Abstract
Shearwaters are among the most abundant seabirds globally and breeding birds often travel thousands of kilometres during foraging trips to productive marine areas. Great Shearwaters Ardenna gravis are endemic breeders of the Tristan da Cunha Archipelago with a population of 5–6 million breeding pairs making them key top predators within the South Atlantic Ocean. We deployed satellite transmitters on 42 breeding and non-breeding shearwaters at two nesting islands and one foraging site in the northern hemisphere to quantify their movements and space use with respect to age, sex, colonies and breeding phases. During the pre-laying period, birds from Gough Island made trips of 2400 km to the Benguela upwelling region off South Africa, or > 4000 km to the Patagonian Shelf; patterns which overlapped with immature birds from the Bay of Fundy, Canada. During the incubation period, males and females from Inaccessible Island showed differences in trip durations, but no difference in foraging ranges: both sexes made trips of 1500–2000 km to the Sub-Antarctic Front or 4000 km to the Patagonian Shelf, among the furthest foraging trips of breeding seabirds observed to date. Although the colonies are separated by only 400 km, during the incubation period, the at-sea distribution of non-breeding birds from Gough Island was spatially segregated from breeding and failed birds from Inaccessible Island, whereas immature birds from Fundy overlapped with birds from both colonies. During the post-breeding period, all tagged populations overlapped on the Patagonian Shelf. Argentina’s Exclusive Economic Zone was used extensively during pre-laying, incubation, and post-breeding periods, highlighting the global importance of the Patagonian Shelf for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds are highly mobile marine predators that may travel vast distances during breeding and non-breeding periods to obtain prey. In particular, birds of the order Procellariiformes, such as shearwaters and petrels, are capable of traveling thousands of kilometers during single foraging trips from their breeding colonies (Pinet et al. 2012; Cleeland et al. 2014; Pollet et al. 2014). Due to these extraordinary abilities, it has been difficult to accurately quantify the spatial and temporal patterns of marine space use from observations alone. Bio-logging technology has revolutionized our ability to answer questions about how individuals and populations partition their use of space in the marine environment (Jouventin and Weimerskirch 1990; Raymond et al. 2010; de Grissac et al. 2016).
Seabirds may partition their marine habitats in a variety of ways to reduce competition among or within species. Species may reduce competition by segregating their diet (Bond et al. 2010; Steenweg et al. 2011; Delord et al. 2016), space use (Masello et al. 2010; Ronconi and Burger 2011), or by breeding at different times (Friesen et al. 2007). Moreover, neighbouring colonies of the same species may also show spatial segregation at sea (Grémillet et al. 2004; Masello et al. 2010; Wakefield et al. 2013; Clay et al. 2016), likely owing to density-dependent resource depletion around their breeding sites (Lewis et al. 2001; Gaston et al. 2007). Intrinsic factors, such as age (Gutowsky et al. 2014) and sex (Phillips et al. 2004; Pinet et al. 2012; Ludynia et al. 2013), may further lead to species-specific patterns of space use and habitat partitioning. Thus, a complete understanding of marine habitat use by avian species requires representative samples of sexes, ages, and colonies, ideally tracked during multiple periods of the annual cycle.
The Great Shearwater Ardenna gravis is a medium-sized seabird with a widespread distribution in the North and South Atlantic Oceans. Apart from a few hundred pairs in the Falkland Islands, they are endemic to the islands of the Tristan da Cunha Archipelago in the center of the South Atlantic, roughly 2500 km west of South Africa and 3500 km east of South America. Nesting occurs within this archipelago, but these birds also undertake impressive transequatorial migrations of over 15,000 km twice a year, moving between the breeding colonies during the austral summer to feeding areas in the North Atlantic during the boreal summer. With a breeding population of approximately 5–6 million pairs (Ryan 2007), the total global population is estimated around 16.5 million individuals, inclusive of pre-breeding age birds (Fishpool and Evans 2001). Feeding mainly on fish and squid, this species is ranked 13th among more than 300 seabird species in terms of total prey consumed by weight (de Brooke 2004). Although they have a conservation status of Least Concern with an apparently stable population (BirdLife International 2017), threats at sea include interactions with offshore infrastructure (Ronconi et al. 2015), mortality in oil spills (Haney et al. 2014), and, especially, bycatch in numerous fisheries throughout their global range (Barnes et al. 1997; Glass et al. 2000; Bugoni et al. 2008; Jiménez et al. 2009; ICES 2013; Hatch et al. 2016). Moreover, mass mortality events of emaciated birds during their northern, post-breeding migration have been increasing in frequency over the past two decades, although the causes of these incidents are unknown (Lee 2009; Haman et al. 2013).
In this study, the spatio-temporal patterns of marine space use by Great Shearwaters in the South Atlantic Ocean were investigated using satellite telemetry of birds tracked from two breeding colonies in the southern hemisphere and one feeding site in the northern hemisphere. We tested for differences in foraging trip characteristics and space use by sex, age classes, breeding locations, and breeding phase. We hypothesized that spatial segregation of neighbouring populations would be strongest during breeding periods (Grémillet et al. 2004; Wakefield et al. 2013) relative to post-breeding periods when individuals are more likely to overlap (Clarke et al. 2010; Catry et al. 2011). During the breeding period, we expected little segregation between sexes for this monomorphic species (e.g. Hedd et al. 2014), but seasonal segregation may be associated with age because immature, non-breeding birds would not be restricted to central-place foraging. We also investigated their spatial overlap within the Exclusive Economic Zones (EEZ) of surrounding countries where birds may be captured as incidental take in fisheries. Specifically, we investigated whether use of shelf waters off South American and Africa differed among colonies or age classes.
Materials and methods
Study species
Great Shearwaters breed over a 7.5-month period from September to April. The phenology includes arrival at the colony in mid-September, courting and mating until mid-October, a 1-month pre-laying exodus, and egg laying upon return in mid-November (Cuthbert 2005; Ryan 2007). Their single egg is incubated for ~ 53 days, hatching in mid-January, and chicks fledge around the last week of April, ~ 108 days after hatching (Elliott 1957; Cuthbert 2005). Sexes are monomorphic, although males average slightly larger in some morphological measurements (Hockey et al. 2005). Both sexes incubate eggs and provision young, with males taking the first incubation shift post-laying (Ronconi unpubl. data) and chick provisioning occurring on average every 3–4 days (Cuthbert 2005). Birds forage by surface seizing and shallow diving typically less than 10 m deep, in cold and warm waters up to 4000 km away from the colony (Ronconi et al. 2010a). Individuals migrate to the northern hemisphere from April through September, although some individuals remain in the North Atlantic until November (Harrison 1985).
