Abstract
Volcanic oceanic archipelagos are fascinating natural laboratories of evolutionary patterns and processes in remote, unique conditions. In the insular marine realm, deepwaters and sea-surface circulation hamper dispersal and, for marine invertebrates, this ability is linked to larval development: planktotrophic organisms disperse easily, whereas non-planktotrophic species usually have restricted ranges. Similarly, bathymetric zonation also influences dispersal: intertidal species are more prone to engage in the process than subtidal/circalittoral species. Therefore, the presence of endemic congeneric non-planktotrophic marine gastropods in two Atlantic archipelagos, hundreds of kilometers apart, inspired a biogeographical hypothesis. It predicts that when two congeneric non-planktotrophic gastropod species, with different bathymetric specific ranges, simultaneously occur and are restricted to two remote archipelagos, the subtidal/circalittoral species is expected to be evolutionarily older than the intertidal species. The present study aims to test this theoretical prediction from a multidisciplinary perspective, with a molecular, Bayesian, fossil-calibrated, phylogenetic analysis of selected Rissoidae species to test the theoretical predictions. We hereby corroborate the earlier speciation of the subtidal/circalittoral Alvania sleursi, compared to the congeneric intertidal Alvania mediolittoralis. Supported by ecological and palaeontological observations in the Azores and Madeira archipelagos, our study provides the first phylogenetic approach to this biogeographical hypothesis, unveiling the evolution of Rissoids in two insular Atlantic systems. In a broader perspective, combining molecular and palaeontological data contributes to better understand past processes that shaped current diversity in Atlantic Archipelagos. This approach can be further replicated in other related non-planktotrophic invertebrates in remote Archipelagos, to corroborate the biogeographical hypothesis in other marine taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Remote volcanic oceanic archipelagos gather ideal conditions to study evolution in ecosystems formed de novo, as life appeared and thrived in unique conditions of isolation (Paulay 1994). The exceptional contexts in which organisms have reached such islands, often located far from potential colonization sources, have intrigued generations of researchers devoted to understand the biogeographic patterns that emerge from marine and terrestrial taxa inhabiting remote insular areas (see Patiño et al. 2017 for a review; Ávila et al. 2018, 2019). Unlike continental taxa that benefit from physical continuity of landmasses for stepwise migration, for marine organisms, the deep waters and the distance between continental landmasses and oceanic islands usually act as barriers for the dispersal of insular young and adult specimens (Ávila 2013).
Dispersal of marine invertebrates
Dispersal ability of marine invertebrates, and consequently its geographical distribution, is directly linked to the duration and mode of larval development, with major consequences at evolutionary and biogeographical levels (Scheltema and Williams 1983; Scheltema 1986a, 1989, 1995; Scheltema et al. 1996). Benthic marine gastropods usually disperse (1) by means of pelagic planktotrophic larvae; (2) by phoresy/attached to bird feathers (common in some intertidal molluscs); or (3) by rafting of egg masses, juveniles or adults of small-sized species, attached to suitable floating materials (Ávila 2006, 2013 and references therein). Accordingly, gastropods with planktotrophic (p) larval development can survive and feed in the water column for extended periods of time, facilitating their dispersal and arrival to remote islands, often maintaining a gene flow rate high enough to prevent speciation of remote, insular populations (García-Talavera 1983; Scheltema and Williams 1983; Scheltema 1986b; Scheltema et al. 1996). Contrastingly, non-planktotrophic (np) gastropods are expected to be geographically confined to smaller areas when compared to planktotrophic ones, as the absence of a free-swimming feeding stage greatly reduces their dispersal capabilities (Scheltema 1986a, 1989, 1995). Therefore, the presence of benthic, non-planktotrophic, shallow-water gastropod species, with a geographical distribution restricted to two remote, oceanic archipelagos (e.g., Azores/Madeira, 840 km apart; see Fig. 1) is not a common feature. One of the authors of this work (R. Cordeiro) compiled and reviewed a database of the 5284 shallow-water (< 50 m depth) gastropod species reported for the entire North Atlantic and adjacent seas (see Supplementary Table S1). Rissoidae (Mollusca: Gastropoda) is one of the most diverse families, with a total of 266 shallow-water species, most of them (~ 77.0%) being np-developers. However, only ten np-rissoid species (3.8%) occur simultaneously and exclusively in two of the Macaronesian Archipelagos (one species in the Azores and Madeira; one in the Azores and Canaries; four in Madeira and Selvagens; one in Madeira and Canaries; and three in Selvagens and Canaries).
Evolutionary history of the family Rissoidae
For decades, the family Rissoidae has been studied to clarify its evolutionary history and relationships among its members. The earlier taxonomic reviews of the family were based mainly on the similarity of conchological characters (Thiele 1929; Wenz 1938; Coan 1964). The classification was subsequently revised by Ponder (1967), with the proposal of four distinct subfamilies. More recently, Ponder (1984) refined the taxonomic position of Rissoidae genera, becoming the most consensual classification scheme for several years by recognizing the subfamilies Rissoinae and Rissoininae. Finally, the phylogenetic status of the superfamily Rissooidea was investigated based on molecular data (16S and 28S; Criscione and Ponder 2013), and the authors proposed the elevation of Rissoininae (sensu Ponder 1984) to the family level. Subsequent work led to the most comprehensive molecular phylogeny of the family Rissoidae to date, based on 16S and 28S markers (Criscione et al. 2017), with a thorough revision of Rissoidae as the former subfamily Rissoinae, excluding the genera Merelina Iredale, 1915 and Lironoba Iredale, 1915. This work provided a new broadly accepted classification scheme for this peculiar family, based on genetic data.
Congeneric non-planktotrophs in remote archipelagos: a theoretical hypothesis
In the Northeast Atlantic Ocean, the evolutionary and biogeographical relationships of shallow-water marine molluscs from volcanic oceanic islands and seamounts are well studied and, particularly, the Azores Archipelago has been considered a model to study the patterns and processes of arrival, colonization, and potential speciation of these species (see Ávila 2005, 2013; Ávila et al. 2008, 2009). Although most np-species are geographically limited to small areas (e.g., the rissoids Alvania angioyi van Aartsen, 1982 endemic to the Azores, and Alvania freitasi Segers, Swinnen & de Prins, 2009 endemic to the Selvagens), some species can occur in more than one archipelago, as mentioned before, or be distributed over large areas as the Northeast Atlantic Ocean [e.g. the intertidal np-rissoid Cingula trifasciata (J. Adams, 1800) has its northern distribution limit in Scandinavia and the southern in Madeira archipelago; Ávila (2005)].
A plausible explanation for these observations would be to consider the speciation of a np-developer in a remote archipelago, where its ancestral first established and later speciated, occupying a suitable, free ecological niche. Through time, the “new” species so far restricted to one remote archipelago would potentially engage in dispersive processes to remote locations (e.g.: another remote archipelago, located far from the area in which the species first appeared) by mediated (e.g., birds) or non-mediated dispersal events. If it successfully established in the new area, a np-species would then have extended its geographical range to more than one remote archipelago. Ávila (2005), and later Ávila et al. (2012), studied in detail the marine benthic microgastropod family Rissoidae in the North Atlantic Ocean, clarifying possible mechanisms for the dispersal of its shallow-water species. A total of 124 rissoids are presently reported from the archipelagos of the Azores, Madeira, Selvagens, Canaries, and Cabo Verde. Of these insular rissoids, the majority (78.9%) possess np-larvae, and their small size (≤ 5 mm) and presence amongst algae, from which they can suspend themselves, make them potential candidates for dispersal by rafting. Thus, when algae are pulled out from the substratum by wave action, intertidal and shallow-water rissoids can be rafted along. However, the initiation of a rafting process does not guarantee that dispersal will be well succeeded, especially in remote volcanic oceanic islands where the distance between the source and sink areas makes the dispersal of young and adult rissoids difficult (Ávila et al. 2012; Ávila 2013).
