an intermediate level of cleaning specialization, despite being a cleaner throughout its nonlarval ontogeny. This highlights the importance of investigating the ecology of cleaner species at multiple temporal and spatial scales to better understand the different processes and aspects influencing the evolution of cleaning behavior specialization.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Code availability
The R scripts used to analyze data and produce figures are available from the corresponding author on request.
References
Arnal C (2000) Diet of broadstripe cleaning gobies on a Barbadian reef. J Fish Biol 57:1075–1082. https://doi.org/10.1006/jfbi.2000.1371
Arnal C, Morand S (2001) Importance of ectoparasites and mucus in cleaning interactions in the Mediterranean cleaner wrasse Symphodus melanocercus. Mar Biol 138:777–784. https://doi.org/10.1007/s002270000494
Arnal C, Morand S, Kulbicki M (1999) Patterns of cleaner wrasse density among three regions of the Pacific. Mar Ecol Prog Ser 177:213–220. https://doi.org/10.3354/meps177213
Arnal C, Côté IM, Sasal P, Morand S (2000) Cleaner–client interactions on a Caribbean reef: influence of correlates of parasitism. Behav Ecol Sociobiol 47:353–358. https://doi.org/10.1007/s002650050676
Arnal C, Côté IM, Morand S (2001) Why clean and be cleaned? The importance of client ectoparasites and mucus in a marine cleaning symbiosis. Behav Ecol Sociobiol 51:1–7. https://doi.org/10.1007/s002650100407
Arnal C, Kulbicki M, Harmelin-Vivien M, Galzin R, Morand S (2002) Patterns of local distribution of Labroides dimidiatus in French Polynesian atolls. Environ Biol Fish 63:9–15. https://doi.org/10.1023/A:1013811205742
Aued AW, Smith F, Quimbayo JP, Cândido DV, Longo GO, Ferreira CEL, Witman JD, Floeter SR, Segal B (2018) Large-scale patterns of benthic marine communities in the Brazilian Province. PLoS ONE 13:e0198452. https://doi.org/10.1371/journal.pone.0198452
Baker R, Buckland A, Sheaves M (2014) Fish gut content analysis: robust measures of diet composition. Fish Fish 15:170–177. https://doi.org/10.1111/faf.12026
Baliga VB, Law CJ (2016) Cleaners among wrasses: phylogenetics and evolutionary patterns of cleaning behavior within Labridae. Mol Phylogenet Evol 94:424–435
Baliga VB, Mehta RS (2019) Morphology, ecology, and biogeography of independent origins of cleaning behavior around the world. Integr Comp Biol 59:625–637. https://doi.org/10.1093/icb/icz030
Bansemer C, Grutter AS, Poulin R (2002) Geographic variation in the behaviour of the cleaner fish Labroides dimidiatus (Labridae). Ethology 108:353–366. https://doi.org/10.1046/j.1439-0310.2002.00777.x
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575. https://doi.org/10.2307/3284227
Campos CEC, Sá-Oliveira JC (2011) Atividade de limpeza e clientes de Elacatinus figaro (Pisces: Gobiidae) nos recifes de coral dos Parrachos de Muriú, Nordeste do Brasil. Biota Neotrop 11:47–51. https://doi.org/10.1590/S1676-06032011000100004
Castelnau FL (1855) Poissons. Animaux nouveaux or rares recueillis pendant l’expédition dans les parties centrales de l’Amérique du Sud, de Rio de Janeiro a Lima, et de Lima au Para; exécutée par ordre du gouvernement Français pendant les années 1843 a 1847. Bertrand, Paris, pp xii–112
Castro CB, Segal B, Negrão F, Calderon EN (2012) Four-year monthly sediment deposition on turbid southwestern Atlantic coral reefs, with a comparison of benthic assemblages. Braz. J. Oceanogr. 60(1):49–63. https://doi.org/10.1590/S1679-87592012000100006
