Abstract
Finding food is crucial to the survival and reproductive success of individuals. Fidelity to previous profitable foraging sites may bring benefits to individuals as they can allocate more time to foraging rather than searching for prey. We studied how environmental conditions influence when lactating long-nosed fur seals (Arctocephalus forsteri) adopt a risky (low fidelity) or conservative (high fidelity) foraging strategy at two intra-annual temporal scales when foraging in a highly variable oceanic environment. Core foraging areas (CFAs; n = 534; 30 × 30 km cells) of consecutive foraging trips were obtained from geolocation tracks of 12 females from summer to winter in 2016 (n = 5) and 2017 (n = 7). We used the spatial variability (standard deviation) of CFAs between or among oceanic foraging trips as a proxy for individual foraging site fidelity (IFSF). Over the entire oceanic foraging period (n = 12), IFSF in the latitudinal axis increased with stronger sea-surface temperature gradient (SSTgrad), but decreased with greater SSTgrad and sea-surface height gradient variability. Over a period of two consecutive oceanic foraging trips (n = 66), IFSF decreased with greater SSTgrad variability in the earlier foraging trip. LNFS show evidence that they use IFSF as a strategy to potentially optimise food acquisition, and that this behaviour is influenced by mesoscale oceanographic parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The marine environment is highly dynamic with physical parameters determining the spatial and temporal distribution of primary productivity, thereby resulting in patchily distributed food resources. Marine predators, therefore, face the challenge of locating the prey which their survival and reproductive success depend on in this heterogeneous environment (Oosthuizen et al. 2015). From an optimal foraging perspective, there may be long-term breeding and survival benefits (Bradshaw et al. 2004) for animals which use prior knowledge about where food is (i.e., predictable) and return to the same foraging area, rather than randomly searching for food (Call et al. 2008). Indeed, many marine species, such as sea birds (Weimerskirch 2007), sharks (Espinoza et al. 2011), whales (Yates et al. 2007), turtles (Tucker et al. 2014), and seals (Oksanen et al. 2014; Arthur et al. 2015; Abrahms et al. 2018a), display individual foraging site fidelity. However, repeated use of the same foraging patch may lead to prey depletion and/or the prey distribution and the density may have changed over time, thereby resulting in site fidelity being a sub-optimal foraging strategy (Pichegru et al. 2010; McIntyre et al. 2017; McHuron et al. 2018). Thus, this illustrates a trade-off between a conservative strategy of sticking to what one already knows and another riskier strategy of switching and searching for new and potentially more profitable foraging patches.
Income-breeding marine predators provisioning offspring (Houston et al. 2007), such as fur seals (Staniland and Boyd 2003), sea lions (Womble et al. 2009), and seabirds (Croll et al. 2006; Rayner et al. 2010), can be considered as central-place foragers, because they alternate between foraging at sea and returning to the colony to feed their nutritionally dependent offspring. When offspring are young, they have a limited ability to fast and restrict the foraging trip duration of parents. As offspring get older and their nutritional demand increases, parents may have to increase their foraging effort by making longer trips to more distant oceanic foraging areas, which may be more energetically profitable than remaining in the coastal foraging areas (Boyd 1999; Kowalczyk et al. 2015). Individual foraging site fidelity may be an important foraging strategy, particularly during the later stages of offspring-provisioning, where energetic demands for self-maintenance and parental investment increase. This crucial period may be important in determining pup-weaning mass which is a significant factor influencing the first year (Georges and Guinet 2000) and the juvenile survival (McMahon et al. 2005; Burkanov et al. 2011), as well as carrying a pup to term as females may also be gestating (Georges and Guinet 2000).
Lactating long-nosed fur seals (LNFS; Arctocephalus forsteri) provide a unique opportunity to investigate individual foraging site fidelity in oceanic environments due to the plasticity of their foraging behaviour over a long 10-month pup-rearing period, and the ability to track seals over consecutive foraging trips. In a typical breeding cycle, adult female LNFS give birth to pups in December (austral summer) and mating takes place shortly after that. Mothers nurse their pups for 8–11 months while simultaneously gestating a foetus (Goldsworthy 2006). During the austral summer, the majority of the lactating females from Kangaroo Island (South Australia) forage on the Australian continental shelf and shelf-break associated with seasonal upwelling (Foo et al. 2019); whereas during the austral winter, the majority of the lactating females forage in oceanic waters associated with the Subtropical Front (Baylis et al. 2008). The marked change from the coastal to the oceanic foraging as lactation progresses is referred to as an alternating foraging strategy (Foo et al. 2019). Foraging site fidelity is generally high in the continental shelf habitats (Baylis et al. 2012; Sommerfeld et al. 2015) and the continental shelf in our study region is narrow, thereby concentrating productivity into a small predictable area. Conversely, the prey in large-scale oceanic habitats are likely to be more dispersed and ephemeral (Kotliar and Wiens 1990) and, thus, foraging site fidelity is generally lower (Baylis et al. 2012). Nonetheless, the greater spatial variability in oceanic regions potentially allows for more intra- and inter-individual variability in foraging strategies to develop and, thus, lead to different levels of individual reproductive success. Indeed, the variability in individual oceanic foraging trip distances and direction has been observed in lactating LNFS (Foo et al. 2019); however, the underlying environmental drivers to this variability are still unclear.
In oceanic regions, several marine predator species (e.g., Cotté et al. 2007; Simmons et al. 2007; Cleeland et al. 2014; Miller et al. 2015) preferentially target mesoscale features, such as eddies and frontal systems, which are relatively productive as they provide intermittent pulses of nutrients to the photic layer for the phytoplankton growth which would attract prey (Williams et al. 2011). Indeed, the oceanic waters associated with the Subtropical Front, located in the south of Australia, are known to have high incidences of eddies, fronts, and filaments (James et al. 2002b; Tomczak et al. 2004), and we expect lactating LNFS foraging to be associated with these oceanographic features. In this study, we investigate the potential environmental drivers to individual foraging site fidelity in lactating LNFS. Specifically, our aims are to (1) determine if sea-surface temperature (SST) and sea-surface height (SSH) gradients are associated with intra-annual individual foraging site fidelity, and (2) quantify any inter-annual differences in foraging site fidelity.
