Abstract
We demonstrate an apparent trait-mediated indirect interaction (TMII) of predators on primary producers in a natural community by altering prey behavior over short and long time scales. Small predatory sea stars (Leptasterias spp.) caused herbivorous snails (Tegula funebralis) added to rocky intertidal tidepools to quickly flee into refuge microhabitats outside tidepools within days, and this was associated with a 58% increase in microalgal growth after 2 weeks. Similarly, removing sea stars caused snails to increase use of tidepools for 1–10 months. After adding sea stars to tidepools, snails quickly fled and then consistently increased use of refuges outside tidepools for 10 months. This was associated with average increases of 59% for microalgal growth over 1 month and 254% for macroalgal growth over 8 months inside tidepools. In 63 unmanipulated tidepools, densities of sea stars and snails were negatively correlated. High densities of snails were associated with unpalatable algal species and bare rock, while high densities of sea stars were associated with palatable algal species, suggesting that this apparent TMII may have community-level impacts. Though multiple lines of evidence suggest TMIIs were likely operating in this system, it was not possible to experimentally partition the relative contributions of TMIIs and density-mediated indirect interactions (DMIIs), so further caging experiments are necessary to distinguish their relative strengths. Overall, we suggest that predators can benefit primary producers by changing prey behavior even when predators and prey are unrestrained by cages or mesocosms, embedded in complex communities, and observed over multiple time scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trophic cascades are one of the central tenets of ecological theory (Hairston et al. 1960; Paine 1980). Their typical mechanism is termed a “density-mediated indirect interaction” (DMII) or “consumptive indirect effect” (CIE; Abrams 1995, 2007; Peacor and Werner 1997; Ohgushi et al. 2012), because predators reduce herbivore densities by consuming them, indirectly benefitting primary producers. However, predators do not only consume prey, but also cause changes in prey traits (e.g., reduced foraging), which can subsequently affect primary producers (Werner and Peacor 2003; Schmitz et al. 2004; Miner et al. 2005). This second mechanism for trophic cascades is an example of a trait-mediated indirect interaction (TMII) or non-consumptive indirect effect (NCIE; Abrams 1995, 2007, Peacor and Werner 1997; Ohgushi et al. 2012). Recent work shows TMIIs can be either partly or nearly entirely responsible for indirect effects within trophic cascades (Peacor and Werner 2001; Preisser et al. 2005; Trussell et al. 2004, but see Weissburg et al. 2014).
Though TMIIs have been detected in many studies, it is imperative to assess their intensity under natural field conditions over the long term. This is readily done when the initiating species rarely kills the mediating species, rendering DMIIs insignificant (e.g., many herbivores and parasites, Callaway et al. 2003; Toscano et al. 2014), or when the mediator’s responses do not involve movement (e.g., physiological or morphological responses, Raimondi et al. 2000). However, it is much more difficult when DMII and TMII effects occur simultaneously and mediators move, as in most trophic cascades. Most TMII studies in these systems are conducted in laboratories or mesocosms, which have the advantage of isolating TMII and DMII effects and manipulating species densities. However, many of these studies lack realism, are short, and may not accurately estimate TMII strength. First, constricting prey inherently limits their options for antipredator behavior, the key mechanism of many tritrophic TMIIs. Second, isolating the focal interactors from the rest of the community may overestimate TMIIs, because indirect effects may attenuate in more complex food webs (Strong 1992; but see Schmitz 1998). Third, resources must often be supplied to prey in mesocosms or are not allowed to grow naturally, making the quantification of the TMII unrealistic (Okuyama and Bolker 2007). Finally, the length of the experiment can drastically affect results; prey can temporarily cease feeding causing initial overestimation of TMII strength, but over time they may become habituated to predation threats or hungry enough to risk foraging causing underestimation of TMII strength (Luttbeg et al. 2003; Okuyama and Bolker 2007). To evaluate TMIIs in nature, TMIIs need to be investigated without restricting movement, within naturally complex communities, using natural prey resources, and over multiple time scales.
Though some studies have overcome some of these limitations and demonstrated trophic cascade TMIIs in natural communities without restricting movement (Turner and Mittelbach 1990; Raimondi et al. 2000; Trussell et al. 2004; Ripple and Beschta 2006; Wada et al. 2013), examples are sparse and it remains important to explore the relevancy of TMIIs to natural systems. The problem with investigating TMIIs in natural communities is that TMIIs cannot be easily separated from DMIIs in most systems. When it is impossible to prevent predation while simultaneously allowing natural predator and prey behavior, it is necessary to marshal multiple lines of supporting evidence to determine whether TMIIs or DMIIs should be operating in a given system. According to criteria established by Peacor and Werner (2001), TMIIs should be more intense than DMIIs in a system when (1) prey respond rapidly to the presence of predators, (2) many more prey respond to predators than can be eaten, and (3) the direct effects on prey and the indirect effects on primary producers are long-lasting (Peacor and Werner 2001). By establishing these criteria using field and laboratory surveys and experiments, the relative intensity of TMIIs can be established and systems where separation of DMIIs and TMIIs is impossible can then be investigated.
We examined a potential tri-trophic TMII by conducting field experiments in naturally occurring tidepool communities over multiple time scales (days, weeks, and months) using unrestricted movement of predators and prey. Thus, we avoided many of the limitations faced by most laboratory and mesocosm studies outlined above. Partitioning TMIIs from DMIIs in this system is challenging. Though caging sea stars would allow us to definitively parse the two mechanisms, it would alter sea star hunting behavior and snail antipredator behavior, which would lead to inaccurate TMII estimates. To obtain the most realistic conditions possible, we instead elected to use uncaged predators at the expense of precise estimates of TMIIs and DMIIs.
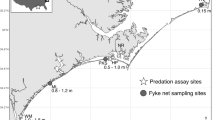
In our tidepool system on the rocky coast near Bodega Bay, California, the small 6-armed predatory sea star, Leptasterias spp. (see Flowers and Foltz 2001 for information on species complex) preys on the abundant herbivorous snail, Tegula funebralis (formerly Chlorostoma funebralis, Bouchet and Rosenberg 2015). The snail is an extremely abundant herbivore that grazes on microalgae and macroalgae and can affect macroalgal biomass and community structure in tidepools (Nielsen 2001). We first determined whether sea stars and snails were negatively associated with one another inside tidepools, whether snails tended to use refuge habitats outside tidepools when sea stars were present, and whether either species was associated with differing algal communities. We then experimentally tested our hypothesis that sea stars induce snails to shift to refuges existing at low tide outside tidepools and reduce grazing inside tidepools, thereby exerting positive TMIIs on naturally growing microalgae and macroalgae over short (< 1 months) and long (8 months) time periods, respectively.
Materials and methods
General considerations
TMIIs are likely stronger than DMIIs in our system because many of the of the criteria of relatively strong TMIIs outlined in the introduction are satisfied (Peacor and Werner 2001): (1) Tegula quickly flee from Leptasterias (Bullock 1953, Yarnall 1964); and (2) few Tegula are eaten by Leptasterias because larger size classes of Tegula are rarely eaten, and Tegula are just one of many species consumed (Bartl 1980; Gravem and Morgan 2016). We further investigated the criteria for strong TMIIs in this system by testing whether Leptasterias cause rapid escape responses by many snails and whether these responses are long-lasting. We ultimately found that TMIIs are likely to be stronger than DMIIs in this system. We thus discuss our findings in the context of TMII literature and interpretation, while acknowledging that DMIIs probably contributed somewhat to the outcomes.
Associations between predators, prey, and community structure
Surveys
To determine whether Leptasterias and Tegula were associated with one another and whether refuge use by snails was associated with sea star density, we surveyed 63 small mid to high intertidal tidepools between 6 and 14-Jul-2009 in Horseshoe Cove, located within the Bodega Marine Reserve in northern California (38°19′N, 123°14′W). We calculated the densities of sea stars and snails as individuals per liter (tide pool volume methods described below), which allowed comparisons among tidepools of different size and served as a proxy for chemical cue concentration of predators. We also counted Tegula in refuge habitats, which we termed the “halo” and defined as a < 15 cm band of emersed rock encircling each tidepool. Leptasterias do not occur in halos during low tide at this site, presumably due to physiological stress.