Tracking data
Birds were tagged at three sites during breeding and non-breeding periods (Table 1). Between 2006 and 2009, 24 birds were captured in August from small vessels with the use of chumming and dip-nets or hoop-nets (Ronconi et al. 2010b) in the Bay of Fundy, Canada (44.47–44.87°N, 66.82–66.52°W). Other studies of Great Shearwaters in the Bay of Fundy and adjacent Gulf of Maine suggest that most (~ 90%) of birds using this area, including the same tagged individuals in this study, are < 3 years old (Powers et al. 2017), therefore, most tagged birds from Fundy were likely immature. In 2009, 22 birds were caught in their burrows at breeding colonies: 6 on Gough Island (40.35°S 9.88°W; population size 1 million pairs) and 8 males and 8 females on Inaccessible Island (37.29°S 12.70°W; 2 million pairs), one of the islands in the Tristan da Cunha Archipelago (Ryan 2007). On Gough, birds were tagged in late September and early October (mean mass at tagging = 789 g ± 30 SD), so their breeding status was not confirmed because pre-breeding age birds may also attend their nest during this time. On Inaccessible, males were tagged on 21st November towards the end of their first incubation shift (mass 797 ± 57 g), and females were tagged between 25 and 30 November upon returning for their first foraging trip post-laying (mass 941 ± 52 g). Birds were sexed by cloacal inspection shortly after egg-laying.
Birds were fitted with battery-powered platform terminal transmitters (PTTs) attached dorsally, between the wings, central to the body mass of the bird. Tags were attached using a combination of waterproof tape (Tesa 4651), glue (Loctite 422), and four sub-cutaneous sutures (Prolene 4-0, Ethicon) as per MacLeod et al. (2008). Three types of PTTs were used: North Star (model 30G, 36 g, n = 3 birds in Fundy 2008), Wildlife Computers (AC1, 19 g, n = 2 birds in Fundy 2008), and SirTrack Wildlife Tracking Solutions (Kiwi Sat 202, 32–34 g, all other sites and years). PTTs represented less than 3% of body mass for non-breeding birds captured in Fundy (mass 1039 ± 90 g, range 920–1280 g) and approximately 3.5–4% of body mass for breeding birds. Duty cycles for tags deployed in Fundy were set at 8-h (on) and 16-h (off), whereas tags deployed on breeding birds were set at 6-h (on) and 42-h (off). During a typical on period this resulted in 2–8 estimated locations through the Argos satellite system (overall 5.1 ± 2.1 SD locations per day per bird). Each observed location included an associated location class (LC) quality ranking which varies in accuracy from less than 1 km (LC-3) to 10 s of km error (LC-A and LC-B) for free-ranging marine animals (Costa et al. 2010). These errors were reduced with the use of state-space models (details below).
Location estimation
To improve the confidence of Argos location estimates and the evenness of sampling intervals along foraging tracks (Reid et al. 2014; Jodice et al. 2016), we fitted a Bayesian switching state-space model (SSSM) to all tracking data using bsam R package (Jonsen et al. 2005, 2013). While a standard state-space model provides improved location estimates and evenness of sampling, the “switching” part of the model provides an estimated behaviour on a scale between 1 and 2, representing transitory or area-restricted search (i.e., foraging) behaviours, respectively (Jonsen et al. 2013; Powers et al. 2017). This behaviour estimate was used to determine, and omit, the portions of the tracks associated with migration (see Analysis section). Estimated locations were derived using the hierarchical first-difference correlated random walk with switching (hDCRWS), which was run in batches on groups of four birds, keeping batches separate for Fundy and Gough/Inaccessible birds. The modelling approach uses a Markov chain Monte Carlo method with 10,000 iterations (thinned by every 10th record) after a burn-in of 40,000 iterations to eliminate the effects of initial values. The model was run with a 6-h time-step so that estimated locations were normalized over a regular interval for the entire tracking period. Previous SSSM analysis found similar results between model outputs when a 3-h time step was applied to duty-cycled tags (8 on and 16 off) and continuously transmitting tags (Powers et al. 2017). However, due to the longer “off” period of the Inaccessible and Gough birds, we opted for a slightly longer (6-h) time-step to produce more generalized tracks. Examples of the application and results of these models for tracking data of Great Shearwaters are illustrated in the supplementary materials of Powers et al. (2017).
Analysis
Tracking data were selected and classified in three steps to focus analysis on non-migratory periods in the southern hemisphere from September to May. First, we classified all tracking data into migratory and non-migratory periods by investigating the “behaviour” state (b) from the SSSM and the direction of movement. Migration initiation was defined by 3 days of continuous movement (b < 1.5) in an expected migratory direction (i.e., southward for Fundy tagged birds and northward for birds tagged at colonies). Migration termination was defined by 2 days of area-restricted search behaviour (b > 1.7) after a prolonged migratory period and after reaching expected destination latitude (< 30° S or > 30° N for south-bound- and north-bound migrations, respectively). Subsequent analysis included only non-migratory periods in the southern hemisphere.
Within the southern hemisphere, tracking data was classified into three phases based on expected timing of breeding phenology (Cuthbert 2005) and observed behaviour of the birds with respect to colony attendance. The “pre-laying” period included birds tracked from Gough in late September until their first return to the colony in early November, and birds tracked from Fundy once their south-bound migration was completed (see above) until their first arrival at any colony in early November. For Gough and Fundy tagged birds which did not return to a colony, the end of the “pre-laying” was defined as 10 November, which coincides approximately with peak egg-laying (Elliott 1957; Cuthbert 2005) and observed patterns of colony visits from Fundy tagged birds (see Results section). The “incubation” period included all days after the “pre-laying” period, until the last visit to the colony or 14 January, which ever came later (all birds stopped attending the colony by the first week of February and, therefore, are presumed to have failed). Hatching was expected around mid-January (Elliott 1957; Cuthbert 2005), therefore, we present no data related to the chick-rearing period. The “post-breeding” period included all remaining locations until the onset of migration (average = 26 May, range 15 May to 05 June, n = 11 birds tracked until migration on-set).