Methods
Study site, animal handling, and instrumentation
The study was undertaken between February and August/September in 2016 and 2017 at Cape Gantheaume, Kangaroo Island, South Australia (36° 04′ S, 137° 27′ E). Forty-five lactating LNFS (2016: n = 15, 2017: n = 30) were randomly selected and captured using a hoopnet. Details of the animal handling and tag attachment methods can be found in a concurrent study by Foo et al. (2019). Seals were weighed (± 0.5 kg), and their body length (nose to tail) and axillary girth were measured (± 1 cm). Geolocation (GLS, Intigeo-C330, 17 × 19 × 8 mm, 3.3 g, Migrate Technology Ltd, Cambridge, UK) loggers were deployed on all individuals and recovered later in the same year between June and August.
The GLS loggers measured ambient light every minute and recorded the maximum value every 4 min. The loggers also sampled sea temperature (0.125 °C resolution; ± 0.5 °C accuracy) after 20 min of being continuously wet and recorded the minimum, maximum, and mean temperature every 4 h. The 2016 loggers sampled ambient temperature every 5 min, and recorded the minimum, maximum, and mean temperature every 4 h. The loggers also sampled the time when an activity (wet or dry)-state change occurred. Each logger was activated and left in an open area at the study site for approximately 5–7 days, either immediately before or after deployment to obtain solar elevation estimates necessary for location calibration.
Location estimation
All data analyses were done using the R program (v3.5.3) (R Core Team 2019). Using the raw light data from GLS loggers, a number of times of twilight period were estimated using the BAStag package (v0.1–3) (Wotherspoon et al. 2016a). Next, we created Markov Chain–Monte Carlo simulations within a Bayesian framework to estimate the final posterior mean of the two primary locations per day while incorporating temperature and land-mask (Sumner et al. 2009; Lisovski et al. 2012) and haul-out constraints; this was done using the SGAT package (v0.1.3) (Wotherspoon et al. 2016b). For the latter, seals were assumed to be back to the colony when the GLS logger was continuously dry for ≥ 4 h as lactating LNFS are not known to haul out at other locations (Page et al. 2006; Baylis et al. 2012). Individual foraging trips and their duration were then extracted from location estimates and checked with wet/dry information from the GLS logger. The accuracy of location estimates, using this method, was 45 ± 29 km (mean ± SD) for a lactating LNFS carrying GLS and GPS tags simultaneously (Foo et al. 2019). As we were only interested in the seal’s lactation period between summer and winter (February–August), the few locations obtained after August from one individual were excluded from further analyses.
Core foraging areas
We assumed that core foraging areas were locations where females spent majority of their time on a foraging trip; we calculated the time spent (h) per 30 × 30 km grid cell, as a proxy for the foraging effort like others have done (Pistorius et al. 2017), for each foraging trip using the trip package (v1.5.0) (Sumner 2016). The size of the grid cell was chosen to account for the error associated with GLS tags while still providing realistic representation of true locations. We found this cell size adequate for creating a measure of foraging site fidelity in subsequent steps (detailed later in “Oceanic Foraging Site Fidelity”). Trips with less than three locations and locations within a 10-km buffer around the colony were removed, because foraging trips are typically, at least, 2 days long (i.e., total of four location points) (Baylis et al. 2008; Foo et al. 2019), and cells near the colony may represent non-foraging periods when females were travelling and/or displaying other kinds of behaviour (e.g., thermoregulation) in waters near the colony (Page et al. 2005). We identified core foraging areas as cells (1) within the 90th percentile of the range of time spent values per foraging trip and (2) within 2° in the latitude north from the southernmost core foraging cell identified for each trip. The latter was done to exclude outlier cells in the 90th percentile that were far away from where many of them tend to cluster. The 90th percentile is a conservative threshold that was chosen to ensure that the selected cells indeed represented foraging behaviour.
Oceanographic data
To describe the physical at-sea environment encountered by the seals in core oceanic foraging areas, we extracted the following environmental variables using the raster (v2.6-7) (Hijmans et al. 2017) and raadtools (v0.5.1) (Sumner 2016) packages: 0.02° bathymetry, daily 0.25° sea-surface temperature gradient (SSTgrad; derived from SST), and daily 0.12° sea-surface height gradient (SSHgrad; derived from SSH). The sources of environmental variables are shown in Table 1. Areas of high SSTgrad and SSHgrad are typically associated with frontal and eddy activities (Sokolov and Rintoul 2007; Bost et al. 2009; Scales et al. 2014). Fronts are horizontal gradients with enhanced water properties (e.g., temperature, salinity, density, etc.) on the water surface that outline transitions between water masses (Belkin et al. 2009). Eddies are circular currents of water that have a change in SSH between its core and boundary (Douglass and Richman 2015), and they can influence chlorophyll-a and SST gradients particularly at their edges (Gaube et al. 2017). All environmental variables were reprojected onto a 0.25 × 0.25° (~ 27–28 km) grid. The mean and standard deviation (SD) of the dynamic environmental variables were calculated from daily values over the period of each foraging trip. SD was used, because the stability of an environment is relevant to fidelity (Arthur et al. 2015). Missing values were removed from calculations. Subsequently, the mean of each dynamic environmental covariate (SSTgrad, SSTgrad_SD, SSHgrad, and SSHgrad_SD) was extracted from cells within a 30-km buffer of each core foraging cell.
Oceanic foraging site fidelity
Foraging trips were classified as “shelf”, which included the continental shelf and shelf-break (bathymetry ≤ 2000 m), or “oceanic” (bathymetry > 2000 m) depending on the mean bathymetry of its core foraging cells. The 2000-m isobath generally marked the end of the shelf-break in this region (Page et al. 2005). Only data from oceanic foraging trips were used in subsequent analyses. Maximum distance from the colony and the duration of each trip were also calculated. Trips were also classified into early autumn (February–March) or late autumn/winter (April–August), as this separates the upwelling and non-upwelling period on the shelf (Foo et al. 2019).