To test whether sea stars, snails, and other invertebrates were associated with the algal community structure and with abiotic factors, we surveyed macroinvertebrates and algae in the same 63 tidepools described above. We estimated cover of common macroalgal species by placing a large gridded quadrat (1 × 1.5 m with 2 × 2 cm cells) over each tidepool and tallying cells > 50% occupied by a given species or bare space (double occupancy was allowed). We calculated the percent cover for each species by dividing the surface area of occupied cells by the surface area of the tidepool (calculated from photos in Image J). We calculated the densities of macroinvertebrates as individuals per liter. For each tide pool, shore level at the surface (Mean ± SD 1.41 ± 0.36 m above MLLW; range 0.60–2.25 m above MLLW) was measured using surveying equipment and USGS benchmarks. Volume (Mean ± SD: 21.57 ± 21.59 L; range 1.2–107.1 L) was determined by measuring water manually pumped from tidepools. Average depth (Mean ± SD 9.2 ± 5.5 cm range 3.2–38.6 cm) was calculated from five random depth measurements. Perimeter (Mean ± SD 2.66 ± 0.95 m; range 1.04– 4.75 m) and surface area (Mean ± SD 0.216 ± 0.137 m2; range 0.051–0.611 m2) were quantified from digital photographs using ImageJ software.
Statistical Analyses
To explore the potential associations and impacts of Tegula and Leptasterias on algal communities, we first classified tidepools into three algal community types using discriminant analyses in JMP software (SAS Institute Inc., Version 9, 2010). We analyzed bivariate correlations between the percent of Tegula in the halo microhabitat, Tegula density, Leptasterias density, and between each of these with the three algal community types (percent cover of articulated coralline algae, Cladophora columbiana, and bare rock with Prionitis lanceolata) in R v3.3.2 using generalized linear models (glm in stats package, R Core Team 2013). Models testing the percent of Tegula in the halo were analyzed using quasibinomial distributions with logit link functions and those testing species densities were analyzed using quasipoisson distributions with log link functions. We then tested our hypothesis that Tegula and Leptasterias were associated with algal community structure using PRIMER-e (Plymouth Routines in Multivariate Ecological Research, Version 6, 2006). Community structure of each tidepool was determined using a normalized and square-root transformed community matrix, and Bray–Curtis similarities. We visualized associations between Tegula and Leptasterias densities and community structure using non-metric multidimensional scaling (NMDS) plots, and overlaid vectors of edible and inedible algal species driving community separation to explore which algal species co-occur with Tegula and Leptasterias. Tegula densities were separated into three evenly distributed low, medium, and high categories as < 1.5 L−1, 1.5–10 L−1, and > 10 L−1, respectively. We suspected that many non-focal factors could be covarying with Tegula and Leptasterias densities and driving associations with community structure. These included abiotic (shore level, volume, area, perimeter, and mean depth) and biotic (densities of abundant macroinvertebrates including periwinkles: Littorina spp. and hermit crabs: Pagurus spp.) factors. To rank and cull these variables, we first ran a distance-based linear model (DistLM) using a R2 selection criterion and 999 permutations and visualized the results using distance-based redundancy analysis (dbRDA) plots. DistLM identified Tegula density, shore level, average depth, volume, Leptasterias spp. Littorina spp., and Pagurus spp. densities as significant correlates (in that order). Finally, to test whether Tegula and Leptasterias densities continued to be associated with community structure after having considered these other important abiotic and biotic factors, we ran a permutational analysis of variance (PERMANOVA) with sequential sums of squares (Type I), using the above terms but with Tegula and Leptasterias densities entered last. We used 999 maximum permutations and excluded 3-way and higher order interactions due to limited degrees of freedom. We expected edible algae to be associated with high Leptasterias but low Tegula densities because of a positive effect of Leptasterias on edible algae.
Predator–prey interactions
Experimental manipulations
To determine whether Leptasterias induced short- and long-term habitat shifts by Tegula and subsequently caused TMIIs on algae, we performed manipulative experiments in 37 of the surveyed tidepools that ranged from 0.77 to 2.25 m above MLLW, 1.9–85.0 L in volume, and 0.05–0.51 m2 in surface area. Each tidepool was designated as either originally “sea star-dominated” (Leptasterias present and < 1 Tegula L−1) or originally “snail-dominated” (Leptasterias absent and > 1 Tegula L−1) using the surveys above. We manipulated Leptasterias and Tegula in 8 treatments with 3–6 tidepools per treatment (Fig. 1). For the originally sea star-dominated tidepools we removed Leptasterias or added Tegula in a factorial design. For the originally snail-dominated tidepools we removed Tegula or added Leptasterias in a factorial design. Among these 8 treatments, snails began experiments in 4 different starting conditions (Fig. 1), including (1) snails marked and added to tidepools (pink snails in treatments 1 and 2), (2) snails in halos (green snails in treatments 1–4), (3) snails inside tidepools (blue snails in treatments 5 and 6), and (4) snails immigrating to tidepools or halos from other areas (purple snails in treatments 1–8). Snails most commonly began experiments in the halos in the originally sea star-dominated tidepools and in the tidepools in the originally snail-dominated tidepools. To avoid tampering with snails and altering their natural behaviors, all snails except those added to tidepools were not marked and were thus indistinguishable within most of the treatments (Fig. 1). However, we were able to independently assess the behaviors of immigrants in isolation using the snail removal treatments (7 and 8). When sea stars were present in tidepools, we expected snails to generally avoid sea stars regardless of their starting condition; we expected snails inside or added to tidepools to flee, snails in refuges outside tidepools to remain there, and snails immigrating from elsewhere to choose refuges rather than tidepools.
The starting conditions of 8 experimental treatments for 37 tidepools (solid ovals) in Horseshoe Cove, California used to test the effects of predatory Leptasterias spp. sea stars on refuge use by Tegula funebralis snails over the short- (1 month) and long-term (10 months) and on microalgae (2 and 4 weeks) and macroalgae (8 months). Refuges were termed “halos” and defined as 15-cm band of emersed rock surrounding tidepools (dashed ovals). Tidepool treatments 1–4 originally contained sea stars with snails predominantly in the halos (“originally sea star-dominated”), and treatments 5–8 originally contained snails but no sea stars (“originally snail-dominated”). Manipulations included (1) snails added, (2) snails added & sea stars removed, (3) control, (4) sea stars removed, (5) sea stars added, (6) control, (7) snails removed & sea stars added, and (8) snails removed (n = 4, 4, 5, 3, 4, 6, 5, and 4, respectively). Snails began in four different starting conditions including snails (1) marked and added to tidepools (pink snails in treatments 1 and 2, black dots indicate markings), (2) starting in halo refuges, which was predominant in originally sea star-dominated tidepools (green snails in treatments 1–4), (3) starting in tidepools, which was predominant in originally snail-dominated tidepools (blue snails in treatments 5 and 6), and (4) immigrants from the surrounding area (purple snails in treatments 1–8). Immigrants and snails starting in halos or tidepools (asterisk) were indistinguishable from one another within treatments. When examining long-term snail behavior, treatments 1 and 3 and 2 and 4 were combined since snails were only added in the early part the experiment
Snails and sea stars were added to tidepools at the natural densities (12.8 snails L−1 and 1.8 sea stars L−1, respectively) that were recorded during surveys of the 63 tidepools described above. This density of Leptasterias added to originally snail-dominated tidepools was maintained throughout the study by replacing escaped sea stars weekly (treatments 5 and 7). All treatments began on July 13, 2009 and snails were added twice to originally sea star-dominated tidepools (treatments 1 and 2), each at the beginning of two 1-month short-term experiments (13-Jul to 19-Aug-2009 and 1 to 28-Oct-2009). To distinguish these snails from snails already in the tidepools, halos, or immigrants, these added snails were marked with fingernail enamel that remained on snail shells for many months. While it is unclear whether marking the snails affected their behavior, we believe these effects were minor compared to behavioral responses to the necessary handling of the snails that were added to the tidepools. This design also allowed us to investigate whether snails in the halos (unmarked snails in treatments 1–4) would respond to the sudden increase in conspecific density by reducing their antipredator behavior, presumably due to the sudden decrease in predation risk (Peacor 2003).