Breeding status was also inferred from observed behaviour of colony attendance. Fundy birds were thought to be “immatures” and Gough birds appeared to be “non-breeders” (see details in the results). Birds tagged on Inaccessible Island were confirmed breeders (eggs laid) but some did not return to the colony after tagging (abandoned) or ceased colony attendance part-way through incubation. Therefore, within the incubation period we classified birds as “breeding” or “failed”, relative to the last visit to the colony.
Individual foraging trips were identified from the tracking data when there was a clear departure and return to the colony, of more than 2 days. This included Gough birds during the pre-laying period and Inaccessible birds during the incubation period. Foraging trip characteristics, including number of days, total distance travelled and maximum distance from colony, were obtained by creating trajectories of SSSM location estimates using adehabitatLT R package (Calenge 2006). Welch Two Sample t-tests or non-parametric Wilcoxon’s rank-sum tests were used to determine if there were significant differences between foraging trip characteristics of birds from Gough and Inaccessible Island, and between males and females from Inaccessible Island.
Spatial analysis was performed on estimated locations from the SSSM analysis, which provided a regular sampling interval required for kernel density analysis. The kernel utilization distribution of tracked birds was estimated using the kernelUD function of adehabitatHR R package (Calenge 2006) with a constant smoothing parameter (h = 2). Given the small sample sizes of individuals tracked during some periods (Table 1), we calculated the kernel areas [50 and 90% utilization distributions (UD)] for different sample sizes (from 1 to the maximum/true sample) and plotted these to see if the size of the distribution area reaches an asymptote (e.g., Gutowsky et al. 2015). For most populations tracked within each breeding period, both the 50 and 90% UD appeared to reach an asymptote with 5–10 tracked birds (Online Supplementary Material 1); therefore, areas used may be slightly underestimated for birds from Gough Island (all periods) and Fundy birds during the post-breeding period when only six tags were still active.
The overlap between distribution estimates were done with the kerneloverlaphr function in adehabitatHR (Calenge 2006) using the Bhattacharyya’s affinity (BA) and the utilization distribution overlap index (UDOI), as recommended by Fieberg and Kochanny (2005). The BA method quantifies the degree of similarity among utilization distributions and is a value bounded between 0 (no overlap) and 1 (complete overlap), whereas the UDOI quantifies space-use sharing where, relative to uniform space use, values < 1 indicate less overlap and values > 1 indicate higher than normal overlap. Additionally, the amount of overlap of tracked points with the EEZ of different countries was calculated as the percentage of points falling within each EEZ.
Results
From 46 tags deployed, we collected more than 25,000 locations (82.3% with Argos LC 0–3) during 4919 bird-tracking days (excluding days when the tags were “off”). Overall mean tracking duration of all individuals was 158.6 days ± 70.0 SD (range 32–361 days). Birds from Gough and Inaccessible were tracked for an average of 196.9 ± 36.3 and 201.9 ± 76.2 days, respectively, compared with an average of 120.2 ± 47.8 days from deployments in the Bay of Fundy. After omitting migratory periods this retained 16,035 locations from 3432 bird-tracking days and 42 birds (omitting 4 birds tracked from Fundy which showed incomplete migrations to the southern hemisphere, Table 1). Argos data processed with state-space models resulted in 21,600 estimated locations used for the kernel utilization density analysis (tracking data are illustrated in Online Supplementary Materials 2).
Of the 18 Bay of Fundy tagged birds which were tracked to the South Atlantic, only one visited a colony during the courtship period (arrived at Nightingale Island 26 Sep) and showed some indication of regular colony attendance during the incubation period (returning 05 Nov). None of the others showed behaviour consistent with breeding patterns, confirming previous suggestions that most Fundy birds are not of breeding age (Powers et al. 2017). First arrival of other birds at colonies coincided roughly with the egg-laying period: Nightingale (n = 5, mean = 18 Nov, range 7 Oct–3 Jan), Inaccessible (n = 6, mean = 13 Nov, range 10–18 Nov), Gough (n = 3, mean = 10 Nov, range 5–13 Nov). Three Fundy birds (17%) showed no colony association. Hereafter, we refer to Fundy tagged birds as “immatures”.
For birds tagged on Gough during the courtship period, five of six individuals returned to the colony but none showed regular colony attendance, so these birds may have been failed breeders or were pre-breeding age birds. Five complete foraging trips were obtained during the pre-laying period. Average pre-laying trip duration was 61.4 ± 13.6 days (range 41–76 days, Table 2). Birds departed within one or 2 days of tag deployment (range 30 Sept–4 Oct) and returned on 12 November (n = 1), 26 November (n = 1), 7 December (n = 2), and 19 December (n = 1). For each bird, transmissions ceased for 1 or 2 cycles upon returning to the island, suggesting they were attending burrows. However, this occurred well after estimated peak laying date for this colony (21 Nov, Cuthbert 2005). Though all individuals appear to have visited or at least approached the colony again in January, no foraging trips during the incubation period were evident; therefore, trip characteristics were not directly comparable to deployments during incubation on Inaccessible Island. Together, these behaviours suggest that birds tagged on Gough were non-breeders, or skipped breeding (possibly due to device effects, see “Discussion”). Hereafter, we refer to Gough-tagged birds as “non-breeders”.
A total of 13 foraging trips (11 individuals) were obtained from Inaccessible birds during the incubation period (Table 2), before apparent nest failures. Average incubation trip duration was 25.1 ± 6.2 days (range 12–34 days). At Inaccessible, the duration of foraging trips during incubation was longer for males (28.2 ± 3.7 days, n = 7) than for females (21.5 ± 6.8 days, n = 6; t = 2.28, df = 11, p = 0.043). Five birds (31%) departed the colony by mid-December and did not return, ten birds (63%) made one or two foraging trips until mid-January, and one bird (ID 96802a) made two trips with the last trip returned to the colony during the first week of February. This suggests that birds abandoned early on or experienced reproductive failure during late incubation (see “Discussion” section for implications of results).