Cheney KL, Côté IM (2001) Are Caribbean cleaning symbioses mutualistic? Costs and benefits of visiting cleaning stations to longfin damselfish. Anim Behav 62:927–933. https://doi.org/10.1006/anbe.2001.1832
Cheney K, Côté I (2003) Do ectoparasites determine cleaner fish abundance? Evidence on two spatial scales. Mar Ecol Prog Ser 263:189–196. https://doi.org/10.3354/meps263189
Cheney KL, Côté IM (2005) Mutualism or parasitism? The variable outcome of cleaning symbioses. Biol Lett 1:162–165. https://doi.org/10.1098/rsbl.2004.0288
Coelho-Souza SA, López MS, Guimarães JRD, Coutinho R, Candella RN (2012) Biophysical interactions in the Cabo Frio upwelling system, Southeastern Brazil. Braz J Oceanogr 60:353–365. https://doi.org/10.1590/s1679-87592012000300008
Côté IM (2000) Evolution and ecology of cleaning symbioses in the sea. Oceanogr Mar Biol 38:311–355
Côté I, Soares M (2011) Gobies as cleaners. In: Kapoor B (ed) The biology of gobies. Science Publishers, pp 532–557
Cote IM, Molloy PP (2003) Temporal variation in cleanerfish and client behaviour: does it reflect ectoparasite availability? Ethology 109:487–499. https://doi.org/10.1046/j.1439-0310.2003.00883.x
Cuvier G, Valenciennes A (1830) Histoire naturelle des poissons. Tome cinquième. Chez F.G, Levrault, Paris
Dunkley K, Ioannou CC, Whittey KE, Cable J, Perkins SE (2019a) Cleaner personality and client identity have joint consequences on cleaning interaction dynamics. Behav Ecol 30:703–712. https://doi.org/10.1093/beheco/arz007
Dunkley K, Ellison AR, Mohammed RS, van Oosterhout C, Whittey KE, Perkins SE, Cable J (2019b) Long-term cleaning patterns of the sharknose goby (Elacatinus evelynae). Coral Reefs 38:321–330. https://doi.org/10.1007/s00338-019-01778-9
Dunkley K, Ward AJW, Perkins SE, Cable J (2020) To clean or not to clean: cleaning mutualism breakdown in a tidal environment. Ecol Evol 10:3043–3054. https://doi.org/10.1002/ece3.6120
Ekau W, Knoppers B (1999) An introduction to the pelagic system of the Northeast and East Brazilian shelf. Arch Fish Mar Res 47:113–132
Ferreira CEL, Floeter SR, Gasparini JL, Ferreira BP, Joyeux JC (2004) Trophic structure patterns of Brazilian reef fishes: a latitudinal comparison. J Biogeogr 31:1093–1106. https://doi.org/10.1111/j.1365-2699.2004.01044.x
Floeter SR, Guimarães RZP, Rocha LA, Ferreira CEL, Rangel CA, Gasparine JL (2001) Geographic variation in reef-fish assemblages along the Brazilian coast. Glob Ecol Biogeogr 10:423–431
Floeter SR, Krohling W, Gasparini JL, Ferreira CEL, Zalmon IR (2007) Reef fish community structure on coastal islands of the southeastern Brazil: the influence of exposure and benthic cover. Environ Biol Fish 78:147–160. https://doi.org/10.1007/s10641-006-9084-6
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA
Francini-Filho RB, Sazima I (2008) A comparative study of cleaning activity of two reef fishes at Fernando de Noronha Archipelago, tropical West Atlantic. Environ Biol Fish 83:213–220. https://doi.org/10.1007/s10641-007-9322-6
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Fulton CJ, Wainwright PC, Hoey AS, Bellwood DR (2017) Global ecological success of Thalassoma fishes in extreme coral reef habitats. Ecol Evol 7:466–472. https://doi.org/10.1002/ece3.2624
Grutter AS (1997) Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia 1997:346. https://doi.org/10.2307/1447754
Grutter AS (1999) Fish cleaning behaviour in Noumea. New Caledonia Mar Freshw Res 50:209. https://doi.org/10.1071/MF97078
Grutter AS (2000) Ontogenetic variation in the diet of the cleaner fish Labroides dimidiatus and its ecological consequences. Mar Ecol Prog Ser 197:241–246