During short-term experiments snails in all 37 tidepools and halos were counted, and the 8 treatments were maintained almost daily for 1 week and then weekly for 3 weeks. The second short-term manipulation was concurrent with experiments testing TMII effects of sea stars on microalgal growth (described below). Other predators such as Pisaster ochraceus, Cancer productus, and Romaleon antennarium were present in the area and may have eaten snails or influenced their behavior. However, (1) we recorded the presence of these predators and removed them at each visit, (2) observations were rare and did not statistically differ in frequency among tidepool treatments, (3) we excluded any time points from analyses where these predators were present in a tidepool, (4) any behavioral effects on snails by these relatively mobile predators appeared fleeting (lasting hours to a few days) compared to Leptasterias that often stayed in the same tidepools for weeks, and (5) these predators should not have strongly influenced refuge use since halo refuges do not exist at high tide when these predators are present and/or active. Other grazers including sea urchins (Strongylocentrotus purpuratus), hermit crabs (Pagurus spp.), littorine snails (Littorina spp.), and limpets (Lottia spp.) were present in the tidepools and may have influenced algal abundances during experiments. Of these taxa, snails and limpets flee from Leptasterias (personal observation), so their responses may have contributed to any TMII effects of sea stars on algae during the experiments, especially in the snail removal treatments. Time constraints prevented us from recording grazer densities at each time step and thus we were unable to include them as a covariate in analyses. Instead we surveyed grazer densities at the start and end (at ~ 4 weeks) of each short-term snail addition to test for differences in responses by non-focal grazers to experimental treatments.
To determine the long-term responses by snails to sea stars, we maintained the above experimental treatments in the same 37 tidepools for 10 months (13-Jul-2009 until 17-May-2010). We maintained sea star densities and removed and counted snails in the snail removals (treatments 7 and 8) approximately weekly, totaling 47 times. Long-term snail behavior was sampled in all 37 tidepools 5 times: during the first week of each short-term experiment (weeks of 13-Jul and 1-Oct-2009, behaviors over each time period were averaged) and on 19-Aug-2009, 28-Oct-2009, and 10-Apr-2010. This long-term manipulation was concurrent with experiments testing for TMII effects of sea stars on macroalgal cover, growth, and recruitment and on microalgal growth (described below).
Though some exchange of snails between tidepools undoubtedly occurred, we assumed that the tidepool manipulations were operating independently from one another for several reasons. First, sea stars and many thousands of snails occurred in the matrix between tidepools that were not part of the experiment, so the vast majority of immigrants to tidepools were not those fleeing from other experimental tidepools. Second, ample snail and sea star habitat occurred in the matrix between our tidepools, including emersed rock, crevices, and many large tidepools that were not part of the experiment, so when a snail left an experimental tidepool it was unlikely to immigrate to a second experimental tidepool quickly. Third, we observed only occasional exchange of marked snails between tidepools and the sea stars added to the tidepools often remained in the same crevice in a given tidepool for many weeks. Finally, many Tegula and other prey species were available to sea stars in tidepools, making it unlikely that sea stars pursued fleeing snails.
Behavioral metrics
We did not track individual snails and they were allowed to freely immigrate to and emigrate from tidepools. Hence, all behaviors are the average for the snail population in a tidepool when measured, though we were able to assess added (marked) snails separately from already present snails and immigrants (unmarked). We used the percentage of snails in the halos to assess average refuge use [(snails in the halo/snails in tidepool and halo) × 100]. This metric likely underestimated refuge use in response to sea stars for two reasons. First, it did not include snails that fled further than the 15-cm-wide halo area or immigrant snails that avoided the tidepool and halo completely. Second, it did not include any use of refuges within tidepools, such as cracks or under and on algal fronds, which were less accessible to predators. To estimate snail abundance, we used snail densities in tidepools per unit volume (L−1) because tidepools varied in size. Increases in snail densities estimated immigration to a tidepool from the halo or surrounding area. Since we could not separate predation from emigration, decreases in snail densities represent combined consumptive and non-consumptive effects. We assess the relative strengths of potential consumptive effects, non-consumptive effects, TMIIs, and DMIIs in the discussion.
Statistical analyses
All analyses of snail behavior were analyzed in R v3.3.2. Because tidepools and snails began experiments under different starting conditions (Fig. 1), we analyzed snail behavior with separate models for (1) marked snails added to originally sea star-dominated tidepools (treatments 1 and 2), (2) unmarked snails in originally sea star-dominated tidepools, which typically began experiments in halos or subsequently immigrated (treatments 1–4), and (3) unmarked snails in originally snail-dominated tidepools, which typically began experiments in tidepools or subsequently immigrated (treatments 5–8). Our snail removal treatment enabled us to investigate behaviors of new immigrants in isolation. We also separately analyzed short- and long-term snail behavior. For models analyzing the percent snails in the halo, we used generalized linear mixed models fit by maximum likelihood (glmer function in lme4 package v1.1–12, Bates et al. 2015) and specified binomial error distributions and logit link functions. For models analyzing snail density inside tidepools, we primarily used generalized linear mixed models using AD model builder (glmmADMB function and package v 0.8.8.3, Fournier et al. 2012) because it enabled us to specify negative binomial error distributions and zero-inflation. The exception was the model analyzing short-term densities of snails marked/added to tidepools, where the response was non-linear so we used a generalized additive mixed model (gamm function in mgcv package v 1.8–15, Wood 2006) and specified a negative binomial error distribution. In all models, we included tidepool as a random factor to account for repeated sampling within tidepools over time. For originally sea star-dominated tidepools, we tested the main and interactive effects of time (days since snail addition began) and sea star treatment on marked/added snails (treatments 1 and 2) and unmarked snails (treatments 1–4) separately. Snail treatment was irrelevant when testing marked/added snails and it was dropped when testing unmarked snails (treatments 1–4) because it contributed very little to models. For originally snail-dominated tidepools (treatments 5–8), we tested the main and interactive effects of time, sea star treatment, and snail treatment. Models were constructed the same for analyses of short-term and long-term behavior except for the long-term snail behavior in originally sea star-dominated tidepools where we dropped snail treatment because no further snails were added (i.e., data for marked and unmarked snails were combined). Our sample sizes were somewhat low, and power analyses using the powerSim() function (R package simr version 10.0.4, Green and MacLeod 2016) revealed low power (ranging from 50.8 to 57.0%) of snail behavior and algae growth models (detailed below). Adjusting the alpha to 0.10 allowed increased power (ranging from 64.7 to 66.9%), so the significance of p values was assessed at α = 0.1 for these models.
Analyses of non-focal grazer including hermit crabs (Pagurus spp.), sea urchins (Strongylocentrotus purpuratus), littorine snails (Littorina spp.), and limpets (Lottia spp.) were performed using mixed general linear models using restricted estimated maximum likelihood (REML) in JMP Pro 13. We analyzed the effects of treatment (8 levels, Fig. 1) and time (2 levels for start and end of the experiment) on the log-transformed densities L−1 of each grazer and included tidepool and addition number as random factors. The exception was limpets, which were only surveyed once before any experiments began, so we analyzed the effects of treatment (8 levels) on log-transformed limpet densities using 1-way ANOVA to investigate any differences in starting densities among treatments.
Impact on algae
Growth of microalgae during short-term experiments
To determine the effects of snails and sea stars on microalgae, we deployed 6 bare porcelain tiles (2.4 × 2.4 cm) using marine epoxy in each of the 37 experimental tidepools during the second short-term snail addition experiment (1–28-Oct-2009). At 2 and 4 weeks, respectively, we collected three tiles in each tidepool. Originally sea star-dominated tidepools with no snails added (treatments 3 and 4) and snail-dominated tidepools with snails removed (treatments 7 and 8) served as controls for algal recruitment under low snail herbivory conditions. We estimated microalgal growth on tiles using chlorophyll-a concentration, which was extracted by placing each tile in acetone for 24 h (as per Morelissen and Harley 2007) and analyzed using a fluorometer (TD-700, Turner Designs) with F4T4.5 B2 lamp with 436 nm excitation and 680 nm emission filters (as per Welschmeyer 1994). Between paired treatments with and without sea stars (1 vs. 2, 3 vs. 4, etc.), we expected sea star presence to benefit microalgae, indicating potential positive TMIIs.