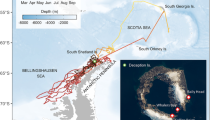
There was no significant difference in maximum distance from the islands between the two sites (W = 44, p = 0.257; Fig. 1, Table 2). During pre-breeding, maximum distance from Gough Island was either 2000–2500 km east to deep water or continental slope waters off the coast of South Africa, or more than 4000 km west to the Patagonian shelf off the coast of Argentina. Incubating birds from Inaccessible Island travelled similar maximum distances: either 1500–2000 km south-east to the Sub-Antarctic Front or approximately 4000 km to the Patagonian Shelf. Maximum distance from the colony was correlated with trip duration for birds during the incubation phase on Inaccessible Island (R2 = 0.275, F1,11 = 5.55, p = 0.038): shorter trips up to ~ 2000 km averaged 21.6 ± 6.7 days, whereas long trips to ~ 4000 km averaged 28.1 ± 4.0 days. Total distance travelled and the maximum distance from the island was not significantly different between males and females from Inaccessible Island (W = 30 and 29, p = 0.20 and 0.25, respectively); both sexes performed shorter and long-distance trips (Table 2, Fig. 1). Consecutive trips were recorded for two individuals: one female conducted two shorter trips and one male conducted two long trips (Table 2).
Foraging trips of Great Shearwaters Ardenna gravis from two breeding colonies. Birds were tracked from a Gough Island during the pre-laying period (n = 5) and b Inaccessible Island during the incubation period with male (blue, n = 6) and female (red, n = 5) differentiated. Colonies are indicated by a pentagon (Inaccessible Island) and black star (Gough Island). Maximum distances are indicated with a yellow star for each foraging trip. Underlying environmental variables include 3000 m bathymetric contours (grey lines), the Sub-Antarctic Front (black-dashed line), and boundaries of exclusive economic zones (EEZ; black solid line)
Great Shearwaters overlapped with the EEZs of five countries, but birds from all tagged populations spent most time within the Argentinean EEZ during all three phases of the breeding period (Table 3). One notable difference among populations was the absence of birds from Inaccessible Island in the South African EEZ, despite the relatively large sample size of birds from this island (Table 3). During the pre-laying period, Gough birds went east of the island towards South Africa or west to the coastal waters off Argentina (Figs. 1 and 2). Fundy birds were found mostly within Argentinean waters, where most of the overlap with Gough birds occurred (Fig. 2a, Table 4). During the incubation period, birds from Fundy and Inaccessible Island were mainly west of the breeding colonies, while Gough birds were mainly east (Fig. 2b). During the incubation period, the overlap between Gough and Inaccessible birds was lower than the overlap of Fundy and Inaccessible birds; breeding and failed birds from Inaccessible had the greatest overlap during this period but UDOI values were still < 1, suggesting lower than uniform space-use sharing (Table 4). During the post-breeding period (15 Jan–May), Great Shearwaters from all sites were west of the breeding colonies, concentrated along the Argentinean coast (Fig. 2c). Birds from all three sites were found close to the coast of Argentina, with a high degree of overlap (> 80%) during the post-breeding period; immature birds from Fundy and non-breeders from Gough showed higher than normal space-use sharing (UDOI = 1.27) during this period (Table 4). Both the kernel utilization distributions (Fig. 2) and the raw tracking data (Fig. 1 and Online Supplementary Material 2) suggest that the southern limit of Great Shearwater foraging distribution may be defined by the Sub-Antarctic Front.
Distribution of Great Shearwaters Ardenna gravis tracked from the Bay of Fundy, Inaccessible Island (pentagon) and Gough Island (star) during: a pre-laying exodus, b incubation and c post-breeding periods. Contours indicate the 50% (solid thicker colour lines) and 90% (dashed thinner colour lines) kernel utilization densities. Breeding status was determined by the behaviour of tagged birds: Fundy and Gough populations are thought to be immatures and/or non-breeders, and Inaccessible birds were classified as “failed” after the last visit to the colony. Underlying environmental variables include 3000 m bathymetric contours (grey lines), the Sub-Antarctic Front (black dashed line), and boundaries of exclusive economic zones (EEZ; black solid line)
Assuming that colony visitations of Fundy tagged birds are indicative of their natal or future breeding sites, we further investigated the distribution of immature (or pre-breeding age) birds with respect to their presumed colony (Fig. 3). During the pre-laying period, all birds overlapped extensively on the Patagonian Shelf (Fig. 3a). During the incubation period, Gough-associated birds showed distributions east of Gough Island, mostly segregated from immature birds of other colonies (Fig. 3b). One immature bird associated with Nightingale Island (near to Inaccessible) showed high usage of waters in the South African EEZ (Fig. 3).
Distribution of immature (non-breeding) Great Shearwaters Ardenna gravis tracked from the Bay of Fundy to the South Atlantic during: a pre-laying exodus (September to 10 November), and b incubation period (11 November to 15 January). Contours indicate the 50% (solid thicker colour lines) and 90% (dashed thinner colour lines) kernel utilization densities. Immature birds were assigned a colony association if tracking data showed a visitation to one of three colonies: Inaccessible (orange), Nightingale (green), Gough (blue); “unknown” is indicated for birds that did not visit any colony (red; see methods and results for details). Underlying environmental variables include 3000 m bathymetric contours (grey lines), the Sub-Antarctic Front (black dashed line), and boundaries of exclusive economic zones (EEZ; black solid line)
Discussion
Individual foraging trips during pre-laying (Gough) and early incubation (Inaccessible) revealed foraging destinations more than 4000 km away. To date, these are among the furthest foraging trips of any breeding seabird (see Table 3 in Pollet et al. 2014), comparable to Short-tailed Shearwaters Ardenna tenuirostris tracked from colonies in Tasmania (mean ~ 2200 km, maximum 5056 km; Cleeland et al. 2014), and much further than other petrel species tracked from Inaccessible Island (Reid et al. 2014). These extraordinary distances highlight the importance of the Patagonian Shelf to this species, even when other foraging areas are available within 2000 km at the Sub-Antarctic Front (Ronconi et al. 2010a) and shelf waters off the coast of South Africa (Crawford et al. 1991; Delord et al. 2014). Moreover, the Patagonian Shelf was extensively used by Great Shearwaters during the pre-laying and post-breeding periods (Fig. 2). However, it is important to consider that most birds tracked in this study appear to have failed breeding by mid- to late-January (see methods), when hatching would have occurred. Therefore, foraging ranges and areas for chick-rearing Great Shearwaters are still unknown and likely differ from results reported here given that chicks are fed at 3–4 day intervals (Cuthbert 2005), suggesting trips of shorter distance and duration. Hatching success for this species is typically 56%, similar to that of other shearwaters (Cuthbert 2005), suggesting that higher nest failure rates that we observed could have been caused by the stress of capture or the added burden of carrying tags weighing 3–3.5% of their body mass, at the upper limit of “acceptable” tag size for shearwaters (Phillips et al. 2003). In particular, on Inaccessible Island, any absence from the burrow or mistimed changeover of incubation duties can result in depredation of eggs by the endemic Tristan Thrush Nesocichla eremita (Ryan and Ronconi 2010). The attachment method with sutures has not been shown to impact breeding success in other species (Pollet et al. 2014; Loring 2016). Smaller tracking devices, such as newer GPS technologies, would provide better information on marine space use during chick-rearing periods for this species. Nonetheless, trip durations during the incubation period reported in this study were similar to birds tagged with much smaller time-depth recorder tags at Inaccessible Island in the same year as the tracking study (Ronconi et al. 2010a), suggesting that observed foraging trips were likely representative of this species during the incubation phase.