Ávila (2006, 2013) suggested the addition of a vertical dimension—the bathymetric/ecological zonation—to the considerations of Scheltema on the relationships between modes of larval development and geographical range distribution (Scheltema 1986b, 1989, 1995). Considering rafting as a key mechanism for the dispersal of epibenthic, shallow-water (< 50 m depth), np-species in Atlantic waters, three main assumptions were pointed out by Ávila (2006): (1) intertidal insular species are more prone to be rafted than subtidal/circalittoral species, due to the increased likelihood of being disturbed by natural events that trigger their passive dispersal (see Fig. 2a); (2) bathymetry/ecological zonation and geographical distribution of species are directly correlated, with intertidal species generally having wider ranges than sublittoral species (e.g., 3–6 m depth) and these, in turn, having wider ranges than subtidal/circalittoral species (e.g., 20–30 m depth) (see Fig. 2b); (3) the chances of a successful dispersal are higher for micromolluscs in which the juveniles/adults are the rafting stage, as they are usually associated with algae and remain attached even after the algal substrate is ripped by wave action. Therefore, the smaller the species, the higher the likelihood of dispersal and range expansion, as rafting is more likely to be well succeeded if the species remains attached to the substrate (Ávila 2006).
Graphical illustration of Ávila’s (2013) biogeographical hypothesis. a Bathymetric range/ecological zonation of intertidal (in green) and subtidal/circalittoral species (in blue); sublittoral species (not represented) have an intermediate bathymetric zonation (3–6 m). Intertidal species are more exposed to disturbing agents (e.g., waves); b intertidal species are expected to have a wider geographical distribution (in green) than sublittoral species (in red), which in turn have a wider range than subtidal/circalittoral species (in blue), due to the likelihood of initiating rafting; c when the premises of Ávila’s (2013) theory are granted, the subtidal/circalittoral species is expected to be evolutionarily older than the intertidal species, conceding enough time for both to disperse successfully
This relation was later completed by Ávila (2013) and resulted in the formulation of a new biogeographical hypothesis, with the addition of evolutionary time as the fourth and last variable influencing the dispersal and evolution of shallow-water marine molluscs. Based on the compilation of the geographical distribution of rissoids in the North Atlantic Ocean by Ávila et al. (2012), this relationship was formulated to address the simultaneous existence of two congeneric np-species—Alvania sleursi (Amati, 1987) and A. mediolittoralis Gofas, 1989—in two nearby archipelagos (Azores and Madeira), which differ only in their bathymetric range (subtidal/circalittoral and intertidal, respectively). Following this premise, as both species presently occur in two nearby archipelagos, it is expected that the one inhabiting deeper water is evolutionarily older than the shallow-water/intertidal species (Ávila 2013). Both species may successfully colonize and establish in the same far-away location under this scenario, but the subtidal/circalittoral species inhabits less disturbed environments being less prone to engage dispersal, whereas the intertidal is fustigated by wave action, inducing rafting more often. Thus, we expect the subtidal/circalittoral lineage to have appeared earlier in the geological scale, granting it more time to successfully expand its distributional range to the same extent as the intertidal congeneric (see Fig. 2c).
This biogeographical hypothesis was later supported by ecological and palaeontological studies in Madeira archipelago and in the Azores, where Santa Maria is the oldest (~ 6.01 Ma; Ramalho et al. 2017) and the only island having known exposed marine fossiliferous outcrops. In Santa Maria Island, fossils of the subtidal/circalittoral rissoid A. sleursi were found in Pliocene (4.78 ± 0.13 to 4.13 ± 0.19 Ma; Ramalho et al. 2017) and Late Pleistocene (last interglacial Marine Isotopic Stage 5e—MIS 5e; 130–116 ka; Ávila et al. 2015) outcrops. For the intertidal species A. mediolittoralis, fossilized remains are only observed in Azorean MIS 5e sediments (Ávila et al. 2015), suggesting it is evolutionarily younger. Despite the earlier emergence of Madeira Islands in the Northeast Atlantic Ocean—Porto Santo ~ 18.8 Ma (Mata et al. 2013) and Madeira’s re-emergence ~ 7 Ma (Ramalho et al. 2015)—the fossiliferous outcrops in this archipelago are assigned to only two geological epochs. Reef limestones from the Pliocene are found in Madeira Island, and bioclastic or bio-edified limestones from the Middle Miocene as well as Pleistocene biogenic carbonate sandstone are present in Porto Santo Island (Mata et al. 2013). Nevertheless, specimens of A. mediolittoralis and A. sleursi are not reported for Madeiran fossiliferous outcrops (Gerber et al. 1989; Ávila et al. 2015).
Notwithstanding its relevance, Ávila’s (2013) hypothesis remains untested from a phylogenetic perspective. Therefore, the main aim of our work is to address this evolutionary/biogeographical link between non-planktotrophic larval development, geographic range, bathymetric/ecological zonation and evolutionary time with molecular phylogenetic tools, targeting A. mediolittoralis, A. sleursi and other rissoids presently occurring in the Azores and/or Madeira archipelagos. The family Rissoidae was chosen as a study model because of the current broad knowledge at the geological and ecological levels, but also due to the high number of representatives, susceptibility to rafting and distributional range in Atlantic islands (Ávila et al. 2012). We hereby propose a multidisciplinary, combined approach of molecular phylogenetics and palaeontological data to unveil evolutionary relationships of insular rissoids and to assess the prediction of an earlier evolutionary divergence of the subtidal/circalittoral lineage.
Materials and methods
Taxon sampling and laboratory analyses
Samples of rissoids naturally occurring in the Azores Archipelago—Alvania angioyi, A. formicarum Gofas, 1989, A. mediolittoralis, A. sleursi, Cingula trifasciata, Crisilla postrema (Gofas, 1990), Rissoa guernei Dautzenberg, 1889 and Setia subvaricosa Gofas, 1990—were retrieved from the marine molluscs reference collection of the Department of Biology of the University of the Azores (DBUA), stored in 96% ethanol and collected in several coastal sites of the archipelago. We have also used samples of Pisinna glabrata (von Mühlfeld, 1824) from DBUA as the outgroup in the phylogenetic reconstructions. DNA was extracted from whole individuals with the commercial kit PureLink® Genomic DNA Mini Kit (Invitrogen™), following the manufacturer’s instructions.
Fragments of the mitochondrial gene cytochrome oxidase subunit I (COI) and the nuclear 28S ribosomal RNA (28S rRNA) were amplified by PCR, performed in 25 µl volumes, entailing 3 μl of DNA, − 10 × buffer MgCl2 free, 2.5 mM MgCl2, 0.2 mM dNTP, 10 μM of each primer, 0.1 μg μl−1 bovine serum albumin (BSA, Promega) and 0.3 U platinum Taq DNA polymerase. PCR conditions and primers are provided as Supplementary Material (Table S2). Electrophoretic runs in 2% (w/v) agarose gel with GelRed (DNA fluorescent dye, BioTarget™) were performed to assess the efficiency of the amplification reactions and the quality of the DNA products. PCR product purification and bi-directional Sanger sequencing with amplification primers were realized by a commercial facility—Genewiz, London, UK.
Sequence data
A manual check of misreads in COI and 28S chromatograms was performed with BioEdit v.7.0.5.3 for Windows (Hall 1999). Sequences of the mitochondrial coding COI gene were translated into amino acids with ExPASy Translate Tool (https://web.expasy.org/translate/), allowing to inspect the existence of stop codons and thus the presence of pseudogenes. For the phylogenetic analyses of COI and 28S genes, we have also included sequences of species reported for the Northeast Atlantic Ocean, deposited in GenBank. All the sequences analyzed for the reconstruction of the gene trees are provided in Supplementary Table S3. Sequences of the mitochondrial COI marker were aligned using Clustal Omega algorithm via Web Services by EMBL-EBI (McWilliam et al. 2013), whereas the alignment of 28S sequences was performed using the MAFFT program (Katoh et al. 2005). Poorly aligned positions and divergent regions that often obscure the phylogenetic signal (Xia and Lemey 2009) were removed from the alignment of the nuclear 28S marker resorting to GBlocks Server v0.91b (Castresana 2000).