We noticed that consecutive oceanic trips were sometimes interrupted by a shelf trip, and individuals may not necessarily return to previous foraging areas on successive foraging trips but do so in later foraging trips; hence, oceanic foraging site fidelity was investigated at two timescales: (1) over the entire oceanic foraging period (i.e., from the first to the last oceanic foraging trip made over the deployment period) and (2) over the period of pairs of consecutive oceanic foraging trips for each individual (Fig. 1). For the former timescale, the SD (variability) in the latitude (Lat_SD) and the longitude (Lon_SD) of all core foraging cells, over the observational period for each individual, were calculated and used as a proxy for foraging site fidelity. For the latter timescale, the average location of core foraging cells for each trip was calculated and the great circle distance (km; hereafter distancefidelity) between the average core locations of pairs of consecutive oceanic trips was used as a proxy for foraging site fidelity. For both fidelity measurements, greater values meant lower foraging site fidelity.
Timeline of the types (oceanic or shelf) of foraging trips made by individual seals. Each observation only indicates the start of the foraging trip. The solid rectangle is an example of the entire oceanic foraging period. The dashed rectangle is an example of a pair of consecutive oceanic foraging trips
Another measure of site fidelity is the tendency for individuals to repeatedly travel in similar directions on multiple trips (Pettex et al. 2012). We calculated the direction of each foraging trip as the bearing between the colony and the furthest location travelled. We used circular statistics from the circular package (v.0.4-93) for the following tests. The persistence of travelling in the same direction over multiple trips was tested for each individual using Rayleigh’s uniformity test. This test provides an r value between 1 and 0, where values closer to 1 indicate greater directionality. We tested the differences in the mean direction of foraging trips between years using the non-parametric Watson–Wheeler test.
Statistical analyses
For the entire oceanic foraging timescale, we calculated the average of each environmental covariate over all core foraging cells for each individual. We then fitted linear models for Lat_SD and Lon_SD against all the environmental predictors and the year. Model selection for linear models was done by sequentially removing the most non-significant term in the model until only significant terms remained. For the pairs of consecutive oceanic foraging trip timescale, we calculated the average of each environmental covariate over all core foraging cells for the earlier trip, since the outcome of the previous foraging trip is more likely to influence the foraging decisions made in the successive trip. We then fitted linear mixed-effect models for distancefidelity against all environmental predictors, season, year, and the number of core foraging cells (proxy for size of core foraging area of the trip), including trip nested within seal identity as a random effect. For linear mixed-effect models, backward model selection was done using Akaike information criterion that was corrected for small-sample sizes (AICc) (Burnham and Anderson 2002). Least significant fixed-effect terms were removed sequentially from the full model to obtain an optimal model with the lowest AICc. Models with delta AICc < 2 were considered not significantly different, and the more parsimonious model was used as the final model. The final model was evaluated for linearity, homogeneity, and normality using standard graphical methods and autocorrelation using the acf() function on model’s residuals. If a temporal correlation existed, an autoregressive function would be added for linear mixed-effect models. The response variable was transformed, and/or a variance component function (only for linear mixed-effect models) was included if it was necessary to fulfil model assumptions. Trip duration, maximum trip distance travelled from colony, and total time spent in core foraging cells per trip were modelled against year and season with trip nested within seal identity as a random effect. This was analysed using a penalised-quasi likelihood linear mixed-effect model with a Gamma distribution and identity link. Results were reported as mean ± SD unless otherwise stated. Statistical significance was determined at p < 0.05.
Results
Location statistics and track summaries
We recovered GLS loggers from 17 adult female seals. However, based on the estimated tracks derived from light data, four seals did not show central-place foraging behaviour (possibly due to pup death or abandonment) and only one seal made shelf foraging trips. These individuals were excluded from subsequent analyses. From the remaining 12 seals (n2016 = 5; n2017 = 7), a total of 3585 at-sea locations were obtained from 159 foraging trips from the early autumn to the winter in both study years (2016 = 99 trips, 2017 = 60 trips; Table 2). Of the total number of foraging trips, 94 were oceanic trips and 65 were shelf trips (Table 2). Oceanic trip duration (Table 3a) and maximum distance travelled (during oceanic trips) (Table 3b) were generally longer and farther in 2017 and in the late autumn/winter.
Environmental drivers to individual oceanic foraging site fidelity
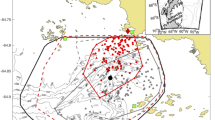
A total of 534 core foraging cells were detected from all the oceanic foraging trips with an average of six core foraging cells per trip (Table 2). The total time spent in the core foraging cells during an oceanic foraging trip was greater in 2017 than in 2016 (Tables 2 and 3c). The distribution of the oceanic core foraging cells tended to occur near mesoscale eddy edges and fronts (as indicated by SSHgrad; Fig. 2). An animation of individual foraging tracks overlayed on top of SSTgrad and SSHgrad in the study region is shown in Supplementary videos 1–4.