We observed some differences in trip duration between males and females, but no differences between sexes in the total or maximum distances travelled. Studies of sexually dimorphic seabirds have revealed sex partitioning of habitat during breeding periods (Phillips et al. 2004, 2011; Ludynia et al. 2013). Sexually monomorphic shearwaters and petrels exhibit behavioural and spatial partitioning between sexes during the pre-laying and egg-laying periods, but not during incubation or chick rearing (Pinet et al. 2012; Hedd et al. 2014). While no pre-laying data of known sex birds were available in this study, the foraging trips of known sex birds from Inaccessible Island (Table 2) were associated with the first trips after egg laying for males, and the second trip of females (since females were tagged after returning to the colony following their first trip post-laying). Therefore, sex differences in trip duration may be related to post-laying recovery of females, whereby females would have taken a longer trip during their first departure after laying (Pinet et al. 2012). The few trips recorded and the very long distances to foraging grounds for both males and females may make it difficult to assess apparent sex-related foraging strategies, if any. Sex-related habitat partitioning in shearwaters is more likely to occur during pre-laying periods (Hedd et al. 2014), or possibly during chick-rearing periods (not tested in this study).
The most striking finding from this study was the apparent at-sea segregation between birds breeding on Gough and Inaccessible Islands, which are only 400 km apart. Although raw tracking data and 90% kernel utilization distributions show general overlap of foraging ranges during the incubation period, overlap indices for “core” foraging areas (50% kernel utilization distributions) were very low between Gough non-breeders and Inaccessible breeding or failed birds. Likewise, the distributions of immature birds tagged in Fundy that associated with Inaccessible and Gough colonies also support the conclusions about the east–west segregation between these neighbouring sites (Fig. 3b). Some of the largest seabird species exhibit spatial segregation of foraging areas among neighbouring colonies (Grémillet et al. 2004, Wakefield et al. 2013). Intraspecific competition and colony size may interact to determine density-dependent foraging ranges for several seabird species (Lewis et al. 2001; Gaston et al. 2013; Jovani et al. 2016), validating tenants of “Ashmole’s halo” effects and central-place foraging theory (Gaston et al. 2007). Thus, when breeding populations of adjacent colonies are very large, as with the millions of Great Shearwaters breeding at our two study islands, one might expect a high degree of spatial segregation to minimize intraspecific competition (Masello et al. 2010). Alternatively, winds may play a crucial role in the movements and space use of shearwaters (Adams and Flora 2010) and the north–south separation between Gough and Inaccessible Islands may expose these populations to different wind regimes, facilitating spatial segregation during the breeding season when birds are strongly tied to the colony. However, Short-tailed Shearwater colonies of even greater population size, separated by similar distances, and exposed to different wind regimes still show considerable overlap in foraging areas (Raymond et al. 2010); thus, an explanation for the segregation between Gough and Inaccessible Island colonies requires further examination.
Despite the segregation between Gough and Inaccessible birds, we observed spatial overlap between failed and breeding birds from Inaccessible Island as well as between immature birds from the Bay of Fundy and adults from Inaccessible or Gough. The Tristan da Cunha Archipelago, including Inaccessible and Nightingale Islands, comprises > 80% of the global population of Great Shearwaters, so if the Fundy birds were a random subset of immature shearwaters, then one would expect greater similarity in spatial overlap between these groups (see also Fig. 3). Necropsies of dead birds and plumage of live birds from the Gulf of Maine and Bay of Fundy suggest that most birds from this region are juvenile and immature birds (Lee 2009; Haman et al. 2013; Powers et al. 2017). Moreover, most birds tracked from Fundy to the South Atlantic showed no clear association with specific colonies until near or after the egg-laying period, supporting the notion that these were pre-breeding age birds. Few studies have tracked juvenile or immature seabirds (Hazen et al. 2012), or contrasted these findings with adult birds. Juvenile albatrosses tracked during the first few months of their life show flight paths and movement behaviours similar to adults (de Grissac et al. 2016), but this may still result in habitat segregation (Gutowsky et al. 2014). Moreover, Short-tailed Albatrosses Phoebastria albatrus showed age-related differences in core foraging areas with first-year birds distributed more broadly than adults (O’Connor 2013). While there is some evidence that age classes of Great Shearwaters may segregate during non-breeding periods in the North Atlantic (Lee 2009; Haman et al. 2013; Powers et al. 2017), our results suggest widespread overlap between age classes in the South Atlantic.