Phylogenetic analyses of COI and 28S
The software PartitionFinder v1.1.1 (Lanfear et al. 2012) was used to determine the best-fit partitioning schemes and models of molecular evolution to each dataset, according to the Akaike’s Information Criterion (Akaike 1973). Data partitioning by codon was applied to COI gene to minimize saturation effects of codon positions on phylogenetic reconstructions (Salemi 2009) and to account for different rates of evolution of each codon position (Pond et al. 2009). The following models of molecular evolution were applied to COI partitions: HKY + G (1st partition), GTR + G (2nd partition), HKY + I (3rd partition). Bayesian inference (BI) and maximum likelihood (ML) methods were used to reconstruct the Rissoidae phylogeny. For BI analyses, performed in MrBayes v3.2.6 software (Ronquist et al. 2012), two independent runs (each with four chains for 2 × 107 generations) were performed. Trees and parameters were sampled every 1000 generations, with the heating parameter set to 0.25. Stationarity was considered to be reached when the average standard deviation of split frequencies was lower than 0.01. Majority-rule consensus trees were estimated from both analyses, after discarding 25% of the total samples as burn-in. ML analyses in Garli v2.0.1 (Zwickl 2006) were performed with a ten independent search replicates and 1000 bootstrap replicates. The evaluation of log-likelihood values across searches allowed to check the convergence between the topologies of the trees generated. SumTrees program of the DendroPy package (Sukumaran and Holder 2010) was used to summarize non-parametric bootstrap support (BS) values for the best tree, after generating a majority-rule consensus tree. Consensus trees inferred for each molecular marker were visualized and rooted—setting P. glabrata as the outgroup for the COI dataset and Anabathron contabulatum (Frauenfeld, 1867) as the outgroup for the 28S dataset—resorting to FigTree v1.4.3 software.
Species tree reconstruction and estimation of divergence times
Species tree reconstruction can be achieved by distinct phylogenetic methodologies. Maximum likelihood methods with concatenated sequences estimate a single species tree that best fits the combined alignment, according to the phylogenetic likelihood function (Felsenstein 1981; Ogilvie et al. 2017). However, inferences drawn from concatenated datasets have been associated with inconsistent results and low reliability, including high bootstrap support even in incorrectly estimated branches (Gadagkar et al. 2005; Kubatko and Degnan 2007), due to the influence of model misspecification or long-branch attraction (Liu et al. 2015; Ogilvie et al. 2017) and the incomplete lineage sorting phenomenon that causes systematic errors on the estimation of branch lengths and divergence times (Mendes and Hahn 2016; Ogilvie et al. 2016, 2017). Therefore, a fully Bayesian multispecies coalescent method—StarBEAST2 framework (Ogilvie et al. 2017)—was applied to the dataset comprising both COI and 28S genes, as implemented in BEAST v2.4.5 software (Bouckaert et al. 2014), for the inference of the evolution of eight rissoid species and their divergence time. This approach, based on the co-estimation of multi-individual multi-locus sequence data, provides a reliable estimation of the species tree by searching and embedding each gene tree within a single shared species tree that likely best reflects the evolutionary history of the taxa (Ogilvie et al. 2017), thus avoiding the issues associated with concatenated datasets (Mendes and Hahn 2016; Ogilvie et al. 2016). Due to the requirements of the chosen phylogeny reconstruction framework, we have only considered rissoid species represented by two or more individuals (Heled and Drummond 2010), for which sequences of both COI and 28S were available (Table 1): Alvania angioyi, A. formicarum, A. mediolittoralis, A. sleursi, Cingula trifasciata, Crisilla postrema, Rissoa guernei, and Setia subvaricosa Gofas, 1990. According to the currently accepted phylogeny of the superfamily Rissooidea, based on molecular data [sensu Criscione and Ponder (2013)], Pisinna glabrata was selected as an outgroup in the species reconstruction for the same reasons.
The software jModelTest v2.1.10 (Darriba et al. 2012) yielded the following substitution models and parameters as the best fitted to the data analyzed, according to the Akaike’s Information Criterion (Akaike 1973): HKY + I + G model, with gamma shape = 0.6100, proportion of invariants = 0.5500 and kappa = 18.7407 for the COI marker; GTR + I + G model, with gamma shape = 0.4710, proportion of invariants = 0.508, rate AC = 0.3118, rate AG = 2.1462, rate AT = 0.7970, rate CG = 0.7967, rate CT = 6.8066 and rate GT = 1.0000 for the 28S marker.
Four fossil calibration points were assigned to estimate the timing of the split events, according to the presence/absence of the species analyzed in fossiliferous outcrops in Santa Maria Island (Azores), dated from the Pliocene and Pleistocene (MIS 5e). Their attribution followed the recommendations of Forest (2009) and Parham et al. (2012) for species tree reconstructions analyses, being defined as the minimum age of the clades to which they were assigned and according to the most recent and valid literature available for the fossil taxa in question (Meireles et al. 2013; Cordeiro et al. 2015; Ávila et al. 2015, 2016; Ramalho et al. 2017), to ensure the accurate temporal placement of the lineage in the phylogenetic analyses. Alvania sleursi is an extant species, also found in fossiliferous sediments dated back to the Pliocene, meaning that it was already inhabiting Santa Maria shores during those periods. One must assume its speciation prior to the formation of the Pliocene palaeo-environment, otherwise, fossil specimens of A. sleursi would not be present there. In Santa Maria, the ancient habitat where A. sleursi existed was covered by lava during a period of transgressive volcanism, promoting the preservation of the Pliocene fossiliferous outcrop. The lava formation on top of the Pliocene sediments was dated as 4.13 ± 0.19 Ma (Ramalho et al. 2017), which was designated as the minimum age for the divergence among A. sleursi, A. mediolittoralis and A. formicarum as, based on the fossil record, A. sleursi already existed but none of its conspecific-related taxa. On the other hand, considering their presence only in more recent fossil sediments from Pleistocene (MIS 5e) (Cordeiro and Ávila 2015; Ávila et al. 2015, 2016), a minimum age of 0.12 Ma (the estimated age of 116 ka was rounded up to 0.12 Ma) was set up for (1) the split between A. formicarum and A. mediolittoralis; (2) the divergence among C. trifasciata and the endemic species A. angioyi and C. postrema; and (3) the split between R. guernei and S. subvaricosa. Although the low number of fossil calibration points used (n = 4) and their inference from fragmented fossil record from a single island are not considered ideal practices on a species tree reconstruction (Parham et al. 2012), the information to calibrate this inference is only available from the fossiliferous outcrops of Santa Maria Island in the Azores Archipelago.
All this information was manually included in the input file for StarBEAST2 (see Supplementary Material File S4), created using BEAUti package manager. We have tested the use of Uncorrelated Lognormal molecular clocks, as well as using the Calibrated Yule model of speciation, but the analyses did not converge in any of the attempts, reaching ESS (effective sample size) values under 50 in most parameters. For that reason, and after evaluating convergence, we have defined a strict clock for both genes, unlinking the molecular clock and tree priors between the markers, and defined Yule model of speciation for 108 generations, with parameters sampled every 10,000 generations. Burn-in was set for 10%, after graphically checking the achievement of stationarity in Tracer v1.6 software (http://beast.bio.ed.ac.uk/Tracer). The convergence and ESS were also assessed with Tracer v1.6. After discarding the burn-in samples, the final species tree with node ages was plotted with TreeAnnotator v2.4.5 (http://tree.bio.ed.ac.uk/software/beast) and visualized with the FigTree v1.4.3 software (http://tree.bio.ed.ac.uk/software/figtree/). Posterior modifications of the phylogeny were performed resorting to Inkscape v0.92 software (http://www.inkscape.org).
Results
Sequenced data and phylogenetic analyses
All the rissoid sequences obtained in this study were deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/), under the accession numbers: MG652373-87, MG652392-422, and MG652429-31 for the COI sequences; MG663173-83, MG663185-209 for the 28S rRNA marker. Sequences were also obtained from Pisinna glabrata: MG652432-36 (COI) and MH047302-4 (28S rRNA). The alignment of COI marker had a total length of 658 bp, whereas the alignment of 28S marker had a length of 1015 bp after the removal of uninformative gaps (total length of 1074 bp prior to the GBlocks analysis).