Hervé M (2017) RVAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9–68. https://cran.r-project.org/package=RVAideMemoire
Huie JM, Thacker CE, Tornabene L (2020) Co-evolution of cleaning and feeding morphology in western Atlantic and eastern Pacific gobies. Evolution 74:419–433. https://doi.org/10.1111/evo.13904
Johnson WS, Ruben P (1988) Cleaning behavior of Bodianus rufus, Thalassoma bifasciatum, Gobiosoma evelynae, and Periclimenes pedersoni along a depth gradient at salt river submarine Canyon. St Croix Environ Biol Fish 23:225–232. https://doi.org/10.1007/BF00004913
Kleypas JA, Mcmanus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159. https://doi.org/10.1093/icb/39.1.146
Leão ZMAN, Dominguez JML (2000) Tropical coast of Brazil. Mar Pollut Bull 41:112–122. https://doi.org/10.1016/S0025-326X(00)00105-3
Lenth RV (2016) Least-squares means: the r package lsmeans. J Stat Softw. https://doi.org/10.18637/jss.v069.i01
Liedke AMR, Barneche DR, Ferreira CEL, Segal B, Nunes LT, Burigo AP, Carvalho JA, Buck S, Bonaldo RM, Floeter SR (2016) Abundance, diet, foraging and nutritional condition of the banded butterflyfish (Chaetodon striatus) along the western Atlantic. Mar Biol 163:1–13. https://doi.org/10.1007/s00227-015-2788-4
Maida M, Ferreira BP (1997) Coral reefs of Brazil overview and field guide. In: Proceedings 8th International Coral Reef Symposium 1:263–274
Morado N, Mota PG, Soares MC (2019) The rock cook wrasse Centrolabrus exoletus aims to clean. Front Ecol Evol. https://doi.org/10.3389/fevo.2019.00182
Morais RA, Ferreira CEL, Floeter SR (2017) Spatial patterns of fish standing biomass across Brazilian reefs. J Fish Biol 91(6):1642–1667. https://doi.org/10.1111/jfb.13482
Narvaez P, Furtado M, Neto A, Moniz I, Azevedo J, Soares M (2015) Temperate facultative cleaner wrasses selectively remove ectoparasites from their client-fish in the Azores. Mar Ecol Prog Ser 540:217–226. https://doi.org/10.3354/meps11522
Oates J, Manica A, Bshary R, Grutter AS (2012) Relationship between roving behaviour and the diet and client composition of the cleaner fish Labroides bicolor. J Fish Biol 81:210–219
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H, Oksanen MJ (2013) Package ‘vegan’. Community ecology package, version, 2(9). https://cran.r-project.org/web/packages/vegan/index.html
de Palmeira ACPA, de Camargo R, de Palmeira RMJ, de Palmeira ACPA, de Camargo R, de Palmeira RMJ (2015) Relação entre a temperatura da superfície do mar e a camada de mistura oceânica sob a passagem de ciclones extratropicais no Atlântico Sudoeste. Rev Bras Meteorol 30:89–100. https://doi.org/10.1590/0102-778620130679
Perry CT, Larcombe P (2003) Marginal and non-reef-building coral environments. Coral Reefs 22:427–432. https://doi.org/10.1007/s00338-003-0330-5
Quimbayo JP, Zapata FA (2018) Cleaning interactions by gobies on a tropical eastern Pacific coral reef. J Fish Biol 92:1110–1125. https://doi.org/10.1111/jfb.13573
Quimbayo JP, Nunes LT, Ozekoski R, Floeter SR, Morais RA, Fontoura L, Bonaldo RM, Ferreira CEL, Sazima I (2017) Cleaning interactions at the only atoll in the South Atlantic. Environ Biol Fish 100:865–875. https://doi.org/10.1007/s10641-017-0612-3
Quimbayo JP, Schlickmann ORC, Floeter SR, Sazima I (2018) Cleaning interactions at the southern limit of tropical reef fishes in the Western Atlantic. Environ Biol Fish 101:1195–1204. https://doi.org/10.1007/s10641-018-0768-5
R Core Team (2020) R: a language and environment for statistical computing. https://www.R-project.org/.