Notwithstanding the possible effects of tags on the breeding performance of Great Shearwaters (see above), our results indicate that the Patagonian Shelf, and the Argentinian EEZ, is of particular importance for this species throughout its breeding and post-breeding season from September to May, for both breeding and non-breeding birds. Likewise, this area is extremely important for other marine top-predators that reside in the South Atlantic (Phillips et al. 2006; Hedd et al. 2014; Gonzalez Carman et al. 2016) or migrate here from breeding areas in the northern hemisphere (Krüger et al. 2017). Several fisheries in Brazil, Uruguay and Argentina take large numbers of shearwaters, petrels, and albatrosses as bycatch (Bugoni et al. 2008; Jiménez et al. 2009, 2010; Favero et al. 2013). The population level impact of these fisheries on Great Shearwaters, along with additional take in gillnet fisheries in the northern hemisphere (ICES 2013; Hatch 2017), is not known, but further analyses of tracking data from different population segments (this study) in conjunction with bycatch data (e.g., Hatch et al. 2016) may reveal which components of the population are most vulnerable to this cumulative bycatch. Refined spatial analysis of these tracking data may better identify fine-scale habitat associations of Great Shearwaters within the Patagonian Shelf ecosystem (e.g., Gonzalez Carman et al. 2016), revealing important areas of marine productivity, regions where bird distributions overlap with fisheries, and areas of international significance to marine biodiversity conservation (Krüger et al. 2017).
References
Adams J, Flora S (2010) Correlating seabird movements with ocean winds: linking satellite telemetry with ocean scatterometry. Mar Biol 157:915–929. https://doi.org/10.1007/s00227-009-1367-y
Barnes KN, Ryan PG, Boix-Hinzen C (1997) The impact of hake Merluccius Spp. longline fishery off South Africa on Procellariiform seabirds. Biol Conserv 82:227–234
BirdLife International (2017) Ardenna gravis. (amended version published in 2016). The IUCN Red List of Threatened Species 2017
Bond AL, McClelland GTW, Jones IL, Lavers JL, Kyser TK (2010) Stable isotopes confirm community patterns in foraging among Hawaiian Procellariiformes. Waterbirds 33:50–58
Bugoni L, Mancini PL, Monteiro DS, Nascimento L, Neves TS (2008) Seabird bycatch in the Brazilian pelagic longline fishery and a review of capture rates in the southwestern Atlantic Ocean. Endanger Species Res 5:137–147
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Catry P, Dias MP, Phillips RA, Granadeiro JP (2011) Different means to the same end: long-distance migrant seabirds from two colonies differ in behaviour, despite common wintering grounds. PLoS One 6:e26079. https://doi.org/10.1371/journal.pone.0026079
Clarke TCR, Diamond AW, Chardine JW (2010) Origin of Canadian Razorbills (Alca torda) wintering in the outer Bay of Fundy confirmed by radio-tracking. Waterbirds 33:541–545. https://doi.org/10.1675/063.033.0414
Clay TA, Manica A, Ryan PG, Silk JRD, Croxall JP, Ireland L, Phillips RA (2016) Proximate drivers of spatial segregation in non-breeding albatrosses. Sci Rep 6:29932
Cleeland JB, Lea M-A, Hindell MA (2014) Use of the Southern Ocean by breeding Short-tailed Shearwaters (Puffinus tenuirostris). J Exp Mar Biol Ecol 450:109–117. https://doi.org/10.1016/j.jembe.2013.10.012
Costa DP, Robinson PW, Arnould JPY, Harrison A, Simmons SE, Hassrick JL, Hoskins AJ, Kirkman SP, Oosthuizen H, Villegas-Amtmann S, Crocker DE (2010) Accuracy of ARGOS locations of pinnipeds at-sea estimated using Fastloc GPS. PLoS One 5:e8677. https://doi.org/10.1371/journal.pone.0008677
Crawford RJM, Ryan PG, Williams AJ (1991) Seabird consumption and production in the Benguela and western Agulhas ecosystems. S Afr J Mar Sci 11:357–375
Cuthbert RJ (2005) Breeding biology, chick growth and provisioning of Great Shearwaters (Puffinus gravis) at Gough Island, South Atlantic Ocean. Emu 105:305–310
de Brooke ML (2004) The food consumption of the world’s seabirds. Proc R Soc B Biol Sci 271(Suppl):S246–S248. https://doi.org/10.1098/rsbl.2003.0153
de Grissac S, Börger L, Guitteaud A, Weimerskirch H, Briggs DR (2016) Contrasting movement strategies among juvenile albatrosses and petrels. Sci Rep 6:26103. https://doi.org/10.1038/srep26103
Delord K, Barbraud C, Bost C-A, Deceuninck B, Lefebvre T, Lutz R, Micol T, Phillips RA, Trathan PN, Weimerskirch H (2014) Areas of importance for seabirds tracked from French southern territories, and recommendations for conservation. Mar Policy 48:1–13. https://doi.org/10.1016/j.marpol.2014.02.019
Delord K, Pinet P, Pinaud D, Barbraud C, De Grissac S, Lewden A, Cherel Y, Weimerskirch H (2016) Species-specific foraging strategies and segregation mechanisms of sympatric Antarctic fulmarine petrels throughout the annual cycle. Ibis 158:569–586. https://doi.org/10.1111/ibi.12365
Elliott HFI (1957) A contribution to the ornithology of the Tristan da Cunha group. Ibis 99:545–586
Favero M, Blanco G, Copello S, Pon JPS, Patterlini C, Mariano-Jelicich R, García G, Berón MP (2013) Seabird bycatch in the Argentinean demersal longline fishery, 2001–2010. Endanger Species Res 19:187–199. https://doi.org/10.3354/esr00478
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manag 69:1346–1359
Fishpool L, Evans M (2001) Important Bird Areas in Africa and Associated Islands. BirdLife International Conservation Series No. 11. Pices Publications and BirdLife International, Newbury and Cambridge
Friesen VL, Smith AL, Gómez-Díaz E, Bolton M, Furness RW, González-Solís J, Monteiro LR (2007) Sympatric speciation by allochrony in a seabird. Proc Natl Acad Sci 104:18589–18594. https://doi.org/10.1073/pnas.0700446104
Gaston AJ, Ydenberg RC, Smith GEJ (2007) Ashmole’s halo and population regulation in seabirds. Mar Ornithol 35:119–126