Examples of sequential foraging tracks (trip number above each plot) from an individual seal (#353). Core foraging cells (90th percentile of time spent in cell of each trip) are red and non-core foraging cells are opaque white. The red triangle represents the colony at Cape Gantheaume, Kangaroo Island. Tracks are overlaid onto a sea-surface height gradient (SSHgrad; units: change in m per 0.25 × 0.25° pixel) and b sea-surface temperature gradient (SSTgrad; units: change in °C per 0.25 × 0.25° pixel). Environmental values show the average values during the period of each foraging trip. High values of SSHgrad and SSTgrad represent areas of strong eddy and frontal activity, respectively. See SFig. 1. for additional examples
During the entire oceanic foraging timescale (n = 12), Lat_SD increased with increasing SSTgrad_SD, but decreased with increasing SSTgrad (Fig. 3, Table 3d), Lat_SD was lower in 2017 than in 2016 (Fig. 3, Table 3d), and Lon_SD decreased with SSHgrad (Fig. 3, Table 3d). The overall average distancefidelity was 195 ± 162 km, n = 66 (range = 12.5–698 km) (Table 2). The global model for distancefidelity, with just seal identity as a random effect, shows to be the most parsimonious random structure (global model with random effect seal, AIC = 185; global model with random effect trip nested within seal, AIC = 187). At the consecutive oceanic foraging timescale, the best model (Table 4) indicated that distancefidelity increased with increasing SSTgrad_SD experienced by an individual during the earlier foraging trip (Fig. 4, Table 3e). The mean bearing across all oceanic foraging trips was different between years (2016: mean = 165 ± 11°, n = 42; 2017: 177 ± 20°, n = 52; Watson–Wheeler: T = 22.8, N = 94, p < 0.001; Table 2). There was a high directional persistence over all oceanic foraging trips for each individual (r value: mean = 0.986 ± 0.011, range = 0.96–0.998, N = 12, all individual p < 0.05; Table 2).
Effect plots of a SSTgrad SD, b SSTgrad, and c year on latitude SD; and d SSHgrad on longitude SD over the entire oceanic foraging period of individual seals. The solid black lines and dots represent the predicted effect. Shaded bands and error bars represent the 95% confidence intervals. Grey circles represent raw data points
Effect of SSTgrad_SD on distancefidelity (distance between mean core foraging areas of consecutive pairs of oceanic foraging trips). The solid black lines and dots represent the predicted effect. Shaded bands and error bars represent the 95% confidence intervals. Grey circles represent raw data points
Discussion
Previous studies on LNFS have reported differences in individual foraging site fidelity between shelf and oceanic habitats (Baylis et al. 2012), but a consensus of understanding of environmental drivers to individual foraging site fidelity is still lacking. Here, we focused on foraging in the oceanic region which typically occurs at the later stage of lactation when female energetic demands are the highest. During this time, lactating females are likely to be operating within their limits, so there is pressure on them to forage as optimally as possible to provide their pups at rates that maximise the pups’ survival probabilities, given the huge amount of energy they have already invested in their pups over the preceding months of lactation. Using continuous foraging tracks of individuals from the summer/early autumn to the winter and over two breeding seasons, we found that individual foraging site fidelity is influenced by the strength and variability of SSTgrad and SSHgrad. This knowledge helps us to identify important foraging hotspots and understand how environmental changes of these hotspots may impact the population dynamics of this top marine predator.
Importance of mesoscale oceanographic features
Individual foraging site fidelity over the entire oceanic foraging period increased (lower Lat_SD and Lon_SD) with greater SSHgrad and SSTgrad, which are typically associated with eddy and frontal activities. The oceanic waters in this study region, which encompasses the Subtropical Front, is known to have a strong eddy field (Tomczak et al. 2004) particularly in winter (Middleton and Bye 2007). From video animations of female foraging tracks superimposed onto SSHgrad, foraging locations during oceanic foraging trips can be seen near or within eddy features (Supplementary video 1-2). Spatial heterogeneity (i.e., strong gradients) associated with fronts and eddies leads to a diversity of habitats, nutrient mixing, and, therefore, areas of high productivity that concentrate prey; thus making them good places to feed especially for animals that are highly motivated to acquire lots of resources (Bost et al. 2009). Hence, it follows that females keep returning to the vicinity of the same profitable foraging area on consecutive trips until profitability declines due to prey depletion, or temporal and spatial variability in prey distribution (Charnov 1976). Other marine predators including the Southern elephant seals (Mirounga leonina) (Campagna et al. 2006; Bailleul et al. 2010; Della Penna et al. 2015; Tosh et al. 2015), Northern elephant seals (Mirounga angustirostris) (Abrahms et al. 2018b), King penguins (Aptenodytes patagonicus) (Cotté et al. 2007), Loggerhead sea turtles (Caretta caretta) (Polovina et al. 2004), Bottlenose dolphins (Tursiops truncates) (Bailey and Thompson 2010), and Antarctic fur seals (Guinet et al. 2001) have all been observed to forage in the proximity of the eddies where the prey tend to aggregate. We note that while our sample size was relatively small (12 individuals), it would not be unrealistic to find an association between individual foraging site fidelity and SSHgrad and SSTgrad, as these oceanographic features are commonly targeted by various marine predators (Bost et al. 2009; Chambault et al. 2017; Reisinger et al. 2018). A preference for foraging in areas with strong oceanographic gradients has also been observed in Macaroni penguins (Eudyptes chrysolophus), Chinstrap penguins (Pygoscelis antarctica), Antarctic fur seals (Arctocephalus gazella) (Lowther et al. 2014), and Northern fur seals (Callorhinus ursinus) (Nordstrom et al. 2013).
In oceanic waters, good foraging patches may be spatially and temporally stable (i.e., predictable) (Staniland et al. 2004). Our results support this—at the entire oceanic foraging and consecutive oceanic foraging trip levels; individual foraging site fidelity decreased with increasing SSTgrad_SD. Similarly, Arthur et al (2015) reported that within-year foraging site fidelity in Antarctic fur seals decreased with greater long-term SST variability in foraging areas. That predictability of the oceanographic environment influenced individual foraging site fidelity at both fine and coarser intra-annual timescales highlights its importance on the foraging strategies of lactating females.
The importance of predictability of SSTgrad may be linked to relatively slow-moving eddies which are characteristic of the Great Australian Bight eddy field (this study region) (Oke et al. 2018). Mesoscale eddies can influence the spatial variability of SST through various mechanisms (Gaube et al. 2015). Observations from supplementary videos 1–2 show certain individuals (e.g., seal IDs #072 and #311) foraging at the same eddy on multiple foraging trips more clearly. Other marine mammals, such as fin (Balaenoptera physalus) and minke whales (Balaenoptera acutorostrata) in the Bay of Fundy, focus their foraging in areas of prey aggregation associated with slower velocity regions within an eddy system (Johnston et al. 2005). Predictability of slow-moving eddies may explain the high individual directional fidelity observed during oceanic foraging trips—a trait which is consistent with the previous work on LNFS at this Kangaroo Island study site (Baylis et al. 2012) and across a diversity of species from Antarctic fur seals (Bonadonna et al. 2001) to northern gannets (Morus bassanus) (Pettex et al. 2012). Nonetheless, this hypothesis requires further investigations to be certain.