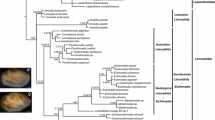
The phylogenetic trees obtained for COI (Fig. 3) and 28S (Fig. 4) displayed similar topologies and acceptable resolution in clarifying systematic positions among Rissoidae taxa. Within each marker, BI and ML analyses produced phylogenetic trees with similar topologies for most relationships, thus only the topology inferred with ML methods is shown (Bayesian Inference gene trees of COI and 28S markers are provided as Supplementary Figures S5 and S6, respectively). Posterior probabilities (PP) tended to be higher than BS values at most nodes, but it is known that PP are typically higher than BS values (Suzuki et al. 2002). The major relationships were concordant between the mtDNA COI (Fig. 3) and nDNA 28S (Fig. 4) phylogenies, as three clades were supported by PP values. Clade A comprised several Alvania spp. in both gene trees (PP 99–100%), whereas in Clade B (PP 96% in the COI phylogeny; PP 88% in the 28S phylogeny) Cingula trifasciata occupied a basal position, closely related to Crisilla postrema and some Alvania spp. Clade C (PP 99% in COI and 28S gene trees) comprised taxa assigned to Pusillina and Rissoa genera, as well as Setia ambigua in the 28S phylogeny. Moreover, the basal phylogenetic position of Setia subvaricosa in relation to the remaining rissoids was supported in the COI gene tree (BS and PP 100%), but for the 28S, its position within Clade C was not supported. In the COI phylogeny, another group comprising Pseudosetia and Onoba genera was also retrieved, but no further considerations were made regarding its phylogenetic position as it was not supported by the analyses. The polyphyly of the genus Alvania was depicted and well-supported in the two markers analyzed.
Maximum likelihood tree obtained for mtDNA COI gene. Values at the nodes correspond to bootstrap support (BS) values and posterior probability (PP). Clades A–C are identified. Accession numbers of sequences are provided after the species designation. Yen (¥) symbol is used to represent support values inferior to 0.7. Colors and names assigned to the clades match between gene trees and species tree
Maximum likelihood tree obtained for nDNA 28S rRNA gene. Values at the nodes correspond to bootstrap support (BS) values and posterior probability (PP). Clades A–C are identified. Accession numbers of sequences are provided after the species designation. Yen (¥) symbol is used to represent support values inferior to 0.7. Colors and names assigned to the clades match between gene trees and species tree
In both phylogenies, A. formicarum, A. mediolittoralis, and A. sleursi were depicted as closely related, with an earlier divergence of the subtidal/circalittoral A. sleursi. In the COI gene tree, the split between A. mediolittoralis and A. formicarum was supported by ML and BI analyses (BS 79%, PP 100%), whereas the older splits were only supported by PP (> 90%). In the 28S phylogeny, splits within Clade A were only supported by PP values (> 85%).
Species tree reconstruction
In the species tree reconstruction using StarBEAST2 framework, the effective sample sizes of every parameter reached values higher than 200, and assessment of parameter statistics in Tracer v1.6 suggested the convergence of the analysis. Posterior probabilities higher than 99% were recovered for all but one node (PP 56.8%, broad credibility interval ranging from 12.82 to 23.17 Ma) (Fig. 5). Confidence intervals were calculated for the age of all the splits inferred and, the older the split event, the lower the associated confidence level.
Rissoidae species tree, estimated according to a fully Bayesian multispecies coalescent method implemented in StarBEAST2 software with datasets of mtDNA COI gene and nDNA 28S rRNA gene. Four calibrations were added to some nodes and represented by a light blue circle. Clades A–C are depicted by different colors; colors and names assigned match between gene trees and species tree. Within Clade A, the colored branches follow the same color scheme as Fig. 2 to represent the ecological zonation of the species: intertidal (green) or subtidal/circalittoral (blue). Posterior probability values are displayed at the nodes and here represented by an asterisk (*) when higher than 99%. Values at the nodes indicate the estimated age of the split event and blue node bars represent 95% of the highest posterior density interval. Numbers within brackets represent the 95% credibility interval associated with the node bars and, thus, split events. The reported geographical distribution of the species is provided under each species name, at the tip of the species tree: eAZO endemic to the Azores, AZO Azores, BRI British Isles, MAD Madeira, POR Portuguese shores, SCA Scandinavia. The appearance of the Azorean Islands is depicted chronologically: SMA Santa Maria, 6.01 Ma (Ramalho et al. 2017); FLW Flores, 2.16 Ma (Azevedo 1998); CVU Corvo, 1.50 Ma (França et al. 2002); SJG São Jorge, 1.32 Ma (Hildenbrand et al. 2014); FAI Faial, 0.85 Ma (Hildenbrand et al. 2014); SMG São Miguel, 0.79 Ma (Sibrant et al. 2015); GRW Graciosa, 0.70 Ma (Sibrant et al. 2014); TER Terceira, 0.40 Ma (Hildenbrand et al. 2014); PIX Pico, 0.27 Ma (Demand et al. 1982)
The species tree reconstruction (Fig. 5) showed early diversification of the studied species during the Early Miocene, ca. 17.52 Ma, resulting in the separation of the lineage that originated Rissoa and Setia genera and the one that leads to the speciation of Alvania, Cingula and Crisilla genera. Curiously, A. angioyi was depicted as more closely related to Crisilla postrema and Cingula trifasciata than to other Alvania species. Three major clades can be distinguished in the species tree: (1) Clade A estimated the first diversification of three species during the Middle Pliocene (ca. 4.34 Ma), by splitting Alvania sleursi and the lineage that later (ca. 360 ka, during the Pleistocene) originated A. formicarum and A. mediolittoralis. As both splits were calibrated, narrow Highest Posterior Density (HPD) intervals were estimated for both split events providing high confidence in this inference: credibility interval of 0.73 Ma for the oldest event and ranging from 0.12 to 0.61 Ma in the youngest; (2) Clade B comprised a first split event (calibrated) during the Late Miocene (ca. 7.67 Ma) between C. trifasciata and the ancestor of A. angioyi and C. postrema, and a divergence between these two taxa around 2.5 Ma later. Both estimations were associated with a low degree of uncertainty, with credibility intervals ranging from 5.04 to 10.12 Ma for the older split, and between 3.5 and 7.33 Ma for the most recent event; and (3) Clade C showed the split between Rissoa guernei and Setia subvaricosa sometime between 20.48 and 5.14 Ma, which decreased the credibility of this inference.
Discussion
Phylogenetic analyses of COI and 28S markers
The phylogenetic analyses performed for Rissoidae taxa supported the monophyly of the family Rissoidae. The genus Alvania was shown to be polyphyletic in both phylogenies, with species assigned clades A and B and in accordance with previous work by Criscione et al. (2017). Within Clade A, we highlight the relationship established between the intertidal A. mediolittoralis and the subtidal/circalittoral A. sleursi, highly supported in both ML and BI analyses (BS and PP > 75% in COI gene tree; PP 99% in 28S gene tree). The systematic position of A. sleursi, currently reported for the Azores and Madeira archipelagos, in a molecular phylogeny of the family Rissoidae had already been hypothesized by Ávila (2013) as expected to occupy a basal position in relation to A. mediolittoralis. This hypothetical systematic position of A. sleursi was corroborated by both COI and 28S phylogenies.
Within Clade B, A. punctura and the Azorean endemic A. angioyi were positioned as sister species in the COI dataset, whereas the endemic taxon was depicted as closely related to the subclade containing A. discors, A. lanciae, A. lineata and A. scabra in the 28S phylogeny. Some controversy is attributed to the classification of the latter four species as Alvania, as they form a lineage distantly related to other Alvania spp. Although no formal attempt has been made to reclassify it, we support Criscione et al.’s (2017) suggestion of revision as Alvaniella Sacco, 1895 or Alvanolira Nordsieck, 1972. On the other hand, Crisilla postrema, currently found in relatively shallow water of the Azores and Madeira (Cordeiro et al. 2015), was positioned as sister species to the Azorean endemic A. angioyi. The type species of Crisilla—C. semistriata (Montagu, 1808)—was considered a subgenus of Alvania for decades due to morphological similarities (Ponder 1984; Gofas 1990). Although poorly supported in the phylogenetic analyses, there are affinities between Crisilla postrema and Alvania angioyi that might call for the need to review their classification. Clade C comprises Pusillina spp., Rissoa spp. and Setia spp., which is concordant with the relationships within Clade E retrieved by Criscione et al. (2017). The phylogenetic position of Setia ambigua suggests it to be more closely related to Rissoa spp. than to the congeneric Azorean endemic S. subvaricosa. Morphological and molecular distinction of S. ambigua relatively to other Setia spp., displaying accentuated differences in the shell features, suggests the need of a taxonomic revision of this taxon, as already stated by Criscione et al. (2017).