Gaston AJ, Elliott KH, Ropert-Coudert Y, Kato A, Macdonald CA, Mallory ML, Gilchrist HG (2013) Modeling foraging range for breeding colonies of thick-billed murres Uria lomvia in the Eastern Canadian Arctic and potential overlap with industrial development. Biol Conserv 168:134–143. https://doi.org/10.1016/j.biocon.2013.09.018
Glass N, Lavarello I, Glass JP, Ryan PG (2000) Longline fishing at Tristan da Cunha: impacts on seabirds. Atl Seabirds 2:49–56
Gonzalez Carman V, Mandiola A, Alemany D, Dassis M, Seco Pon JP, Prosdocimi L, Ponce De Leon A, Mianzan H, Acha EM, Rodrıguez D, Favero M, Copello S (2016) Distribution of megafuanal species in the Southwestern Atlantic: key ecological areas and opportunities for marine conservation. ICES J Mar Sci Sci 73:1579–1588. https://doi.org/10.1093/icesjms/fsw019
Grémillet D, Dell’Omo G, Ryan P, Peters G, Ropert-Coudert Y, Weeks S (2004) Offshore diplomacy or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of Cape gannets from neighbouring colonies. Mar Ecol Prog Ser 268:265–279. https://doi.org/10.3354/meps268265
Gutowsky SE, Tremblay Y, Kappes MA, Flint EN, Klavitter J, Laniawe L, Costa DP, Naughton MB, Romano MD, Shaffer SA (2014) Divergent post-breeding distribution and habitat associations of fledgling and adult Black-footed Albatrosses Phoebastria nigripes in the North Pacific. Ibis 156:60–72. https://doi.org/10.1111/ibi.12119
Gutowsky SE, Leonard ML, Conners MG, Shaffer SA, Jonsen ID (2015) Individual-level variation and higher-level interpretations of space use in wide-ranging species: an albatross case study of sampling effects. Front Mar Sci 2:93. https://doi.org/10.3389/fmars.2015.00093
Haman KH, Norton TM, Ronconi RA, Nemeth NM, Thomas AC, Courchesne SJ, Segars A, Keel MK (2013) Great Shearwater (Puffinus gravis) mortality events along the eastern coast of the United States. J Wildl Dis 49:235–245. https://doi.org/10.7589/2012-04-119
Haney JC, Geiger HJ, Short JW (2014) Bird mortality from the Deepwater Horizon oil spill. I. Exposure probability in the offshore Gulf of Mexico. Mar Ecol Prog Ser 513:225–237. https://doi.org/10.3354/meps10991
Harrison P (1985) Seabirds: an identification guide, 2nd edn. Houghton Miff Company, Boston
Hatch JM (2017) Comprehensive estimates of seabird-fishery interactions for the US Northeast and mid-Atlantic. Aquat Conserv Mar Freshw Ecosyst. https://doi.org/10.1002/aqc.2812
Hatch JM, Wiley D, Murray KT, Welch L (2016) Integrating Satellite-Tagged Seabird and Fishery-Dependent Data: A Case Study of Great Shearwaters (Puffinus gravis) and the U.S. New England Sink Gillnet Fishery. Conserv Lett 9:43–50. https://doi.org/10.1111/conl.12178
Hazen E, Maxwell S, Bailey H, Bograd S, Hamann M, Gaspar P, Godley B, Shillinger G (2012) Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar Ecol Prog Ser 457:221–240. https://doi.org/10.3354/meps09857
Hedd A, Montevecchi WA, Phillips RA, Fifield DA, Garthe S (2014) Seasonal sexual segregation by monomorphic sooty shearwaters Puffinus griseus reflects different reproductive roles during the pre-laying period. PLoS One 9:e85572. https://doi.org/10.1371/journal.pone.0085572
Hockey PAR, Dean WRJ, Ryan PG (2005) Roberts’ Birds of Southern Africa, 7th edn. The Trustees of the John Voelcker Bird Book Fund, Cape Town
ICES (2013) Report of the Workshop to Review and Advise on Seabird Bycatch (WKBYCS), 14–18 October 2013, Copenhagen, Denmark. ICES CM 2013/ACOM:77, p 79
Jiménez S, Domingo A, Brazeiro A (2009) Seabird bycatch in the Southwest Atlantic: Interaction with the Uruguayan pelagic longline fishery. Polar Biol 32:187–196. https://doi.org/10.1007/s00300-008-0519-8
Jiménez S, Abreu M, Pons M, Ortiz M, Domingo A (2010) Assessing the impact of the pelagic longline fishery on albatrosses and petrels in the southwest Atlantic. Aquat Living Resour 23:49–64. https://doi.org/10.1051/alr/2010002
Jodice PGR, Ronconi RA, Rupp E, Wallace GE, Satgé Y (2016) First satellite tracks of the Endangered black-capped petrel. Endanger Species Res 29:23–33. https://doi.org/10.3354/esr00697
Jonsen ID, Mills Flemming J, Myers RA (2005) Robust state-space modeling of animal movement data. Ecology 86:2874–2880
Jonsen ID, Basson M, Bestley S, Bravington MV, Patterson TA, Pedersen MW, Thomson R, Thygesen UH, Wotherspoon SJ (2013) State-space models for bio-loggers: a methodological road map. Deep Sea Res Part II 88:34–46. https://doi.org/10.1016/j.dsr2.2012.07.008
Jouventin P, Weimerskirch H (1990) Satellite tracking of wandering albatrosses. Nature 343:746–748
Jovani R, Lascelles B, Garamszegi LZ, Mavor R, Thaxter CB, Oro D (2016) Colony size and foraging range in seabirds. Oikos 125:968–974. https://doi.org/10.1111/oik.02781
Krüger L, Ramos JA, Xavier JC, Grémillet D, González-Solís J, Kolbeinsson Y, Militão T, Navarro J, Petry MV, Phillips RA, Ramírez I, Reyes-González JM, Ryan PG, Sigurðsson IA, Van Sebille E, Wanless RM, Paiva VH (2017) Identification of candidate pelagic marine protected areas through a seabird seasonal-, multispecific- and extinction risk-based approach. Anim Conserv. https://doi.org/10.1111/acv.12339
Lee DS (2009) Mass die-offs of Greater Shearwaters in the western North Atlantic: effects of weather patterns on mortality of a trans-equatorial migrant. Chat 73:37–47
Lewis S, Sherratt TN, Hamer KC, Wanless S (2001) Evidence of intra-specific competition for food in a pelagic seabird. Nature 412:816–819. https://doi.org/10.1038/35090566
Loring PH (2016) Evaluating digital VHF technology to monitor shorebird and seabird use of offshore wind energy areas in the Western North Atlantic. PhD thesis, University of Massachusetts Amherst, Amherst
Ludynia K, Dehnhard N, Poisbleau M, Demongin L, Masello JF, Voigt CC, Quillfeldt P (2013) Sexual segregation in rockhopper penguins during incubation. Anim Behav 85:255–267