Since the starting conditions of the organisms were different, we analyzed originally sea star-dominated (treatments 1–4) and snail-dominated (treatments 5–8) tidepools separately using mixed generalized linear models. We used glmmADMB function and package for the sea-star dominated tidepools because this allowed us to specify negative binomial distributions (to account for over-dispersion in the data), and used glmer function in lme4 package specifying a gamma distribution for the snail-dominated tidepools. We analyzed the effects of week (2 or 4 weeks), sea star presence and snail treatment on chlorophyll-a, with tidepool included as a random factor. In only the originally sea star-dominated tidepools, too many tiles were lost after 4 weeks, so only the 2-week tile collection was analyzed and time was dropped from the model. We calculated the magnitude of the TMII as the difference in microalgal cover between paired predator present versus absent treatments within each snail treatment (i.e., between light and dark paired bars in Fig. 6) at 2 and 4 weeks.
Cover of macroalgae
Macroalgal surveys were conducted between 6 and 8-Jul-2009 (just before the experimental manipulations began) as part of the tidepool surveys outlined above, and they were repeated 1 month later on 4 and 5-Aug-2009 to determine the effects of Leptasterias and Tegula manipulations on macroalgal cover in the 37 experimental tidepools. Additional macroalgal cover surveys were planned, but harbor seals (Phoca vitulina) killed the algae in many tidepools in fall 2009. To test if snail and sea star treatments changed cover of individual macroalgal species in tidepools over 1 month, we ran multivariate analyses of variance (MANOVA) in JMP on change in percent cover of bare rock and the 19 common macroalgal species. We also analyzed the effects of survey date and sea star and snail treatments on algal community structure, with tidepool number (nested within overall treatment) included to control for repeated measures among tidepools (PERMANOVA using Bray–Curtis similarity matrices and 999 maximum permutations). For both these analyses originally sea star-dominated and snail-dominated tidepools were analyzed separately.
Growth and recruitment of macroalgae during long-term experiments
We also tested the effects of Tegula and Leptasterias on macroalgal growth and recruitment in cleared plots since growing tissue and early life stages may be more vulnerable to Tegula herbivory. In each of the 37 experimental tidepools, we denuded 4 circular plots (5.08 cm diameter) with a small blowtorch between 17 and 22-Sept-2009. Individual recruits were defined as individuals that settled within plots and growing new thalli or crusts (typically Mazzaella flaccida, Mastocarpus papillatus, and encrusting coralline, red, or green algae). Encroaching algae was defined as existing nearby algae that had grown into the plot (typically Cladophora columbiana, articulated coralline algae, and encrusting coralline or red algae). After ~ 8 months (between 26-Apr and 17-May-2010), the number and percent cover of individual algal recruits and encroaching algae were surveyed, with percent cover calculated using a gridded circular quadrat (5.08 cm diameter with 24 0.84 × 0.84 cm cells). We analyzed the effects of sea star and snail treatments on the number and cover of algal recruits and the cover of encroaching algae in each clearing plot using a mixed general linear model fit using restricted estimated maximum likelihood (REML) in JMP and included tidepool number nested within sea star and snail treatments as a random factor to control for non-independence of plots within the same tidepool. Between paired treatments with and without sea stars (1 vs. 2, 3 vs. 4, etc.), we expected sea star presence to benefit macroalgae, indicating potential positive TMIIs.
Results
Predator and prey relation to community structure
Discriminant analyses identified three distinct tidepool community types (Fig. 2a; Wilk’s λin: F(2,60) = 13.45, P < 0.001) that were dominated by (1) articulated coralline algae, (2) Cladophora columbiana, and (3) bare rock and Prionitis lanceolata. Tegula density was positively correlated with bare rock cover (glm: t1,62 = 3.81, P < 0.001, Log10 (Tegula density) = 1.45 + 0.03*percent cover of bare rock and negatively correlated with articulated coralline algae cover (glm: t1,62 = − 2.94, P = 0.005, Log10 (Tegula density) = 2.52–0.14*coralline percent cover). In contrast, Leptasterias density was positively correlated with articulated coralline algal cover (glm: t1,62 = 2.89, P = 0.005, Log10 (Leptasterias density) = − 2.50 + 0.03* coralline % cover). Neither species was correlated with Cladophora cover.
a Canonical score plot showing the different algal community types classified by discriminant analysis on 63 tidepools. Inner ellipses show the 95% confidence interval for group means (cross-hairs), and outer ellipses show the normal 50% contours. Vector overlays depict the algal species strongly driving group separation. b Non-metric multidimensional scaling (NMDS) plot of community dissimilarity with vector overlays depicting common algal species driving dissimilarity. Tidepools were categorized by Tegula density being low, medium and high (< 1.5 L−1, 1.5–10 L−1, and > 10 L−1, respectively) and labeled with light, medium, and dark tones, respectively. Leptasterias presence and absence in tidepools are indicated by red and blue tones, respectively. c Distance-based redundancy analysis (dbRDA) plot depicting algal community dissimilarity. Vector overlays depict the abiotic factors and animals most strongly associated with algal community structure
Leptasterias and Tegula appeared to be associated with differing algal communities (Fig. 2b). The highly edible algal species Ulva lactuca and Mazzaella spp. were associated with low densities of Tegula and the presence of Leptasterias. These animal densities were also associated with inedible Phyllospadix scouleri and coralline algae. The edible algal species Cladophora, Porphyra perforata, and Endocladia muricata tended to be associated with lower densities of Tegula and an absence of Leptasterias, while bare rock and the inedible algal species Mastocarpus papillatus, Prionitis lanceolata and red crustose algae were associated with high densities of Tegula regardless of Leptasterias presence.
When exploring the abiotic and biotic covariates, we found that Tegula density, shore level, average depth, volume, Leptasterias density, periwinkle density (Littorina spp.), and hermit crab density (Pagurus spp.) were associated with algal community structure, in that order (Fig. 2c; DISTLM: F1,54 = 16.22, 15.54, 7.74, 6.33, 5.45, 4.98, and 2.50, respectively, and P < 0.001 for all factors except Pagurus spp. where P = 0.033). Area and perimeter of tidepools were marginally nonsignificantly correlated (DISTLM: F1,54 = 2.34, P = 0.053) and not correlated (DISTLM: F1,54 = 1.77, P = 0.116) with algal community structure, respectively. Tegula and Leptasterias densities continued to be associated with algal community structure (PERMANOVA: F1,27 = 4.62, P = 0.001 and F1,27 = 3.46, P = 0.007, respectively) even having already considered the effects of shore level, depth, volume, and Pagurus spp. and Littorina spp. densities. Further, low density of Tegula was associated with different algal communities than were medium and high densities of Tegula (PERMANOVA post hoc analyses: t27 = 2.20, P < 0.001 and t27 = 2.24, P < 0.001, respectively).
Predator–prey interactions
Surveys
Increased Leptasterias density in tidepools was correlated with both an increase of snails in halos and reduced densities of snails in tidepools (Fig. 3a, b; glm: t50 = 3.46, P = 0.001, logit (proportion Tegula in halo) = − 1.00 + 8.10*Leptasterias density; and glm: t61 = − 2.48, P < 0.017, Log10 (Tegula density) = 2.36–4.66*Leptasterias density, respectively).