Evolutionary history of the Azorean Rissoidae species
The species tree obtained in this study revealed that the lineage that later originated Alvania angioyi, A. formicarum, A. mediolittoralis, A. sleursi, Cingula trifasciata, Crisilla postrema, Rissoa guernei, and Setia subvaricosa, first started to diversify during the Early Miocene, ca. 17.52 Ma. However, this estimate is not reliable as the low support for this node (PP 56.8%) broadens the credibility interval to ages ranging from 12.82 to 23.17 Ma. Rissoidae-like organisms are known since the Lower Jurassic (Conti et al. 1993; Criscione and Ponder 2013; Criscione et al. 2017) and some genera were already diversified in the Central Paratethys during the Miocene (Kowalke and Harzhauser 2004), thus, despite not being able to assertively define an age for this divergence event, we believe it is possible that the divergence of these lineages occurred sometime within the estimated range, as it agrees with the fossil record of rissoids worldwide. The concordance in the typology of the species tree inferred (Fig. 5) and the gene trees (Figs. 3, 4), all revealing a clade comprising A. sleursi, A. mediolittoralis and A. formicarum with an earlier divergence of the subtidal/circalittoral A. sleursi, increases the confidence in its phylogenetic position among the rissoids.
Different confidence intervals were retrieved for the several splits inferred. Large credibility intervals (e.g., Clade C) obscure the estimation of a trustworthy age for the split event analyzed, turning inferences regarding the taxonomic position and temporal range of existence of the species involved quite dubious. Even when calibrated, older splits were associated with lower confidence levels. This scenario might reflect a discrepancy between the placement of the calibration point and its true position in the phylogeny, as the further the calibration point is assigned relative to the node of interest, the greater the uncertainty associated with the estimations (Forest 2009). As this work constitutes a phylogenetic study, split events reflect the timing of divergence of the lineages that later originated modern assemblages and not the time of arrival of rissoids or their ancestors to the Azores Archipelago’s vicinity.
Nevertheless, the divergence dates and relationships proposed by the species’ tree obtained seem reasonable based on the worldwide fossil record of the family Rissoidae, the geological history of the Azores Archipelago, and the evolutionary history revealed by the main patterns retrieved from mtDNA COI and nDNA 28S phylogenies (see Figs. 3, 4). As expected, the relationships inferred in the gene trees are, sometimes, not concordant among phylogenies (e.g., in the COI phylogeny Setia subvaricosa is not included in the clade comprising Rissoa guernei, but it is in the 28S gene tree). Nuclear and mitochondrial molecules have different evolutionary rates, which influence the evolutionary history inferred by a gene tree (Degnan and Rosenberg 2006). Particularly, due to its faster evolutionary rate, mitochondrial markers are expected to show higher saturation levels than the nuclear genes and will, therefore, obscure the estimations of older relationships by overestimating them. In salamanders, the overestimation of the divergence events by mitochondrial markers has reached levels of 3–10 times for recent events and 2–3 times for ancient ones, when compared with the nuclear markers (Zheng et al. 2011), whereas in a study of ray-fishes it ranged from 1 to 1.5 times (Hurley et al. 2007). Overestimation is not exclusive to mitochondrial markers, as it also occurs in nuclear genes, which require longer times to coalesce. Nevertheless, these biases are particularly misleading in cases of few calibration points and studies of deep phylogenies in higher taxonomic levels, where the time span is wide and the lineages are considerably old (Zheng et al. 2011).
Due to stochastic events during speciation, discordance between the topologies of the gene trees and the species tree is expected under the coalescent model, especially when assuming the most frequently observed gene tree typology as an estimate of the species tree (Degnan and Rosenberg 2006; Kubatko and Degnan 2007). Reasons pointed out as responsible for the discordance between gene trees and species trees include horizontal transfer, incomplete lineage sorting, and gene duplication and extinction (Maddison 1997). The larger the time between speciation events, associated with longer internal branches in the species tree, and the older the species origins reduce the chances for gene species tree discord. However, in the case of recent diversifications, the time elapsed is insufficient to grant the fixation of gene lineages by genetic drift, and discordance between gene and species tree will arise due to deep coalescence of gene lineages from non-closely related species (Knowles 2009). Nevertheless, when comparing the major patterns of the species tree with the ones inferred in the gene trees, the Clades A–C are found in the three phylogenies despite some differences in the relationships within. To avoid the incorrect use of gene trees as a proxy for species phylogeny and to obtain a more reliable estimation of the “true” topology of the rissoid’s phylogeny, we opted to apply StarBEAST2 framework, which allows an integrated analysis of each marker’s evolution and embeds each tree within a most likely single shared species tree. With this approach, we avoided the interference of biasing phenomena such as overestimation by mitochondrial markers and incomplete lineage sorting (Ogilvie et al. 2017). This constitutes the first time that a species tree is obtained for rissoids in the Azores, as well as the first approach to its evolution in an integrated perspective that includes clues about past environments, given by the palaeontological record.
Testing Ávila’s (2013) biogeographical hypothesis
Throughout this study, Ávila’s (2013) theory was tested by the reconstruction of the evolutionary history of eight Rissoidae species (Fig. 5), including the two species which observations encouraged the early formulation of the idea. Within the species tree inferred, Clade A must be emphasized to test this hypothesis, as it comprises A. mediolittoralis and A. sleursi, two np-species currently found in the Azores and Madeira archipelagos. Nowadays, A. mediolittoralis is commonly found in the intertidal algal turf, with much rarer reports from depths of up to 24 m (Ávila 2005, 2013; Cordeiro et al. 2015), whereas A. sleursi frequently occurs in deeper waters from 15 to 25 m down to 40 m depth (Ávila 2013; Cordeiro et al. 2015).
The inferred species tree indicates that the divergence between the lineages that originated A. sleursi and the shallow-water species occurred 4.8–4.13 Ma ago. By this time, the volcanic edifice of Santa Maria Island (Azores), which first emerged at ~ 6.01 Ma (Ramalho et al. 2017), is believed to have been under a period of waning volcanism, erosion, and subsidence leading to the formation of a truncated, top-flat seamount (Ramalho et al. 2017). Thus, during this period, Santa Maria’s edifice became a shallow-water sandy shoal where volcanic activity was rare, mostly submarine (Ramalho et al. 2017). A wide variety of marine environments existed in this guyot, yielding diverse organisms typical from open-water marine environments (see Uchman et al. 2018 and references therein) and benthic species from shallower waters, including rissoids and other molluscs (Ávila et al. 2015).
According to Ávila’s (2013) hypothesis, subtidal/circalittoral species would be less prone to be rafted than intertidal ones, which would be expected to have also wider geographical distributions. Despite these predictions, both species are currently found in the same geographical areas—they are common species in all the islands of the Azores and are reported for the coastal areas of the Madeira Islands (Cordeiro et al. 2015). The palaeontological record of both archipelagos holds valuable clues to interpret the results of the phylogenetic approach used in this study and better understand the current distribution of the non-planktotrophic A. mediolittoralis and A. sleursi.