The predominance of SSTgrad on individual foraging site fidelity in the latitudinal axis might be due to the cyclical north–south movement of the Subtropical Front, a feature which can affect the stability of the mixed layer in the surrounding water column and, thus, influence the spatial variability of eddies and fronts (Tomczak et al. 2004). Conversely, the predominance of SSHgrad in the longitudinal axis might be due to the westward drift of eddies due to strong westward currents from the south of Tasmania in the winter (James et al. 2002a; Oke et al. 2018).
Inter-annual variability in foraging site fidelity
Individual foraging site fidelity in the foraging latitude was greater in 2017 than in 2016, and this may have contributed to heavier winter pup mass in 2017 (12.6 ± 2.25 SD kg) than in 2016 (11 ± 2.11 kg SD) (Foo et al. 2019). Additionally, LNFS pup production at Cape Gantheaume in the 2017–2018 breeding season (following season after the fieldwork for this study ended) was the largest ever recorded at the time (20% increase from the previous season, whereas there was a 3% decrease in pup production between 2015–2016 and 2016–2017 seasons), and the average summer (2 months old) pup mass was greater in the 2017–2018 (6.69 ± 1.31 kg SD) than in 2016–2017 (6.55 ± 1.46 kg SD) breeding season (S. Goldsworthy, pers comm), which has been on a steady decline since the late 1990s (Goldsworthy et al. 2016). These observations suggest that 2017 was, especially, a good year for the pup growth rates (higher winter mass) and carrying the pups to term. The mid-to-late stage of gestation plays a crucial role in determining the reproductive success of LNFS as significant foetal mortality occurs during this period (McKenzie et al. 2005). By returning to the same profitable foraging site repeatedly, females can maximise the time spent on foraging and minimise the time spent on searching for prey. Coincidentally, 2017 females also had a longer time spent in core foraging areas per trip, and longer and farther oceanic foraging trips. Prior knowledge of the profitable areas is particularly beneficial for long foraging trips, which are energetically expensive, as the high variability in the individual foraging trip route and habitat may lead to mass loss for adults (Call et al. 2008) and, thus, lower the parental investment in gestation.
Conclusion
Lactating LNFS in South Australia display higher intra-annual individual foraging site fidelity to areas with strong and stable oceanographic gradients. At the individual level, females also tend to be persistent in the direction of their oceanic foraging trips from the colony. Future research on the persistence of individual foraging site fidelity over multiple years would give us insight into how this strategy might help them to cope with possible severe environmental changes.
Data availability
The datasets analysed during the current study are available from the corresponding author on a reasonable request.
References
Abrahms B, Hazen EL, Bograd SJ, Brashares JS, Robinson PW, Scales KL, Crocker DE, Costa DP (2018a) Climate mediates the success of migration strategies in a marine predator. Ecol Lett 21:63–71. https://doi.org/10.1111/ele.12871
Abrahms B, Scales KL, Hazen EL, Bograd SJ, Schick RS, Robinson PW, Costa DP (2018b) Mesoscale activity facilitates energy gain in a top predator. Proc R Soc B Biol Sci 285:20181101. https://doi.org/10.1098/rspb.2018.1101
Arthur B, Hindell M, Bester M, Trathan P, Jonsen I, Staniland I, Oosthuizen WC, Wege M, Lea MA (2015) Return customers: foraging site fidelity and the effect of environmental variability in wide-ranging antarctic fur seals. PLoS ONE 10:e0120888. https://doi.org/10.1371/journal.pone.0120888
Bailey H, Thompson P (2010) Effect of oceanographic features on fine-scale foraging movements of bottlenose dolphins. Mar Ecol Prog Ser 418:223–233. https://doi.org/10.3354/meps08789
Bailleul F, Cotté C, Guinet C (2010) Mesoscale eddies as foraging area of a deep-diving predator, the southern elephant seal. Mar Ecol Prog Ser 408:251–264. https://doi.org/10.3354/meps08560
Baylis AMM, Page B, Goldsworthy SD (2008) Effect of seasonal changes in upwelling activity on the foraging locations of a wide-ranging central-place forager, the New Zealand fur seal. Can J Zool 86:774–789. https://doi.org/10.1139/Z08-055
Baylis AMM, Page B, McKenzie J, Goldsworthy SD (2012) Individual foraging site fidelity in lactating New Zealand fur seals: continental shelf vs. oceanic habitats. Mar Mammal Sci 28:276–294. https://doi.org/10.1111/j.1748-7692.2011.00487.x
Belkin IM, Cornillon PC, Sherman K (2009) Fronts in large marine ecosystems. Prog Oceanogr 81:223–236. https://doi.org/10.1016/j.pocean.2009.04.015
Bonadonna F, Lea MA, Dehorter O, Guinet C (2001) Foraging ground fidelity and route-choice tactics of a marine predator: The Antarctic fur seal Arctocephalus gazella. Mar Ecol Prog Ser 223:287–297. https://doi.org/10.3354/meps223287
Bost CA, Cotte C, Bailleul F, Cherel Y, Charrassin JB, Guinet C, Ainley DG, Weimerskirch H (2009) The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J Mar Syst 78:363–376. https://doi.org/10.1016/j.jmarsys.2008.11.022