Short-term experiments
Snails avoided sea stars regardless of whether (1) snails were marked and added to sea star-dominated tidepools (treatments 1 and 2; Fig. 4a, b); (2) snails were present inside and/or immigrating to originally snail-dominated tidepools where we added sea stars (treatments 5 and 6; Fig. 4c, d), or (3) snails were newly immigrating to originally snail-dominated tidepools where we had removed snails and added sea stars to tidepools (treatments 7 and 8; Fig. 4c, d). Snails added to tidepools (treatments 1 and 2) containing sea stars fled to refuges more quickly than those added to tidepools without sea stars (Fig. 4a; glmm: time × sea star treatment: z = 2.19, P = 0.029; 44.8% average increase in refuge use). Further, when sea stars were removed, densities of snails added to tidepools stayed much higher (647.7% higher average density) and did not decrease as quickly over time compared to tidepools where sea stars were present (Fig. 4b; gamm: time × sea star treatment: t = − 4.342, P < 0.001). Some modest effects of removing sea stars on unmarked snails, which typically started in halos of or were immigrants to originally sea star-dominated tidepools, were noted (treatments 1–4; Figs. 4a, b; 27.7% average decrease in refuge use and 250.4% average density increase) but the effects of sea star removal were not significant (glmm on refuge use: time × sea star treatment: z = 1.29, P = 0.197; glmm on density: time × sea star treatment: z = − 0.41, P = 0.680). The lack of effect may be in part due to low numbers of these snails. Snails initially in the halos did not decrease refuge use or move into tidepools following the addition of conspecifics (glmm on refuge use: snail treatment: z = 1.31, P = 0.192, time × snail treatment: z = − 0.08, P = 0.935; glmm on density: snail treatment: z = 0.14, P = 0.890, time × snail treatment: z = 0.00, P = 1.000) indicating that they did not respond to the sudden decrease in relative predation risk.
Refuge habitat use and densities of snails during two replicate short-term experiments (1 month each). Fits are plotted using glm binomial (a, c), linear (right panel of b, d) or gam loess (left panel of b) fits. Effects of sea star presence (closed turquoise symbols, solid lines) and absence (open black symbols, dashed lines) on behavior was examined for snails under different starting conditions. Snails were either (1) marked and added to originally sea star-dominated tidepools (left panels of a, b, n = 4 and 4 tidepools with and without sea stars, respectively), (2) not added to originally sea star-dominated tidepools and consisted of unmarked snails that typically started in refuge habitats of or immigrated to tidepools during experiments (right panels of a, b, n = 5 and 3 tidepools with and without sea stars, respectively), (3) removed consistently from originally snail-dominated tidepools so consisted of unmarked snails newly immigrating to tidepools between removals (left panels of c, d, n = 6 and 4 tidepools with and without sea stars, respectively), or (4) not removed from originally snail-dominated tidepools and consisted of unmarked snails that typically started inside or immigrated to tidepools during experiments (n = 4 and 6 tidepools with and without sea stars, respectively)
For unmarked snails that typically started inside of or immigrated to originally snail-dominated tidepools (treatments 5–8), adding Leptasterias generally caused snails to increase refuge use throughout the experiment (Fig. 4c; glmm: sea star treatment: z = 1.90, P = 0.057; time × sea star treatment: z = -0.20, P = 0.839). Though not statistically different from one another (snail treatment × sea star treatment: z = − 1.55, P = 0.122), this was more apparent when snails were newly immigrating (i.e., where snails were removed; treatments 7 and 8, 119.0% greater average refuge use with sea stars present versus absent) and less apparent when snails were not removed (treatments 5 and 6, 28.7% greater average refuge use with sea stars present versus absent). Adding sea stars caused a modest decrease in snail density in these tidepools over time compared to no decrease when sea stars were not added (Fig. 4d; time × sea star treatment: z = − 3.21, P = 0.001; 22.8%, and 33.7% average density decrease for treatments with and without snails removed, respectively). As expected, snail removals reduced snail densities in tidepools (Fig. 4d; snail treatment: z = 2.54, P = 0.011 69.7% average density decrease).
We found no effects of our experimental manipulations on the densities of three non-focal grazers (treatment x time: F7,82 = 1.40, P = 0.219 for hermit crabs, F7,83 = 0.34, P = 0.902 for sea urchins, and F7,83 = 0.35, P = 0.931 for littorine snails). Though there was some indication that limpet densities at the start of the experiments were higher in some treatments (anova: treatment: F7,26 = 2.77, P = 0.027), post hoc analyses showed no significant differences among treatments (P > 0.068 for all pairwise comparisons). At the start of the experiments, Tegula were 2.4-, 8.7-, 39.1-, and 921.8-fold more abundant than littorines, limpets, hermit crabs, and sea urchins, respectively. They were also generally much larger than the hermit crabs, limpets or littorines.
Long-term experiments
Over the 10-month experiment, removals of sea stars from originally sea star-dominated tidepools (treatments 1–4) consistently decreased refuge use by all snails (Fig. 5a; 22.5% average decrease; glmm: sea star treatment: z = − 1.94, P = 0.053). While removing sea stars was associated with a 219.6% increase in average snail density, this was not significant (Fig. 5b; glmm: sea star treatment: z = − 0.54, P = 0.59).
Refuge habitat use and densities of snails over the long-term experiment (10 months) in 37 tidepools. Effects of sea star presence (closed turquoise symbols, solid lines) and absence (open black symbols, dashed lines) on behavior of snails under different experimental conditions. All snails in originally sea-star dominated tidepools were combined since snail addition treatments were not performed over the long term, and these tidepools were observed five times (a, b, n = 9 and 7 tidepools with and without sea stars, respectively). Snails were removed approximately weekly from originally snail-dominated tidepools and so consisted of snails newly immigrating to tidepools between removals, and these tidepools were observed 47 times (left panels of c, d, left panels, n = 6 and 4 tidepools with and without sea stars, respectively). Snails that were not removed from originally snail-dominated tidepools consisted of unmarked snails that typically started inside or immigrated to tidepools during experiments, and these were observed five times (c, d, right panels, n = 4 and 6 tidepools with and without sea stars, respectively)
Further, adding sea stars to originally snail-dominated tidepools (treatments 5−8) consistently and strongly increased refuge use by snails newly immigrating to tidepools (i.e., where snails were removed; Fig. 5c; glmm: sea star × snail treatment: z = 2.46, P = 0.014; 201.7% average increase). However, adding sea stars did not statistically increase refuge use in tidepools where snails were not removed (Fig. 5c; 20.5% average increase;). Adding sea stars did not decrease snail densities over the long term (Fig. 5d; sea star treatment: z = 1.00, P = 0.317; 23.9% and 19.2% average density decreases), though detection of any potential differences may have been impeded by very high variability in snail densities among tidepools. As expected, long-term snail removals decreased snail densities in tidepools from an average of 11.2–2.8 snails L−1 (Fig. 5d; snail treatment: z = -2.20, P = 0.028, 74.9% average density decrease).
Impacts on algae
Growth of microalgae during short-term experiments
When snails were added to originally sea star-dominated tidepools (treatments 1 and 2), microalgae growth increased by 36.5% after 2 weeks in tidepools with sea stars compared to those where sea stars were removed (Fig. 6a; glmm: sea star × snail treatment: z = 2.89, P = 0.004). Unsurprisingly, snail addition decreased microalgal growth (Fig. 6a; glmm: snail treatment: z = − 1.91, P = 0.056). However, when no snails were added, sea stars had an unexpected negative effect on microalgal growth (Fig. 6a). However, snail densities in these latter tidepools were extremely low (Fig. 4b), so it is unlikely that Tegula mediated this negative effect. Since we often observed sea stars crawling over microalgae in the laboratory without disturbing it, we also doubt that this was a direct effect of sea stars on algae. Rather, this was likely due to other factors such as the environment or other grazers.
The effect (mean ± SE) of sea star and snail manipulation on algae. a Microalgal growth after 2 weeks in originally sea star-dominated tidepools (n = 4 tidepools for all treatments). b Microalgal growth after 2 and 4 weeks in originally snail-dominated tidepools (n = 4, 5, 5, and 4 tidepools in order shown). c Encroaching macroalgal cover in cleared plots after 8 months in originally snail-dominated tidepools (n = 5, 4, 4, and 5 tidepools in order shown). Snails were removed approximately weekly
Adding sea stars to originally snail-dominated tidepools increased microalgal growth by an average of 63.8% and 70.2% for tidepools where snails were and were not removed, respectively (treatments 5–8; Fig. 6b; glmm: sea star treatment: t = 1.90, P = 0.058; sea star x snail treatment: t = − 0.47, P = 0.638). This was consistent at each time point (2 and 4 weeks) (Fig. 6b; time × sea star treatment: t = − 1.35, P = 0.176). As expected, algae were more abundant when snails were removed and increased over time (Fig. 6b; glmm: snail treatment: t = − 1.75, P = 0.080; time: t = − 3.2, P = 0.001).