The finding of fossilized specimens of A. sleursi (Meireles et al. 2013) on Pliocene sediments (4.78 ± 0.135 to 4.13 ± 0.19 Ma; Ramalho et al. 2017) of Santa Maria Island suggests that the divergence of the lineages encompassed within Clade A occurred earlier than the fossilization event, and that, by that time, viable populations of A. sleursi were already established in the Azores. As all the species assigned to Clade A are endemic to one (the Azores) or two (the Azores and Madeira) Atlantic Archipelagos and are not found in fossiliferous outcrops outside of the Azores, it is plausible to assume that they do not constitute relicts of a former wider distribution. Therefore, and considering the climatic and oceanographic conditions of the last 5 Ma, we hypothesize that this divergence occurred in the Azores vicinities, where the ancient lineage diversified in, at least, three species—A. sleursi, A. mediolittoralis and A. formicarum—that established in the Azores and have only recently dispersed to Madeira. The finding of A. sleursi representatives also in MIS 5e sediments, with an age of 130–116 ka (Ávila 2005; Cordeiro and Ávila 2015; Ávila et al. 2015, 2016) suggests that this subtidal/circalittoral rissoid has been inhabiting this region for a considerable time, where it supposedly established long before A. mediolittoralis speciated. The posterior divergence of lineages that originated A. formicarum and A. mediolittoralis is estimated to have taken place ca. 360 ka ago, during the Pleistocene. Considering that representatives of both species are found in MIS 5e highstand deposits of Santa Maria Island (Ávila et al. 2009, 2015, 2016), the age estimated for the split event leading to their speciation can be accepted. The divergence is also believed to have happened in the Azores Archipelago, as A. formicarum is endemic to this region, with no knowledge of previous wider distribution (Cordeiro et al. 2015).
Despite the older age of Porto Santo Island, its Miocene fossiliferous outcrops, mainly composed by massive coral reefs, rhodoliths, echinoderms and some molluscs (Mata et al. 2013), are similar in taxonomic composition to Santa Maria’s Pliocene outcrops (Ávila et al. 2016 and references therein), with the exception of the massive coral reefs, which are only represented at Santa Maria by small, solitary corals. Although efforts to evaluate the palaeontological record of Madeira Archipelago have been made, there are still no reports of fossilized A. mediolittoralis and A. sleursi in Madeiran region (Gerber et al. 1989; Ávila et al. 2015). This absence suggests that these molluscs were still not present in the latter archipelago during the Pliocene or Pleistocene, when they had already established in the Azores (Ávila et al. 2015, 2016), having reached the Madeira Archipelago later. Therefore, based on the evolutionary history inferred and data provided by ecological and palaeontological observations, we propose early speciation within the Azores Archipelago’s vicinity, followed by their dispersal to Madeira.
From an evolutionary perspective, this phylogenetic approach allowed us to test if the subtidal/circalittoral species was indeed longer lived than the intertidal species. Clade A revealed that the oldest split event was the one leading to the divergence of A. sleursi, recently followed by the speciation of the intertidal A. mediolittoralis during the Pleistocene. The early speciation of the subtidal/circalittoral species provided enough time for individuals to engage dispersal, even though less prone to do so, settle and successfully colonize another archipelago, and thus, to widen its geographical range. For the first time, Ávila’s theoretical biogeographical hypothesis, proposed to explain the simultaneous existence of two congeneric np-species with different bathymetric ranges in two nearby archipelagos, is hereby corroborated with genetic data from rissoids, the marine mollusc gastropods that inspired its formulation. Observations in distinct scientific fields—phylogenetics, paleontology and ecology—contribute to increase the robustness of its theory. Further studies involving other marine invertebrates with similar biogeographical patterns should be addressed to further sustain Ávila’s (2013) hypothesis.
Conclusions
Out of 266 shallow-water (< 50 m depth) rissoid species reported for the entire North Atlantic and adjacent seas, a total of 12 rissoid species are reported simultaneously and exclusively for three archipelagos (Madeira, Selvagens, and the Canaries). This shows that the dispersal of formerly endemic species to one of the archipelagos, albeit infrequent, is probably a more common event than previously thought. A consequence of this work is the prediction that, for marine species having similar ecological traits (ecological zonation depths, mode of larval development, habitat), those having wider geographical ranges are expected to be older (in an evolutionary sense) in comparison with species that occur in only two, or are restricted to just one, archipelagos.
Extending and testing of this biogeographical hypothesis to other marine phyla will impact our current knowledge of inter-archipelagic patterns and processes of dispersal, as well as the evolutionary times of speciation of shallow-water marine organisms living in remote volcanic oceanic islands, thus providing a more complete and robust understanding for recent marine island biogeography theory (see Ávila et al. 2018, 2019).
Data availability
All the mitochondrial COI and nuclear 28S sequences analyzed during the current study are deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/). COI sequences are deposited in GenBank with the accession numbers: MG652373-8 (Alvania angioyi), MG652379-80 (A. formicarum), MG652381-5 (A. mediolittoralis), MG652386-7 (A. sleursi), MG652395-407 (Cingula trifasciata), MG652392-4 (Crisilla postrema), MG652408-13 and MG652419-21 (Rissoa guernei), MG652414-8 and MG652422 (R. cf. guernei), MG652429-31 (Setia subvaricosa), and MG652432-36 (Pisinna glabrata). Sequences of 28S rRNA gene are available under the following accession numbers: MG663173-8 (A. angioyi), MG663179 (A. formicarum), MG663180-1 (A. mediolittoralis), MG663182-3 (A. sleursi), MG663189-96 (C. trifasciata), MG663185-8 (C. postrema), MG663197-203, MG663207 and MG663209 (R. guernei), MG663204-6 and MG663208 (R. cf. guernei), MG663215-6 (S. subvaricosa), and MH047302-4 (P. glabrata).
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Proceedings of the 2nd international symposium on information theory. Akademiai Kiado, Budapest, pp 267–281
Ávila SP (2005) Processos e padrões de dispersão e colonização nos Rissoidae (Mollusca: Gastropoda) dos Açores. Dissertation, University of the Azores, Ponta Delgada
Ávila SP (2006) Oceanic islands, rafting, geographical range and bathymetry: a neglected relationship? Occas Publ Irish Biogeogr Soc 9:22–39
Ávila SP (2013) Unravelling the patterns and processes of evolution of marine life in oceanic islands: a global framework. In: Fernández-Palacios JM, Nascimento L, Hernández J, Clemente S, González A, Díaz-González JP (eds) Climate change perspectives from the atlantic: past, present and future. Universidad de La Laguna, Tenerife, pp 95–125
Ávila SP, Madeira P, Mendes N, Rebelo A, Medeiros A, Gomes C, García-Talavera F, da Silva CM, Cachão M, Hillaire-Marcel C, Martins AMF (2008) Mass extinctions in the Azores during the last glaciation: fact or myth? J Biogeogr 35:1123–1129. https://doi.org/10.1111/j.1365-2699.2008.01881.x
Ávila SP, da Silva CM, Schiebel R, Cecca F, Backeljau T, Martins AMF (2009) How did they get here? Palaeobiogeography of the Pleistocene marine Molluscs of the Azores. Bull la Soc Géologique la Fr 180:295–307. https://doi.org/10.2113/gssgfbull.180.4.295