MacLeod CJ, Adams J, Lyver P (2008) At-sea distribution of satellite-tracked Grey-faced Petrels, Pterodroma macroptera gouldi, captured on the Ruamaahua (Aldermen) Islands, New Zealand. Pap Proc R Soc Tasmania 142:73–88
Masello JF, Mundry R, Poisbleau M, Demongin L, Voigt CC, Wikelski M, Quillfeldt P (2010) Diving seabirds share foraging space and time within and among species. Ecosphere 1:art19. https://doi.org/10.1890/es10-00103.1
O’Connor A (2013) Distributions and fishery associations of immature short-tailed albatrosses (Phoebastria albatrus) in the North Pacific. MSc thesis, Oregon State University, Corvallis, OR
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc Lond Ser B 271:1283–1291
Phillips RA, Silk JRD, Croxall JP, Afanasyev V (2006) Year-round distribution of white-chinned petrels from South Georgia: relationships with oceanography and fisheries. Biol Conserv 129:336–347
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Pinet P, Jaquemet S, Phillips RA, Le Corre M (2012) Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim Behav 83:979–989. https://doi.org/10.1016/j.anbehav.2012.01.019
Pollet IL, Ronconi RA, Jonsen ID, Leonard ML, Taylor PD, Shutler D (2014) Foraging movements of Leach’s storm-petrels Oceanodroma leucorhoa during incubation. J Avian Biol 45:305–314. https://doi.org/10.1111/jav.00361
Powers KD, Wiley DN, Allyn AJ, Welch L, Ronconi RA (2017) Movements and foraging habitats of great shearwaters Puffinus gravis in the Gulf of Maine. Mar Ecol Prog Ser 574:211–226. https://doi.org/10.3354/meps12168
Raymond B, Shaffer SA, Sokolov S, Woehler EJ, Costa DP, Einoder L, Hindell M, Hosie G, Pinkerton M, Sagar PM, Scott D, Smith A, Thompson DR, Vertigan C, Weimerskirch H (2010) Shearwater foraging in the Southern Ocean: the roles of prey availability and winds. PLoS One 5:e10960. https://doi.org/10.1371/journal.pone.0010960
Reid TA, Ronconi RA, Cuthbert RJ, Ryan PG (2014) The summer foraging ranges of adult spectacled petrels Procellaria conspicillata. Antarct Sci 26:23–32. https://doi.org/10.1017/S0954102013000266
Ronconi RA, Burger AE (2011) Foraging space as a limited resource: inter- and intra-specific competition among sympatric pursuit-diving seabirds. Can J Zool 89:356–368. https://doi.org/10.1139/z11-006
Ronconi RA, Ryan PG, Ropert-Coudert Y (2010a) Diving of great shearwaters (Puffinus gravis) in cold and warm water regions of the South Atlantic Ocean. PLoS One 5:e15508
Ronconi RA, Swaim ZT, Lane HA, Hunnewell RW, Westgate AJ, Koopman HN (2010b) Modified hoop-net techniques for capturing birds at sea and comparison with other capture methods. Mar Ornithol 38:23–29
Ronconi RA, Allard KA, Taylor PD (2015) Bird interactions with offshore oil and gas platforms: review of impacts and monitoring techniques. J Environ Manag 147:34–45. https://doi.org/10.1016/j.jenvman.2014.07.031
Ryan PG (2007) Field guide to the animals and plants of Tristan da Cunha and Gough Island. Pisces Publications, Newbury
Ryan PG, Ronconi RA (2010) The Tristan Thrush Nesocichla eremita as seabird predator. Ardea 98:247–250
Steenweg RJ, Ronconi RA, Leonard ML (2011) Seasonal and age-dependent dietary partitioning between the Great Black-backed and Herring Gulls. Condor 113:795–805. https://doi.org/10.1525/cond.2011.110004
Wakefield ED, Bodey TW, Bearhop S, Blackburn J, Colhoun K, Davies R, Dwyer RG, Green JA, Grémillet D, Jackson AL, Jessopp MJ, Kane A, Langston RHW, Lescroël A, Murray S, Le Nuz M, Patrick SC, Péron C, Soanes LM, Wanless S, Votier SC, Hamer KC (2013) Space partitioning without territoriality in gannets. Science 341:68–70
Acknowledgements
We thank the Administrator and Island Council of Tristan for permission to visit and work on Inaccessible Island. Logistical support in the South Atlantic was supplied by the South African National Antarctic Programme (SANAP), and Tristan’s Conservation Department; we especially thank N. Glass, and Captain C. October and the crew of the Edinburgh. Logistical support in the Bay of Fundy was supplied by the Grand Manan Whale and Seabird Research Station (GMWSRS), and we especially thank L. Murison, Z. Swaim, H. Lane, C. McKinstry, A. Neimanis and the many field assistants that worked at GMWSRS. Thank you to M. Martin, D. Veit, S. Johnston, J. Chardine, J. Adams, T. Diamond, and M. Leonard for their guidance, support and roles in satellite tag acquisition. We thank two anonymous reviewers for comments which improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Provided by the Environmental Damages Fund (Environment Canada), New Brunswick Wildlife Trust Fund, National Geographic Society, Ocean Fund, Canadian Wildlife Service, James L. Baillie Memorial Fund (Bird Studies Canada), US Fish and Wildlife Service, BirdLife International, North Star Science & Technology, and the GMWSRS. RAR was supported by a scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Killam Trusts, Dalhousie University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. Permits to capture and tag birds were obtained through the Canadian Wildlife Service (SC2429, SC2483, SC2543, SC2599, SC2655) and the Bird Banding Office (10,480-S). Animal Ethics approvals were obtained through the University of North Carolina Wilmington (protocols #2005-003 and #2007-007) and Dalhousie University (#09-041).
Additional information
Responsible Editor: V. H. Paiva.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ronconi, R.A., Schoombie, S., Westgate, A.J. et al. Effects of age, sex, colony and breeding phase on marine space use by Great Shearwaters Ardenna gravis in the South Atlantic. Mar Biol 165, 58 (2018). https://doi.org/10.1007/s00227-018-3299-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3299-x