Cover of macroalgae
No effects of sea star or snail treatments on established macroalgae were observed over the very short time period (~ 1 month) between macroalgal surveys. MANOVA results analyzing the eight treatment effects on macroalgal cover by species showed no significant changes for any species (sea star × snail treatment × original tidepool dominant: Wilk’s \(\lambda\): F3,25 = 1.01, P = 0.491). Similarly, PERMANOVA analyses showed no significant effects of snail treatment or sea star treatment on community structure for either snail-dominated or sea star-dominated tidepools (overall treatment: F3,14 = 0.45, P = 0.928, and F3,11 = 0.78, P = 0.689, respectively). Thus, we were unable to perform tests to investigate whether edible versus inedible algal species were affected by the experimental manipulations. See the discussion for our qualitative observations among algae species.
Growth and recruitment of macroalgae during long-term experiments
We found no effect of sea star removal from originally sea star-dominated tidepools on growing macroalgae encroaching into clearings (treatments 1–4; sea star treatment: F1,37 = 0.81, P = 0.373), perhaps because snail densities were low regardless of sea star treatment (Fig. 5b). In contrast, when we added sea stars to originally snail-dominated tidepools, the cover of macroalgae encroaching into clearing plots increased by 252% and 197% in tidepools where snails were and were not removed, respectively (treatments 5–8; Fig. 6c; sea star treatment: F1,44 = 5.26, P = 0.025; sea star × snail treatment: F1,44 = 0.46, P = 0.498). Though the absolute cover of encroaching algae only reached 20.4% and 10.2% cover over 8 months with sea stars present (for tidepools with snails removed or not removed, respectively), the original undisturbed macroalgae cover ranged from 21 to 45% cover, so recovery was fairly substantial.
Because sample sizes were small when species were considered individually, no treatment effects were detected for any individual macroalgal species for either originally Tegula- or sea star-dominated tidepools (MANOVA: Wilks’ \(\lambda\): F3,64 = 1.02, P = 0.443 and F3,57 = 1.42, P = 0.106, respectively). However, the edible algae Cladophora columbiana was the most common algae recorded, and it grew most in tidepools where snails were removed and sea stars were added (Mean ± SE 17.3 ± 7.6% cover compared to < 6.9% cover for the other seven treatments). The number and cover of new algal recruits were not affected by sea stars in the originally snail-dominated (sea star treatment: F1,44 = 0.33, P = 0.570 and F1,36 = 0.13, P = 0.723, respectively) or sea star-dominated tidepools (sea star treatment: F1,37 = 0.19, P = 0.664 and F1,32 = 0.51, P = 0.478, respectively), likely due to low algal recruitment (averaging less than 1 per plot) during the experiment.
Discussion/conclusion
The intensity of TMIIs
Although we did not experimentally quantify DMIIs, TMIIs appeared to play a role in structuring rocky intertidal algal communities under natural conditions. Leptasterias caused Tegula to use refuges outside of tidepools and presumably graze less over short (days–weeks) and long (weeks–months) time frames, which in turn was associated with positive effects on both microalgae and macroalgae in tidepools over 1 month and 8 months, respectively. Though the observed effects on algae are a combination of TMII and DMII, we believe it is reasonable to attribute the results primarily to TMIIs because (1) prey rapidly responded to predators, (2) many more prey responded than could be eaten, and (3) effects on both the behavioral responses and on algae were long-lasting (Peacor and Werner 2001). That said, our observed effects of sea stars and snails on algae were in part due to consumptive effects and to DMII because some snails were likely eaten by sea stars during the experiments.
Regarding the 1st criterion above, snails added to tidepools containing sea stars typically began climbing upward within minutes until they reached the waterline or emerged from the water, thereby evading sea stars that apparently always remained submerged (personal observation). By the next day, the majority of snails had fled to halo refuges or left the area when sea stars were present (Fig. 4a). Further, effects of sea stars on snails in other treatments occurred within days (Fig. 4). This is supported by similar immediate responses by Tegula to Leptasterias in other studies (Yarnall 1964; Gravem and Morgan 2016; Morgan et al. 2016).
Adding sea stars to tidepools often elicited escape responses by hundreds of snails in a given tidepool observation (Figs. 4, 5), but we believe that the probable number eaten is lesser than the number responding, which would elicit strong TMIIs (2nd criterion above, Peacor and Werner 2001). A low potential for predation may exist for many reasons. First, there is an abundance of other prey species at this site, which comprised 76% of Leptasterias diets, while Tegula comprised 24% during a snapshot survey in 2009 (Gravem and Morgan 2016). Further, Tegula can easily escape most Leptasterias encounters (personal observation) and throughout the long-term field manipulation we observed only five predation attempts on Tegula of the 294 sea star observations. This low frequency is especially striking because Leptasterias preying on Tegula had very long average handling times of 128 h in laboratory experiments (Gravem and Morgan 2016), though laboratory experiments do not always accurately estimate feeding habits in the field (Ruesink 2000). Further, even when Tegula are prevented from escaping Leptasterias by confining predator–prey pairs together in very small tanks in the laboratory, most medium and nearly all large snails remain uneaten after > 2 weeks (Gravem and Morgan 2016, 60.0% of medium > 12 mm diameter snails and 93.6% of large > 18 mm diameter snails survived). At this site, the practically invulnerable large snails (> 18 mm) comprised 29% of the population during the experiment and have higher grazing rates than small snails (Best 1964; Gravem and Morgan 2016), so they should meditate relatively strong TMIIs but weak DMIIs. Despite the low risk, all snails, even larger ones, respond strongly to Leptasterias contact and waterborne cues by fleeing or grazing less in the laboratory and field (Gravem and Morgan 2016). In our experiments, most snails likely responded to waterborne cues from sea stars that could reach hundreds of snails in a short time. Further, ours and other studies suggest that Tegula are able to distinguish between Leptasterias and other predatory sea stars such as Pisaster (Gravem and Morgan 2017; Yarnall 1964). So, the observed fear response is not likely a general response to sea star cues.
Sea stars added to tidepools also induced long-lasting refuge use by snails, which is a characteristic of relatively strong TMIIs in a system (3rd criterion above, Peacor and Werner 2001). This result was similar to other studies showing strong effects of predators on gastropod habitat use or grazing (Bernot and Turner 2001; Trussell et al. 2002, 2004; Matassa and Trussell 2011; Wada et al. 2013). This was likely a sustained change in the average behavior of the snail population rather than a permanent habitat shift for particular individuals since it is probable that different individuals were sampled each time. Individual Leptasterias were often observed in the same location for months even without caging, which apparently maintained sustained behavioral responses by the snail population.
The duration and natural circumstances of these experiments further demonstrate that TMIIs, which have been well established in laboratory and mesocosm experiments, may be also operating in natural communities. First, sea star-induced refuge use by snails and apparently subsequent benefits to algae were consistent over short and long time scales. The duration of the experiments ensured that the apparent TMIIs in these tidepools were not an artifact of prey temporarily abstaining from grazing or only exhibiting a short-term response to predators (Luttbeg et al. 2003; Werner and Peacor 2003; Okuyama and Bolker 2007). Also, the consistency in refuge use and apparent long-term benefit to macroalgae when sea stars were added to tidepools showed that the snail population did not become habituated to sea star cues, which would cease the mediation of any potential TMII (Luttbeg et al. 2003; Werner and Peacor 2003; Okuyama and Bolker 2007). Second, the effects on algae occurred without caging or restricting the hunting behaviors of sea stars or predator avoidance behaviors of snails. Thus, snails were not exposed to unnaturally strong predator cues that could have caused overestimation of TMII strength. Also, snails were not starved, which could have induced them to risk grazing in tidepools resulting in underestimation of TMII strength (Long and Hay 2012; Weissburg et al. 2014). Third, the apparent TMIIs detected were relevant for the ecosystem because the experiment used algae growing naturally, not algae introduced to the system or out-planted from the laboratory (Okuyama and Bolker 2007).