Boyd IL (1999) Foraging and provisioning in Antarctic fur seals: interannual variability in time-energy budgets. Behav Ecol 10:198–208. https://doi.org/10.1093/beheco/10.2.198
Bradshaw C, Higgins J, Michael K, Wotherspoon S, Hindell M (2004) At-sea distribution of female southern elephant seals relative to variation in ocean surface properties. Ices J Mar Sci 61:1014–1027. https://doi.org/10.1016/j.icesjms.2004.07.012
Burkanov V, Gurarie E, Altukhov A, Mamaev E, Permyakov P, Trukhin A, Waite J, Gelatt T (2011) Environmental and biological factors influencing maternal attendance patterns of Steller sea lions (Eumetopias jubatus) in Russia. J Mammal 92:352–366. https://doi.org/10.1644/10-MAMM-A-194.1
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Call KA, Ream RR, Johnson D, Sterling JT, Towell RG (2008) Foraging route tactics and site fidelity of adult female northern fur seal (Callorhinus ursinus) around the Pribilof Islands. Deep Sea Res Part II Top Stud Oceanogr 55:1883–1896. https://doi.org/10.1016/j.dsr2.2008.04.022
Campagna C, Piola AR, Rosa M, Lewis M, Fernández T (2006) Southern elephant seal trajectories, fronts and eddies in the Brazil/Malvinas Confluence. Deep Res Part I Oceanogr Res Pap 53:1907–1924. https://doi.org/10.1016/j.dsr.2006.08.015
Chambault P, Roquet F, Benhamou S, Baudena A, Pauthenet E, de Thoisy B, Bonola M, Dos Reis V, Crasson R, Brucker M, Le Maho Y, Chevallier D (2017) The Gulf Stream frontal system: a key oceanographic feature in the habitat selection of the leatherback turtle? Deep Res Part I Oceanogr Res Pap 123:35–47. https://doi.org/10.1016/j.dsr.2017.03.003
Charnov EL (1976) Optimal foraging, marginal value theorem. Theor Popul Biol 9:129–136. https://doi.org/10.1016/0040-5809(76)90040-x
Cleeland JB, Lea MA, Hindell MA (2014) Use of the Southern Ocean by breeding short-tailed shearwaters (Puffinus tenuirostris). J Exp Mar Bio Ecol 450:109–117. https://doi.org/10.1016/j.jembe.2013.10.012
Cotté C, Park YH, Guinet C, Bost CA (2007) Movements of foraging king penguins through marine mesoscale eddies. Proc R Soc B Biol Sci 274:2385–2391. https://doi.org/10.1098/rspb.2007.0775
Croll DA, Demer DA, Hewitt RP, Jansen JK, Goebel ME, Tershy BR (2006) Effects of variability in prey abundance on reproduction and foraging in chinstrap penguins (Pygoscelis antarctica). J Zool 269:506–513. https://doi.org/10.1111/j.1469-7998.2006.00090.x
Della Penna A, De Monte S, Kestenare E, Guinet C, D’Ovidio F (2015) Quasi-planktonic behavior of foraging top marine predators. Sci Rep 5:18063. https://doi.org/10.1038/srep18063
Douglass EM, Richman JG (2015) Analysis of ageostrophy in strong surface eddies in the Atlantic Ocean. J Geophys Res Ocean 120:1490–1507. https://doi.org/10.1002/2014JC010350
Espinoza M, Farrugia TJ, Lowe CG (2011) Habitat use, movements and site fidelity of the gray smooth-hound shark in a restored California estuary. J Exp Mar Bio Ecol 401:63–74. https://doi.org/10.1016/j.jembe.2011.03.001
Foo D, McMahon C, Hindell M, Goldsworthy S, Bailleul F (2019) Influence of shelf oceanographic variability on alternate foraging strategies in long-nosed fur seals. Mar Ecol Prog Ser 615:189–204. https://doi.org/10.3354/meps12922
Gaube P, Chelton DB, Samelson RM, Schlax MG, O’Neill LW (2015) Satellite observations of mesoscale eddy-induced ekman pumping. J Phys Oceanogr 45:104–132. https://doi.org/10.1175/jpo-d-14-0032.1
Gaube P, Barcelo C, McGillicuddy DJ, Domingo A, Miller P, Giffoni B, Marcovaldi N, Swimmer Y (2017) The use of mesoscale eddies by juvenile loggerhead sea turtles (Caretta caretta) in the southwestern Atlantic. PLoS ONE 12:e0172839. https://doi.org/10.1371/journal.pone.0172839
Georges JY, Guinet C (2000) Maternal care in the Subantarctic fur Seals on Amsterdam Island. Ecology 81:295–308. https://doi.org/10.1890/0012-9658(2000)081[0295:MCITSF]2.0.CO;2
Goldsworthy SD (2006) Maternal strategies of the New Zealand fur seal: evidence for interannual variability in provisioning and pup growth strategies. Aust J Zool 54:31–44. https://doi.org/10.1071/ZO05041
Goldsworthy SD, Bailleul F, Shaughnessy PD, Mackay AI, Reinhold S-L, Stonnill M, Lashmar K (2016) Monitoring of pinniped populations on Kangaroo Island: 2015/16. Report to the Department of Environment Water and Natural Resources, London
Guinet C, Dubroca L, Lea MA, Goldsworthy S, Cherel Y, Duhamel G, Bonadonna F, Donnay JP (2001) Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems. Mar Ecol Prog Ser 219:251–264. https://doi.org/10.3354/meps219251
Hijmans RJ, van Etter J, Cheng J, Mattiuzzi M, Summer M, Greenberg JA, Lamigueiro OP, Bevan A, Racine EB, Shortridge A, Ghosh A (2017) Geographic data analysis and modeling. https://cran.r-project.org/web/packages/raster/index.html. Accessed 3 Mar 2019
Houston AI, Stephens PA, Boyd IL, Harding KC, McNamara JM (2007) Capital or income breeding? A theoretical model of female reproductive strategies. Behav Ecol 18:241–250. https://doi.org/10.1093/beheco/arl080