Ávila SP, Goud J, Martins AMF (2012) Patterns of diversity of the Rissoidae (Mollusca: Gastropoda) in the Atlantic and the Mediterranean Region. Sci World J. https://doi.org/10.1100/2012/164890 (Article ID:164890)
Ávila SP, Melo C, Silva L, Ramalho RS, Quartau R, Hipólito A, Cordeiro R, Rebelo AC, Madeira P, Rovere A, Hearty PJ, Henriques D, da Silva CM, Martins AMDF, Zazo C (2015) A review of the MIS 5e highstand deposits from Santa Maria Island (Azores, NE Atlantic): palaeobiodiversity, palaeoecology and palaeobiogeography. Quat Sci Rev 114:126–148. https://doi.org/10.1016/j.quascirev.2015.02.012
Ávila SP, Cachão M, Ramalho RS, Botelho AZ, Madeira P, Rebelo AC, Cordeiro R, Melo C, Hipólito A, Ventura MA, Lipps JH (2016) The palaeontological heritage of Santa Maria Island (Azores: NE Atlantic): a re-evaluation of Geosites in GeoPark Azores and their use in Geotourism. Geoheritage 8:155–171. https://doi.org/10.1007/s12371-015-0148-x
Ávila SP, Cordeiro R, Madeira P, Silva L, Medeiros A, Rebelo AC, Melo C, Neto AI, Haroun R, Monteiro A, Rijsdijk K, Johnson ME (2018) Global change impacts on large-scale biogeographic patterns of marine organisms on Atlantic oceanic islands. Mar Pollut Bull 126:101–112. https://doi.org/10.1016/j.marpolbul.2017.10.087
Ávila SP, Melo C, Sá N, Quartau R, Rijsdijk K, Ramalho RS, Berning B, Cordeiro R, de Sá NC, Pimentel A, Baptista L, Medeiros A, Gil A, Johnson ME (2019) Towards a “sea-level sensitive marine island biogeography” model: the impact of glacio-eustatic oscillations in global marine island biogeographic patterns. Biol Rev. https://doi.org/10.1111/brv.12492
Azevedo J (1998) Geologia e Hidrogeologia da Ilha das Flores (Açores-Portugal). Dissertation, University of Coimbra, Coimbra
Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol 10:e1003537. https://doi.org/10.1371/journal.pcbi.1003537
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552. https://doi.org/10.1093/oxfordjournals.molbev.a026334
Coan E (1964) A proposed revision of the Rissoacean families Rissoidae, Rissoinidae, and Cingulopsidae (Mollusca: Gastropoda). Veliger 6:164–171
Conti MA, Monari S, Oliverio M (1993) Early rissoid gastropods from the Jurassic of Italy: the meaning of first appearances. Scr Geol 2:67–74
Cordeiro R, Ávila SP (2015) New species of Rissoidae (Mollusca, Gastropoda) from the Archipelago of the Azores (northeast Atlantic) with an updated regional checklist for the family. Zookeys 480:1–19. https://doi.org/10.3897/zookeys.480.8599
Cordeiro R, Borges JP, Martins AMF, Ávila SP (2015) Checklist of the littoral gastropods (Mollusca Gastropoda) from the Archipelago of the Azores (NE Atlantic). Biodivers J 6:855–900
Criscione F, Ponder WF (2013) A phylogenetic analysis of Rissooidean and Cingulopsoidean families (Gastropoda: Caenogastropoda). Mol Phylogenet Evol 66:1075–1082. https://doi.org/10.1016/j.ympev.2012.11.026
Criscione F, Ponder WF, Köhler F, Takano T, Kano Y (2017) A molecular phylogeny of Rissoidae (Caenogastropoda: Rissooidea) allows testing the diagnostic utility of morphological traits. Zool J Linn Soc 179:23–40. https://doi.org/10.1111/zoj.12447
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Degnan JH, Rosenberg NA (2006) Discordance of species trees with their most likely gene trees. PLoS Genet 2:762–768. https://doi.org/10.1371/journal.pgen.0020068
Demand J, Fabriol R, Gerard F, Lundt F, Chovelon P (1982) Prospection géothermique, íles de Faial et de Pico (Açores). Rapport géologique, geochimique et gravimétrique. Technical Report BRGM 82 SGN 003 GTH
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376. https://doi.org/10.1007/BF01734359
Forest F (2009) Calibrating the tree of life: fossils, molecules and evolutionary timescales. Ann Bot 104:789–794. https://doi.org/10.1093/aob/mcp192
França Z, Nunes J, Cruz J, Duarte J, Forjaz V (2002) Preliminary study of the Corvo Island volcanism, Azores. 3o Assembleia Luso-Espanhola de Geodesia e Geofísica S09:727–730
Gadagkar S, Rosenberg MS, Kumar S (2005) Inferring species Phylogenies from multiple genes: concatenated sequence tree versus consensus gene tree. J Exp Zool 304B:64–74. https://doi.org/10.1002/jez.b.21026
García-Talavera F (1983) Los moluscos gasterópodos anfiatlánticos: estudio paleo y biogeográfico de las especies bentónicas litorales. Universidad de La Laguna, La Laguna
GEBCO (2008) The GEBCO_2008 Grid, version 20100927. http://www.gebco.net
Gerber J, Hemmen J, Groh K (1989) Eine pleistozäne marine Molluskenfauna on Porto Santo (Madeira-Archipel). Mitt dtsch malakozool Ges 44:19–30
Gofas S (1990) The littoral Rissoidae and Anabathridae of São Miguel, Azores. In: Martins AMF (ed) The marine fauna and flora of the azores. (Proceedings of the First International Workshop of Malacology, Vila Franca Do Campo, São Miguel, Azores). Açoreana, Supplement 2, pp 97–134
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Heled J, Drummond AJ (2010) Bayesian inference of species trees from multilocus data. Mol Biol Evol 27(3):570–580. https://doi.org/10.1093/molbev/msp274
Hildenbrand A, Weis D, Madureira P, Marques F (2014) Recent plate re-organization at the Azores Triple Junction: evidence from combined geochemical and geochronological data on Faial, S. Jorge and Terceira volcanic islands. Lithos 210–211:27–39. https://doi.org/10.1016/j.lithos.2014.09.009
Hurley I, Mueller R, Dunn K, Schmidt E, Friedman M, Ho R, Prince V, Yang Z, Thomas M, Coates M (2007) A new time-scale for ray-finned fish evolution. Proc R Soc B 274:489–498. https://doi.org/10.1098/rspb.2006.3749
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518. https://doi.org/10.1093/nar/gki198
Knowles LL (2009) Estimating species trees: methods of phylogenetic analysis when there is incongruence across genes. Syst Biol 58:463–467. https://doi.org/10.1093/sysbio/syp061
Kowalke T, Harzhauser M (2004) Early ontogeny and palaeoecology of the Mid-Miocene rissoid gastropods of the Central Paratethys. Acta Palaeontol Pol 49:111–134
Kubatko LS, Degnan JH (2007) Inconsistency of phylogenetic estimates from concatenated data under coalescence. Syst Biol 56:17–24. https://doi.org/10.1080/10635150601146041
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1675–1701
Liu L, Xi Z, Wu S, Davis CC, Edwards SV (2015) Estimating phylogenetic trees from genome-scale data. Ann N Y Acad Sci 1360:36–53. https://doi.org/10.1111/nyas.12747
Maddison WP (1997) Gene trees in species trees. Syst Biol 46:523–536. https://doi.org/10.1093/sysbio/46.3.523
Mata J, Fonseca PE, Prada S, Rodrigues D, Martins S, Ramalho RS, Madeira J, Cachão M, da Silva CM, Matias MJ (2013) O Arquipélago da Madeira. In: Dias R, Araújo A, Terrinha P, Kullberg JC (eds) Geologia de Portugal, 1st edn. Escolar Editora, Lisboa, pp 691–746
McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R (2013) Analysis tool web services from the EMBL-EBI. Nucleic Acids Res 41:W597–W600
Meireles RP, Quartau R, Ramalho RS, Rebelo AC, Madeira J, Zanon V, Ávila SP (2013) Depositional processes on oceanic island shelves—evidence from storm-generated Neogene deposits from the mid-North Atlantic. Sedimentology 60:1769–1785. https://doi.org/10.1111/sed.12055
Mendes FK, Hahn MW (2016) Gene tree discordance causes apparent substitution rate variation. Syst Biol 65:711–721. https://doi.org/10.1093/sysbio/syw018
Ogilvie HA, Heled J, Xie D, Drummond AJ (2016) Computational performance and statistical accuracy of ∗BEAST and comparisons with other methods. Syst Biol 65:381–396. https://doi.org/10.1093/sysbio/syv118
Ogilvie HA, Bouckaert RR, Drummond AJ (2017) StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Mol Biol Evol 34:2101–2114. https://doi.org/10.1093/molbev/msx126