Finally, the apparent TMII remained strong even while the focal interactors coexisted with other intertidal species, so it was not attenuated when embedded in a complex community as others have predicted (Schmitz 1998; Strong 1992). Though we observed no effects of our treatments on the densities of non-focal grazer taxa during the experiments, our sea star manipulations may have caused these grazers to reduce grazing and they likely contributed to the apparent TMIIs on algae. The presence of other grazers in the system diminishes our ability to isolate the effects of Tegula, but this was unavoidable in the natural setting of the experiment. However, two of the grazers (hermit crabs and sea urchins) do not respond to Leptasterias (personal observation) so their grazing rates should not have been affected by the sea star manipulations. Further, Tegula were 2.4- and 8.7-fold more abundant than limpets and littorine snails, respectively, and were generally much larger. Paired with our finding that Tegula removal increased microalgal growth, these observations suggest that Tegula was the dominant grazer in this system and likely the primary driver of the observed effects on algae.
Context dependence
The starting conditions of snails affected both their response to sea stars (Figs. 4, 5), and the strength of the apparent TMII on algae (Fig. 6). Strong effects on both microalgae and macroalgae were observed when we added sea stars to originally snail-dominated tidepools (Fig. 6b). These positive effects on algae occurred in tidepools where snails were and were not removed, but the mechanism appeared to differ; Snails newly immigrating to snail removal tidepools increased refuge use over the short and long term (Figs. 4c, and 5c, left panels) and had reduced densities in tidepools over the short term (Fig. 4d, left panel) likely benefiting algae. In contrast, in tidepools where snails were not removed, these snails displayed only modest increases in refuge use and no decreases in density over the short-term, with no changes over the long-term (Figs. 4c, d, 5c, d, right panels), or discernibly decrease in density in tidepools (Figs. 4f and 5d, right panels). But, apparent TMIIs still occurred over the short term (Fig. 6b) suggesting that grazing rates may have decreased in response to predators, as found in many other tritrophic systems (Werner and Peacor 2003). Thus, apparent TMIIs may have occurred via two antipredator behaviors: habitat shifts, and reduced grazing (Kats and Dill 1998).
Potential effects of TMIIs on algal communities
Although we were unable to experimentally assess the effects of Leptasterias and Tegula on macroalgal community structure, the positive apparent TMIIs between Leptasterias and growing macroalgae (Fig. 6c) and the association of Leptasterias and Tegula with different macroalgal communities (Fig. 2b, c) suggest Leptasterias and Tegula may alter algal community structure. The clearance experiment suggested that Leptasterias may moderately enhance the seasonal growth or recovery after disturbance of macroalgae. Among species, the surveys suggested that Ulva and Mazzaella, which are highly preferred by Tegula (Aquilino et al. 2012), were associated with the presence of sea stars in concert with low snail density (Fig. 2b, light red circles) suggesting sea stars may benefit these algae. Sea star presence with low snail densities (Fig. 2b, light red circles) was also associated with morphologically complex and inedible Phyllospadix and articulated coralline algae, which may be caused by the sea stars using these algae as habitat (personal observation) rather than a result of low snail herbivory. Further, bare rock, red encrusting algae and chemically-defended inedible algae Prionitis and Mastocarpus were positively associated with Tegula regardless of Leptasterias presence (Fig. 2b, darker blue and darker red circles) suggesting these surface conditions may dominate under heavy grazing pressure. Finally, the edible algal species Porphyra, Cladophora, and Endocladia (Aquilino et al. 2012) tended to be associated with low to medium densities of Tegula and an absence of Leptasterias (Fig. 2b, light and medium blue circles), suggesting they are only able to withstand moderate grazing pressure. Overall, the multivariate analyses suggested that Leptasterias and Tegula densities were correlated with macroalgal community structure, even having considered the probable importance of covarying abiotic and biotic factors (Fig. 2c). We note that these are all correlative findings and long-term caging experiments are necessary to determine whether Leptasterias spp. can indirectly affect algal community structure. On the other hand, there is experimental evidence using long-term manipulations that Tegula herbivory has strong impacts on tidepool algal communities (Nielsen 2001).
While our study demonstrates the potential for Leptasterias to exert positive TMIIs on tidepool algae, the overall effect of Leptasterias and Tegula on all macroalgae in the system is less clear. For example, Leptasterias scaring snails out of a tidepool may indirectly negatively affect algae outside tidepools (i.e., in the halo or in the matrix between tidepools). This type of effect, where predators cause cascades in adjacent habitats, is known as “remote control” and is commonly observed in TMII studies (e.g., Grabowski and Kimbro 2005, Trussell et al. 2006). We investigated remote control in our experimental tidepools by deploying porcelain tiles both in tidepools and in halos for 7 weeks, but found no effects of sea star or snail presence on microalgal growth, suggesting that the halos are not heavily utilized as foraging habitat by snails (unpublished data). In a similar fashion, the snails fleeing Leptasterias must emigrate elsewhere and eventually forage, resulting in a weaker overall TMII in the system than was observed inside the tidepools (Abrams 2008). Further, snails may re-enter tidepools over time, enticed by suddenly available resources, resulting in a cyclical pattern in predicted prey density and TMII and DMII strengths in a habitat patch, as modeled by Abrams (2008). Thus, because the sign and strength of the TMII and DMII likely change with the spatial and temporal scale considered, the overall effect of Leptasterias presence on algae in the rocky intertidal system remains unknown. Regardless, our study suggests that when Leptasterias consistently inhabit tidepools, they alter snail behavior for up to 10 months and may exert positive TMIIs on tidepool algae.
In conclusion, predators may cause TMII trophic cascades in unconstrained natural conditions by altering behavior of prey, rather than by eating prey. Overall, predators caused extended habitat shifts by prey and were associated with both short- and long-term increases for primary producers. Though it was not possible to separate the relative intensity of TMII and DMII, TMIIs were likely relatively strong because prey rapidly responded to predators, many more prey responded than could be eaten, not all sizes of prey were vulnerable, and effects on the behavioral responses of prey were long-lasting. These effects occurred without restricting predator or prey movement, within naturally complex communities, using natural prey resources, and over multiple time scales. Although the per-capita consumption rates typically are the primary mechanism of species interactions in population and food web models (Bolker et al. 2003; Persson and De Roos 2003), the present study emphasizes the need to incorporate behavior to gain an inclusive, realistic estimation of the cascading effects of predators on communities.
Data availability
The datasets generated during and/or analysed during the current study are available as a repository in GitHub. https://github.com/gravems/Data_Gravem-and-Morgan_2019_Trait-mediated-indirect-effects-in-a-natural-tidepool-system.