James C, Tomczak M, Helmond I, Pender L (2002a) Summer and winter surveys of the subtropcal front of the southeastern Indian Ocean 1997–1998. J Mar Syst 37:129–149. https://doi.org/10.1016/s0924-7963(02)00199-9
James C, Tomczak M, Helmond I, Pender L (2002b) Summer and winter surveys of the Subtropical Front of the southeastern Indian Ocean 1997–1998. J Mar Syst 37:129–149. https://doi.org/10.1016/s0924-7963(02)00199-9
Johnston DW, Thorne LH, Read AJ (2005) Fin whales Balaenoptera physalus and minke whales Balaenoptera acutorostrata exploit a tidally driven island wake ecosystem in the Bay of Fundy. Mar Ecol Prog Ser 305:287–295. https://doi.org/10.3354/meps305287
Kotliar NB, Wiens JA (1990) Multiple scales of patchiness and patch structure: a hierarchical framework for the study of heterogeneity. Oikos 59:253. https://doi.org/10.2307/3545542
Kowalczyk ND, Reina RD, Preston TJ, Chiaradia A (2015) Environmental variability drives shifts in the foraging behaviour and reproductive success of an inshore seabird. Oecologia 178:967–979. https://doi.org/10.1007/s00442-015-3294-6
Lisovski S, Hahn S, Hodgson D (2012) GeoLight- processing and analysing light-based geolocator data inR. Methods Ecol Evol 3:1055–1059. https://doi.org/10.1111/j.2041-210X.2012.00248.x
McHuron EA, Hazen E, Costa DP (2018) Constrained by consistency? Repeatability of foraging behavior at multiple timescales for a generalist marine predator. Mar Biol 165:122. https://doi.org/10.1007/s00227-018-3382-3
McIntyre T, Bester MN, Bornemann H, Tosh CA, de Bruyn PJN (2017) Slow to change? Individual fidelity to three-dimensional foraging habitats in southern elephant seals, Mirounga leonina. Anim Behav 127:91–99. https://doi.org/10.1016/j.anbehav.2017.03.006
McKenzie J, Parry LJ, Page B, Goldsworthy SD (2005) Estimation of pregnancy rates and reproductive failure in New Zealand Fur Seals (Arctocephalus Forsteri). J Mammal 86:1237–1246. https://doi.org/10.1644/05-mamm-a-085r.1
McMahon CR, Hindell MA, Burton HR, Bester MN (2005) Comparison of southern elephant seal populations, and observations of a population on a demographic knife-edge. Mar Ecol Prog Ser 288:273–283. https://doi.org/10.3354/meps288273
Middleton JF, Bye JAT (2007) A review of the shelf-slope circulation along Australia’s southern shelves: Cape Leeuwin to Portland. Prog Oceanogr 75:1–41. https://doi.org/10.1016/j.pocean.2007.07.001
Miller PI, Scales KL, Ingram SN, Southall EJ, Sims DW (2015) Basking sharks and oceanographic fronts: quantifying associations in the north-east Atlantic. Funct Ecol 29:1099–1109. https://doi.org/10.1111/1365-2435.12423
Nordstrom CA, Battaile BC, Cotté C, Trites AW (2013) Foraging habitats of lactating northern fur seals are structured by thermocline depths and submesoscale fronts in the eastern Bering Sea. Deep Sea Res Part II Top Stud Oceanogr 88–89:78–96. https://doi.org/10.1016/j.dsr2.2012.07.010
Oke PR, Griffin DA, Rykova T, de Oliveira HB (2018) Ocean circulation in the Great Australian Bight in an eddy-resolving ocean reanalysis: the eddy field, seasonal and interannual variability. Deep Res Part II Top Stud Oceanogr 157–158:11–26. https://doi.org/10.1016/j.dsr2.2018.09.012
Oksanen SM, Ahola MP, Lehtonen E, Kunnasranta M (2014) Using movement data of Baltic grey seals to examine foraging-site fidelity: implications for seal-fishery conflict mitigation. Mar Ecol Prog Ser 507:297–308. https://doi.org/10.3354/meps10846
Oosthuizen WC, Bester MN, Altwegg R, McIntyre T, De Bruyn PJN (2015) Decomposing the variance in Southern Elephant seal weaning mass: partitioning environmental signals and maternal effects. Ecosphere 6:1–22. https://doi.org/10.1890/ES14-00508.1
Page B, McKenzie J, Goldsworthy SD (2005) Inter-sexual differences in New Zealand fur seal diving behaviour. Mar Ecol Prog Ser 304:249–264. https://doi.org/10.3354/meps304249
Page B, McKenzie J, Sumner MD, Coyne M, Goldsworthy SD (2006) Spatial separation of foraging habitats among New Zealand fur seals. Mar Ecol Prog Ser 323:263–279. https://doi.org/10.3354/meps323263
Pettex E, Lorentsen SH, Grémillet D, Gimenez O, Barrett RT, Pons JB, Le Bohec C, Bonadonna F (2012) Multi-scale foraging variability in Northern gannet (Morus bassanus) fuels potential foraging plasticity. Mar Biol 159:2743–2756. https://doi.org/10.1007/s00227-012-2035-1
Pichegru L, Ryan PG, Crawford RJM, van der Lingen CD, Grémillet D (2010) Behavioural inertia places a top marine predator at risk from environmental change in the Benguela upwelling system. Mar Biol 157:537–544. https://doi.org/10.1007/s00227-009-1339-2
Polovina JJ, Balazs GH, Howell EA, Parker DM, Seki MP, Dutton PH (2004) Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish Oceanogr 13:36–51. https://doi.org/10.1046/j.1365-2419.2003.00270.x
Rayner MJ, Hartill BW, Hauber ME, Phillips RA (2010) Central place foraging by breeding Cook’s petrel Pterodroma cookii: foraging duration reflects range, diet and chick meal mass. Mar Biol 157:2187–2194. https://doi.org/10.1007/s00227-010-1483-8
R Core Team (2019) R: a language and environment for statistical computing.