Parham JF, Donoghue PCJ, Bell CJ, Calway TD, Head JJ, Holroyd PA, Inoue JG, Irmis RB, Joyce WG, Ksepka DT, Patané JSL, Smith ND, Tarver JE, Van Tuinen M, Yang Z, Angielczyk KD, Greenwood JM, Hipsley CA, Jacobs L, Makovicky PJ, Müller J, Smith KT, Theodor JM, Warnock RCM, Benton MJ (2012) Best practices for justifying fossil calibrations. Syst Biol 61:346–359. https://doi.org/10.1093/sysbio/syr107
Patiño J, Whittaker RJ, Borges PAV, Fernández-Palacios JM, Ah-Peng C, Araújo MB, Ávila SP, Cardoso P, Cornuault J, de Boer EJ, de Nascimento L, Gil A, González-Castro A, Gruner DS, Heleno R, Hortal J, Illera JC, Kaiser-Bunbury CN, Matthews TJ, Papadopoulou A, Pettorelli N, Price JP, Santos AMC, Steinbauer MJ, Triantis KA, Valente L, Vargas P, Weigelt P, Emerson BC (2017) A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J Biogeogr 44:963–983. https://doi.org/10.1111/jbi.12986
Paulay G (1994) Biodiversity on oceanic islands: its origin and extinction. Integr Comp Biol 34:134–144. https://doi.org/10.1093/icb/34.1.134
Pond SLK, Poon AFY, Frost SDW (2009) Estimating selection pressures on alignments of coding sequences. In: Lemey P, Salemi M, Vandamme A (eds) The phylogenetic handbook: a practical approach to phylogenetic analysis and hypothesis, 2nd edn. Cambridge University Press, Cambridge, pp 419–451
Ponder WF (1967) Classification of Rissoidae and Orbitestellidae with descriptions of some new taxa. Trans R Soc N Zeal Zool 9:193–224
Ponder WF (1984) A review of the Genera of the Rissoidae (Mollusca: Mesogastropoda: Rissoacea). Rec Aust Museum Suppl 4:1–221. https://doi.org/10.3853/j.0812-7387.4.1985.100
Portuguese Hydrographic Institute (2014) World shoreline. https://www.hidrografico.pt/recursos/files/download_gratuito/Linha_costa_internacional.zip
Ramalho RS, da Silveira AB, Fonseca PE, Madeira J, Cosca M, Cachão M, Fonseca MM, Prada SN (2015) The emergence of volcanic oceanic islands on a slow-moving plate: the example of Madeira Island, NE Atlantic. Geochem Geophys GeosyS 16:522–537. https://doi.org/10.1002/2014GC005657
Ramalho RS, Helffrich G, Madeira J, Cosca M, Thomas C, Quartau R, Hipólito A, Rovere A, Hearty PJ, Ávila SP (2017) Emergence and evolution of Santa Maria Island (Azores)—the conundrum of uplifted islands revisited. Geol Soc Am Bull 129:372–391. https://doi.org/10.1130/B31538.1
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Salemi M (2009) Genetic distances and nucleotide substitution models. In: Lemey P, Salemi M, Vandamme A (eds) The phylogenetic handbook: a practical approach to phylogenetic analysis and hypothesis, 2nd edn. Cambridge University Press, Cambridge, pp 126–140
Scheltema RS (1986a) Long-distance dispersal by planktonic larvae of shoal-water benthic invertebrates among central Pacific islands. Bull Mar Sci 39:241–256
Scheltema RS (1986b) On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bull Mar Sci 39:290–322
Scheltema RS (1989) Planktonic and non-planktonic development among prosobranch gastropods and its relationship to the geographic range of species. In: Ryland JS, Tyles PA (eds) Reproduction, genetics and distribution of marine organisms. Olsen and Olsen, Fredensborg, pp 183–188
Scheltema RS (1995) The relevance of passive dispersal for the biogeography of Caribbean mollusks. Am Malacol Bull 11:99–115
Scheltema RS, Williams IP (1983) Long-distance dispersal of planktonic larvae and the biogeography and evolution of some Polynesian and western Pacific mollusks. Bull Mar Sci 33:545–565
Scheltema RS, Williams IP, Lobel PS (1996) Retention around and long-distance dispersal between oceanic islands by planktonic larvae of benthic gastropod Mollusca. Am Malacol Bull 12:67–75
Sibrant A, Marques F, Hildenbrand A (2014) Construction and destruction of a volcanic island developed inside an oceanic rift: Graciosa Island, Terceira Rift, Azores. J Volcanol Geotherm Res 284:32–45. https://doi.org/10.1016/j.jvolgeores.2014.07.014
Sibrant A, Hildenbrand A, Marques F, Weiss B, Boulesteix T, Hübscher C, Lüdmann T, Costa A, Catalão J (2015) Morpho-structural evolution of a volcanic island developed inside an active oceanic rift: S. Miguel Island (Terceira Rift, Azores). J Volcanol Geotherm Res 301:90–106. https://doi.org/10.1016/j.jvolgeores.2015.04.011
Sukumaran J, Holder M (2010) DendroPy: a Python library for phylogenetic computing. Bioinformatics 26:1569–1571
Suzuki Y, Glazko GV, Nei M (2002) Overcredibility of molecular phylogenies obtained by Bayesian phylogenetics. Proc Natl Acad Sci USA 99:16138–16143. https://doi.org/10.1073/pnas.212646199
Thiele J (1929) Handbuch der Systematischen Weichtierkunde. Gustav Fischer, Jena
Uchman A, Torres P, Johnson ME, Berning B, Ramalho RS, Rebelo AC, Melo CS, Baptista L, Madeira P, Cordeiro R, Ávila SP (2018) Feeding traces of recent ray fish and occurrences of the trace fossil Piscichnus waitemata from the Pliocene of Santa Maria Island, Azores (Northeast Atlantic). Palaios 33:361–375. https://doi.org/10.2110/palo.2018.027
Wenz W (1938) Gastropoda. Teil 1, Allgemeiner Teil und Prosobranchia. In: Schindewolfe OH (ed) Handbuch der Paläozoologie, vol 6. Gebrüer Bornträger, Berlin, pp 1–231
Xia X, Lemey P (2009) Assessing substitution saturation with DAMBE. In: Lemey P, Salemi M, Vandamme A (eds) The phylogenetic handbook: a practical approach to phylogenetic analysis and hypothesis. Cambridge University Press, Cambridge, pp 615–630
Zheng Y, Peng R, Kuro-o M, Zeng Z (2011) Exploring patterns and extent of bias in estimating divergence time from mitochondrial DNA sequence data in a particular lineage: a case study of Salamanders (Order Caudata). Mol Biol Evol 28:2521–2535. https://doi.org/10.1093/molbev/msr072
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. University of Texas, Austin
Acknowledgements
This work was supported by Fundação para a Ciência e Tecnologia, IP (Grant number SFRH/BD/135918/2018 to L.B.; research contract IF/00465/2015 to S.P.A.); by Fundo Regional para a Ciência e Tecnologia (Grant number M3.1.a/F/100/2015 to C.S.M.); by FEDER funds through the Operational Programme for Competitiveness Factors—COMPETE and national funds through Fundação para a Ciência e Tecnologia, IP (projects UID/BIA/50027/2013, POCI-01-0145-FEDER-006821); by regional funds through Direção Regional para a Ciência e Tecnologia (DRCT-M1.1.a/005/Funcionamento-C-/2016); and by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF) under project MarInfo (NORTE-01-0145-FEDER-000031). This work was also supported by FEDER funds (in 85%) and by funds of the Regional Government of the Azores (15%) through Programa Operacional Açores 2020, in the scope of the project “AZORESBIOPORTAL—PORBIOTA”: ACORES-01-0145-FEDER-000072. We thank the reviewers for comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The sequences used in the molecular phylogenies of COI and 28S were mainly retrieved from the GenBank database. The new sequences were obtained from specimens deposited in the Marine Molluscs Reference Collection of the Department of Biology of the University of the Azores (DBUA). Sampling was not performed for this work. All applicable national and/or institutional guidelines for the use of collection material were followed. Only invertebrates were used in this study.
Additional information
Responsible Editor: T. Reusch.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baptista, L., Santos, A.M., Cabezas, M.P. et al. Intertidal or subtidal/circalittoral species: which appeared first? A phylogenetic approach to the evolution of non-planktotrophic species in Atlantic Archipelagos. Mar Biol 166, 88 (2019). https://doi.org/10.1007/s00227-019-3536-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3536-y