References
Abrams PA (1995) Implications of dynamically variable traits for identifying, classifying and measuring direct and indirect effects in ecological communities. Am Nat 146:112–134. https://doi.org/10.1086/285789
Abrams PA (2007) Defining and measuring the impact of dynamic traits on interspecific interactions. Ecology 88(10):2555–2562. https://doi.org/10.1890/06-1381.1
Abrams PA (2008) Measuring the population-level consequences of predator-induced prey movement. Evol Ecol Res 10:333–350
Aquilino KM, Coulbourne ME, Stachowicz JJ (2012) Mixed species diets enhance the growth of two rocky intertidal herbivores. Mar Ecol Prog Ser 468:179–189. https://doi.org/10.3354/meps09893
Bartl S (1980) A comparison of the feeding behavior of the six-rayed seastar, Leptasterias hexactis, and from two intertidal habitats. Bodega Marine Laboratory Cadet Hand Library, unpublished student report, Problems in Marine Biology course
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bernot RJ, Turner AM (2001) Predator identity and trait-mediated indirect effects in a littoral food web. Oecologia 129:139–146. https://doi.org/10.1007/s004420100705
Best B (1964) Feeding activities of Tegula funebralis. The Veliger 6:42–45
Bolker B, Holyoak M, Krivan V, Rowe L, Schmitz O (2003) Connecting theoretical and empirical studies of trait-mediated interactions. Ecology 84:1101–1114
Bouchet P, Rosenberg G (2015) Tegula funebralis. In: MolluscaBase (2015). Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=534190. Accessed 15 Sep 2015
Bullock TH (1953) Predator recognition and escape responses of some intertidal gastropods in presence of starfish. Behaviour 5:130–140. https://doi.org/10.1163/156853953x00078
Callaway RM, Pennings SC, Richards CL (2003) Phenotypic plasticity and interactions among plants. Ecology 84:1115–1128
Flowers JM, Foltz DW (2001) Reconciling molecular systematics and traditional taxonomy in a species-rich clade of sea stars (Leptasterias subgenus hexasterias). Mar Biol 139:475–483. https://doi.org/10.1007/s002270100595
Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J (2012) AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249
Grabowski JH, Kimbro DL (2005) Predator-avoidance behavior extends trophic cascades to refuge habitats. Ecology 86:1312–1319
Gravem SA, Morgan SG (2016) Prey state alters trait-mediated indirect interactions in rocky tidepools. Funct Ecol 30:1574–1582. https://doi.org/10.1111/1365-2435.12628
Gravem SA, Morgan SG (2017) Shifts in intertidal zonation and refuge use by prey after mass mortalities of two predators. Ecology 98:1006–1015
Green P, MacLeod CJ (2016) SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol 7:493–498
Hairston NG, Smith FE, Slobodkin LB (1960) Community structure, population control and competition. Am Nat 94:421–425
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Long JD, Hay ME (2012) The impact of trait-mediated indirect interactions in marine communities. In: Ohgushi T, Schmitz OJ, Holt RD (eds) Trait-mediated indirect interactions: ecological and evolutionary perspectives. Cambridge University Press, New York, pp 47–68
Luttbeg B, Rowe L, Mangel M (2003) Prey state and experimental design affect relative size of trait- and density-mediated indirect effects. Ecology 84:1140–1150. https://doi.org/10.1890/0012-9658(2003)084%5b1140:PSAEDA%5d2.0.CO;2
Matassa CM, Trussell GC (2011) Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92:2258–2266. https://doi.org/10.1890/11-0424.1
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692. https://doi.org/10.1016/j.tree.2005.08.002
Morelissen B, Harley CDG (2007) The effects of temperature on producers, consumers, and plant-herbivore interactions in an intertidal community. J Exp Mar Biol Ecol 348:162–173. https://doi.org/10.1016/j.jembe.2007.04.006
Morgan SG, Gravem SA, Lipus AC, Grabiel M, Miner BG (2016) Trait-mediated indirect interactions among residents of rocky shore tidepools. Mar Ecol Prog Ser 552:31–46
Nielsen KJ (2001) Bottom-up and top-down forces in tide pools: test of a food chain model in an intertidal community. Ecol Monogr 71:187–217. https://doi.org/10.1890/0012-9615(2001)071%5b0187:BUATDF%5d2.0.CO;2
Ohgushi T, Schmitz OJ, Holt RD (2012) Trait-mediated indirect interactions: ecological and evolutionary perspectives. Cambridge University Press, New York
Okuyama T, Bolker BM (2007) On quantitative measures of indirect interactions. Ecol Lett 10:264–271. https://doi.org/10.1111/j.1461-0248.2007.01019
Paine RT (1980) Food webs: linkage, interaction strength and community infrastructure- the 3rd Tansley lecture. J Anim Ecol 49:667–685. https://doi.org/10.2307/4220
Peacor SD (2003) Phenotypic modifications to conspecific density arising from predation risk assessment. Oikos 100:409–415
Peacor SD, Werner EE (1997) Trait-mediated indirect interactions in a simple aquatic food web. Ecology 78:1146–1156. https://doi.org/10.2307/2265865
Peacor SD, Werner EE (2001) The contribution of trait-mediated indirect effects to the net effects of a predator. Proc Natl Acad Sci USA 98:3904–3908. https://doi.org/10.1073/pnas.071061998
Persson L, De Roos AM (2003) Adaptive habitat use in size-structured populations: linking individual behavior to population processes. Ecology 84:1129–1139. https://doi.org/10.1890/0012-9658(2003)084%5b1129:AHUISP%5d2.0.CO;2
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? the effects of intimidation and consumption in predator-prey interactions. Ecology 86:501–509. https://doi.org/10.1890/04-0719
Raimondi PT, Forde SE, Delph LF, Lively CM (2000) Processes structuring communities: evidence for trait-mediated indirect effects through induced polymorphisms. Oikos 91:353–361
Ripple WJ, Beschta RL (2006) Linking wolves to willows via risk-sensitive foraging by ungulates in the northern Yellowstone ecosystem. For Ecol Manag 230:96–106. https://doi.org/10.1016/j.foreco.2006.04.023
Ruesink JL (2000) Intertidal mesograzers in field microcosms: linking laboratory feeding rates to community dynamics. J Exp Mar Biol Ecol 248:163–176. https://doi.org/10.1016/s0022-0981(00)00170-2
Schmitz OJ (1998) Direct and indirect effects of predation and predation risk in old-field interaction webs. Am Nat 151:327–342
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–163. https://doi.org/10.1111/j.1461-0248.2003.00560.x
Strong DR (1992) Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73:747–754. https://doi.org/10.2307/1940154
R Core Team (2013) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/. Accessed 7 Sept 2017 (ISBN: 3-900051-07-0)
Toscano BJ, Newsome B, Griffen BD (2014) Parasite modification of predator functional response. Oecologia 175:345–352
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245
Trussell GC, Ewanchuk PJ, Bertness MD, Silliman BR (2004) Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia 139:427–432. https://doi.org/10.1007/s00442-004-1512-8
Trussell GC, Ewanchuk PJ, Matassa CM (2006) Habitat effects on the relative importance of trait- and density-mediated indirect interactions. Ecol Lett 9:1245–1252. https://doi.org/10.1111/j.1461-0248.2006.00981.x
Turner AM, Mittelbach GG (1990) Predator avoidance and community structure-interactions among piscivores, planktivores, and plankton. Ecology 71:2241–2254. https://doi.org/10.2307/1938636
Wada Y, Iwasaki K, Yusa Y (2013) Changes in algal community structure via density- and trait-mediated indirect interactions in a marine ecosystem. Ecology 94:2567–2574. https://doi.org/10.1890/12-0725.1
Weissburg M, Smee DL, Ferner MC (2014) The sensory ecology of nonconsumptive predator effects. Am Nat 184:141–157. https://doi.org/10.1086/676644
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr 39:1985–1992
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. https://doi.org/10.1890/0012-9658(2003)084%5b1083:AROTII%5d2.0.CO;2
Wood SN (2006) Generalized additive models: an introduction. R Chapman and Hall/CRC, New York
Yarnall JL (1964) The responses of Tegula funebralis to starfishes and predatory snails (Mollusca: Gastropoda). Veliger 6:56–58
Acknowledgements
For assistance in the field and laboratory, thanks to Sarah Traiger, Olivia Turnross, Aiko Michot, Alex von Boer, Jonathan Demmer, Ryanne Ardisana, Amy Fonarow, Andrew Chen, Preston Malm, and Mimi Gravem. Thanks to Blake Brown, Sarah Hameed, and Erin Satterthwaite for support. Thanks to Drs. Seth Miller, Mark Novak, Susan Williams, Andy Sih, Eric Sanford, Brian Gaylord, Jarrett Byrnes, Ben Dalziel and our anonymous reviewers for providing constructive comments on experimental design and/or the manuscript. This is a contribution of Bodega Marine Laboratory and dedicated to the memory of Dr. Susan Williams, whose many direct and indirect effects are intense, profound, and will be long-lasting.
Funding
This study was funded by California SeaGrants R/FISH218, R/MPA14 and R/MPA24 awarded to Steven Morgan, National Science Foundation Grants OCE-1334448 and OCE-0927196 awarded to Steven Morgan, National Science Foundation GK-12 Grant 0841297 awarded to Susan Williams, the Conchologists of America, the Mildred E. Mathias Foundation, Henry A. Jastro fellowship, the UC Davis Graduate Group in Ecology, and the Bodega Marine Laboratory Fellowship.
Author information
Authors and Affiliations
Contributions
SG and SM conceived the project idea. SG collected and analyzed the data and was the primary author. SM provided constant guidance and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving. animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All collections and manipulations of animals in the study were in compliance with the California Department of Fish and Game collecting permit SC-4688 to Steven Morgan and approved by the University of California Natural Reserve System.
Additional information
Responsible Editor: P. Gagnon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by B. Miner and undisclosed experts.
Rights and permissions
About this article
Cite this article
Gravem, S.A., Morgan, S.G. Trait-mediated indirect effects in a natural tidepool system. Mar Biol 166, 23 (2019). https://doi.org/10.1007/s00227-019-3469-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3469-5