Rocha LA, Rosa IL, Feitoza B (2000) Sponge-dwelling fishes of Northeastern Brazil. Environ Biol Fish 59:453–458. https://doi.org/10.1023/A:1026584708092
Sazima I, Moura RL, Rosa RS (1997) Elacatinus figaro sp.n (Perciformes: Gobiidae), a new cleaner goby from the coast of Brazil. Aqua J Ichthyol Aquat Biol 2:33–38
Sazima I, Moura RL, Sazima C (1999) Cleaning activity of juvenile angelfish, Pomacanthus paru, on the reefs of the Abrolhos Archipelago, Western South Atlantic. Environ Biol Fish 56:399–407. https://doi.org/10.1023/A:1007531925845
Sazima I, Sazima C, Francini-Filho RB, Moura RL (2000) Daily cleaning activity and diversity of clients of the barber goby, Elacatinus figaro, on rocky reefs in southeastern Brazil. Environ Biol Fish 59:69–77
Sikkel P, Fuller C, Hunte W (2000) Habitat/sex differences in time at cleaning stations and ectoparasite loads in a Caribbean reef fish. Mar Ecol Prog Ser 193:191–199. https://doi.org/10.3354/meps193191
Sikkel PC, Cheney KL, Côté IM (2004) In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim Behav 68:241–247. https://doi.org/10.1016/j.anbehav.2003.10.023
Soares MC, Cardoso SC, Côté IM (2007) Client preferences by Caribbean cleaning gobies: food, safety or something else? Behav Ecol Sociobiol 61:1015–1022. https://doi.org/10.1007/s00265-006-0334-6
Soares MC, Côté IM, Cardoso SC, Bshary R (2008) The cleaning goby mutualism: a system without punishment, partner switching or tactile stimulation. J Zool 276:306–312. https://doi.org/10.1111/j.1469-7998.2008.00489.x
Spalding M, Spalding MD, Ravilious C, Green EP (2001) World atlas of coral reefs. Univ of California Press
Steindachner F (1878) Ichthyologische beiträge. VI Sitzungsber Akad Wiss Wien 77:379–392
Vaughan DB, Grutter AS, Costello MJ, Hutson KS (2016) Cleaner fishes and shrimp diversity and a re-evaluation of cleaning symbioses. Fish Fish 18:698–716. https://doi.org/10.1111/faf.12198
White JW, Grigsby CJ, Warner RR (2007) Cleaning behavior is riskier and less profitable than an alternative strategy for a facultative cleaner fish. Coral Reefs 26:87–94. https://doi.org/10.1007/s00338-006-0161-2
Whiteman EA, Côté IM (2002) Cleaning activity of two Caribbean cleaning gobies: intra- and interspecific comparisons. J Fish Biol 60:1443–1458. https://doi.org/10.1006/jfbi.2002.1947
Whiteman EA, Côté IM (2004) Dominance hierarchies in group-living cleaning gobies: causes and foraging consequences. Anim Behav 67:239–247. https://doi.org/10.1016/j.anbehav.2003.04.006
Willis TJ (2001) Visual census methods underestimate density and diversity of cryptic reef fishes. J Fish Biol 59:1408–1411. https://doi.org/10.1111/j.1095-8649.2001.tb00202.x
Xavier R, Mazzei R, Pérez-Losada M, Rosado D, Santos JL, Veríssimo A, Soares MC (2019) A risky business? Habitat and social behavior impact skin and gut microbiomes in Caribbean cleaning gobies. Front Microbiol 10:716. https://doi.org/10.3389/fmicb.2019.00716
Acknowledgments
We would like to thank Guilherme O. Longo and the SISBIOTA network for the assistance in the field, Virginia Paola for the ectoparasites identification, and two anonymous reviewers for their helpful comments and suggestions. RM was supported by a Master’s scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). TCM was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) with a Nota 10 fellowship (# E-26/202.858/2016). JLL was supported by a research fellowship from CNPq. CELF is supported by CNPq and FAPERJ grants. CAMMC was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). M.C.S. is currently supported by National Funds through Fundação para a Ciência e a Tecnologia (DL57/2016/CP1440/CT0019).
Funding
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) through research funds granted to CELF.
Author information
Authors and Affiliations
Contributions
RM, MCS, and CELF designed the study. RM, TCM, and CAMMC collected and analyzed the data. JLL identified the ectoparasites. RM wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethics approval
Research methods and capture of specimens at protected sites within the study areas were under authorization of the Brazilian environmental agency ICMBIO/SISBIO (Permit numbers: 33688-1 and 32652-1).
Additional information
Responsible Editor: D. Goulet.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewers: V. Baliga and an undisclosed expert.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazzei, R., Mendes, T.C., Cordeiro, C.A.M.M. et al. Diet and abundance of the barber goby Elacatinus figaro on Brazilian marginal reefs: ecological predictors and reliance on cleaning interactions. Mar Biol 168, 64 (2021). https://doi.org/10.1007/s00227-021-03856-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03856-5