Reisinger RR, Raymond B, Hindell MA, Bester MN, Crawford RJM, Davies D, de Bruyn PJN, Dilley BJ, Kirkman SP, Makhado AB, Ryan PG, Schoombie S, Stevens K, Sumner MD, Tosh CA, Wege M, Whitehead TO, Wotherspoon S, Pistorius PA (2018) Habitat modelling of tracking data from multiple marine predators identifies important areas in the Southern Indian Ocean. Divers Distrib 24:535–550. https://doi.org/10.1111/ddi.12702
Scales KL, Miller PI, Embling CB, Ingram SN, Pirotta E, Votier SC (2014) Mesoscale fronts as foraging habitats: composite front mapping reveals oceanographic drivers of habitat use for a pelagic seabird. J R Soc Interface 11:20140679–20140679. https://doi.org/10.1098/rsif.2014.0679
Simmons SE, Crocker DE, Kudela RM, Costa DP (2007) Linking foraging behaviour of the northern elephant seal with oceanography and bathymetry at mesoscales. Mar Ecol Prog Ser 346:265–275. https://doi.org/10.3354/meps07014
Sokolov S, Rintoul SR (2007) Multiple jets of the antarctic circumpolar current South of Australia. J Phys Oceanogr 37:1394–1412. https://doi.org/10.1175/JPO3111.1
Sommerfeld J, Kato A, Ropert-Coudert Y, Garthe S, Wilcox C, Hindell MA (2015) Flexible foraging behaviour in a marine predator, the Masked booby (Sula dactylatra), according to foraging locations and environmental conditions. J Exp Mar Bio Ecol 463:79–86. https://doi.org/10.1016/j.jembe.2014.11.005
Staniland IJ, Boyd IL (2003) Variation in the foraging location of Antarctic fur seals (Arctocephalus gazella) and the effects on diving behavior. Mar Mammal Sci 19:331–343. https://doi.org/10.1111/j.1748-7692.2003.tb01112.x
Staniland IJ, Reid K, Boyd IL (2004) Comparing individual and spatial influences on foraging behaviour in Antarctic fur seals Arctocephalus gazella. Mar Ecol Prog Ser 275:263–274. https://doi.org/10.3354/meps275263
Sumner MD (2016) raadtools: Tools for synoptic environmental spatial data. https://github.com/AustralianAntarcticDivision/raadtools. Accessed 3 Mar 2019
Sumner MD, Wotherspoon SJ, Hindell MA (2009) Bayesian estimation of animal movement from archival and satellite tags. PLoS ONE 4:1–13. https://doi.org/10.1371/journal.pone.0007324
Tomczak M, Pender L, Liefrink S (2004) Variability of the subtropical front in the Indian Ocean south of Australia. Ocean Dyn 54:506–519. https://doi.org/10.1007/s10236-004-0095-6
Tosh CA, de Bruyn PJN, Steyn J, Bornemann H, van den Hoff J, Stewart BS, Plötz J, Bester MN (2015) The importance of seasonal sea-surface height anomalies for foraging juvenile southern elephant seals. Mar Biol 162:2131–2140. https://doi.org/10.1007/s00227-015-2743-4
Tucker AD, MacDonald BD, Seminoff JA (2014) Foraging site fidelity and stable isotope values of loggerhead turtles tracked in the Gulf of Mexico and northwest Caribbean. Mar Ecol Prog Ser 502:267–279. https://doi.org/10.3354/meps10655
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Res Part II 54:211–223. https://doi.org/10.1016/j.dsr2.2006.11.013
Williams GD, Hindell M, Houssais MN, Tamura T, Field IC (2011) Upper ocean stratification and sea ice growth rates during the summer-fall transition, as revealed by Elephant seal foraging in the Adelie Depression, East Antarctica. Ocean Sci 7:185–202. https://doi.org/10.5194/os-7-185-2011
Womble JN, Sigler MF, Willson MF (2009) Linking seasonal distribution patterns with prey availability in a central-place forager, the Steller sea lion. J Biogeogr 36:439–451. https://doi.org/10.1111/j.1365-2699.2007.01873.x
Wotherspoon S, Sumner M, Lisovski S (2016a) Basic data processing for light based geolocation archival tags. https://github.com/SWotherspoon/BAStag/. Accessed 3 Mar 2019
Wotherspoon S, Sumner M, Lisovski S (2016b) Solar/satellite geolocation for animal tracking. https://github.com/SWotherspoon/SGAT. Accessed 3 Mar 2019
Yates O, Black AD, Palavecino P (2007) Site fidelity and behaviour of killer whales (Orcinus orca) at Sea Lion Island in the Southwest Atlantic. Lat Am J Aquat Mamm 6:89–95. https://doi.org/10.5597/lajam00112
Acknowledgements
This study was supported through grants from the Sea World Research and Rescue Foundation, the Holsworth Wildlife and Research Endowment, and the Lirabenda Endowment Fund (issued by the Field Naturalists Society of South Australia). We thank the Department of Environment, Water and Natural Resources for their logistical support. We are also deeply grateful for the volunteers who assisted us in the fieldwork. All animal handling and experimentation were undertaken with the approval from the Primary Industries and Regions South Australia animal ethics committee (application 32-12), Department of Environment, Water and Natural Resources (permit A24684) and the University of Tasmania animal ethics committee (permit A0015176). We thank the reviewers for their valuable feedback that helped to improve this manuscript.
Funding
This study was funded by the Sea World Research and Rescue Foundation (SWR/10/2016), the Holsworth Wildlife and Research Endowment (H0023641), and the Lirabenda Endowment Fund (issued by the Field Naturalists Society of South Australia; no Grant Number).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures, performed in studies involving animals, were in accordance to the ethical standards of the institution or practice at which the studies were conducted (University of Tasmania animal ethics approval permit number: A0015176).
Additional information
Responsible Editor: D.E. Crocker.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by B. Abrahms and undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 2689 kb)
Supplementary file2 (MP4 3543 kb)
Supplementary file3 (MP4 2311 kb)
Supplementary file4 (MP4 3428 kb)
Rights and permissions
About this article
Cite this article
Foo, D., Hindell, M., McMahon, C. et al. Environmental drivers of oceanic foraging site fidelity in central place foragers. Mar Biol 167, 76 (2020). https://doi.org/10.1007/s00227-020-03685-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03685-y