Abstract
Little is known about the diet of planktonic tunicates such as salps, which can comprise around 80% of zooplankton abundance under bloom conditions. The gut contents of solitary and aggregate phases of the salps Thalia democratica and Salpa fusiformis were analysed with scanning electron microscopy (SEM), high-performance liquid chromatography (HPLC) and stable isotope analyses (SIA) to describe their diets under field conditions. The gut contents contained representatives of diatoms, dinoflagellates, haptophyte flagellates (Order Prymnesiales and Coccolithophorales), prasinophytes (Order Chlorodendrales) and, in a few instances, copepods (Crustacea). SEM confirmed the presence of many species of phytoplankton in the salp guts and was broadly supported by HPLC and SIA. The dominant peaks in the HPLC chromatograms corresponded to fucoxanthin, alloxanthin, chlorophyll b and β-carotene, indicating the ingestion of diatoms, cryptophytes and green algae. Solitary stages of both S. fusiformis and T. democratica fed on items that were not common in the phytoplankton samples, namely coccolithophores and copepods, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salps are periodically important grazers in temperate marine ecosystems and sometimes form ‘blooms’ that are capable of depleting phytoplankton production (Boero et al. 2013). Blooms of thaliaceans such as salps and doliolids may appear suddenly, last for a short time, occur over vast scales, suddenly disappear and are not always recurrent on a regular basis (Kawahara et al. 2006; Paffenhöfer 2013). Earlier opinions that salps represented a dead-end in the food chain (McClintock et al. 2008) and were, therefore, unimportant have been replaced by the view that salps and other thaliaceans contribute significantly to marine food webs, including sequestration of carbon in the sediments via their large faecal pellets (Henschke et al. 2016).
Salp blooms along the east coast of Tasmania have reached abundances over 2000 individuals m−3 (Ahmad Ishak 2014), in response to increased flow of East Australian Current (EAC) extension water southwards along the coast. The EAC is predicted to continue to strengthen, carrying warm water from the tropical north of Australia and making the Tasman Sea a marine hotspot where water temperatures are increasing more than two times the global rate (~2.28 °C per decade, Ridgway 2007). If salps continue to bloom during periods of increased penetration of the EAC, then the marine food web off Tasmania could change from being crustacean-dominated to an alternative pathway of salp-dominance. This will produce both bottom–up effects on higher trophic levels and top–down impacts on phytoplankton. Currently, little is known about the feeding ecology of salps in Tasmanian waters.
To link salps to their dietary sources we employed three methods to describe their gut contents and their trophic position in the marine food web: scanning electron microscopy (SEM), high-performance liquid chromatography (HPLC) and stable isotopes analysis (SIA) were used to ascertain trophic position. SEM has been used to describe gut contents of Ilhea racovitzai and Salpa thompsoni from the Southern Ocean (Harbou et al. 2011) and Cyclosalpa bakeri in the Woods Hole region (Madin and Purcell 1992). Both studies revealed the presence of diatom remains, along with other algal fragments, faecal pellets and silicoflagellates. The faecal pellets of C. bakeri were also examined using HPLC and identifiable pigments were extracted revealing the presence of chlorophylls a and c and their derivatives (Madin and Purcell 1992).
Stable isotope ratios, primarily δ13C and δ15N, provide a useful tool for estimating the trophic position of salps (via δ15N) and determining their primary source of carbon (Post 2002). Briefly, carbon isotope ratios of animal tissues reflect those in their diet and experience minor trophic enrichment of 0–1‰ per trophic level and, therefore, can be useful for tracing carbon pathways and sources of primary productivity (Hobson and Welch 1992). Nitrogen isotope ratios undergo a stepwise enrichment between prey and consumer tissues and δ15N are used as a trophic position indicator (Minagawa and Wada 1984).
In this study, our aim was to describe the diets and trophic positions of two species of salp that are occurring more frequently in the marine hot spot region of the Tasman Sea (Ahmad Ishak 2014). We compared the gut contents of the salps with phytoplankton sampled from the water column in Storm Bay, Tasmania, and used stable isotopes to infer the trophic positions of both solitary and aggregate stages of Salpa fusiformis and Thalia democratica.
Methods
Field collections
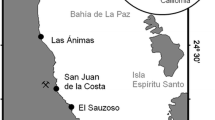
Salps were collected with a 2-m long Bongo net (200 µm mesh, 75 cm mouth diameter), fitted with a soft, non-filtering cod end (1 L capacity) and with a flow meter in the mouth of the net. The nets were towed vertically through the water column, at a speed of <1 ms−1, from a depth of 50 m. The contents of one net were preserved in the 5% buffered formaldehyde and counted in the laboratory under a Leica M165C stereo-microscope. If necessary, those samples were split so that at least 400 salps were counted. Abundances were based on the contents of single nets. Solitary adults (oozoids) and aggregates (blastozooids) of T. democratica and S. fusiformis were caught from Site 2 in Storm Bay, Tasmania (Fig. 1), on 12 December 2013 and 23 January 2014. The salps were preserved in 95% non-denatured ethanol for SEM, while others were frozen in liquid nitrogen for HPLC and stable isotope analyses. To describe the phytoplankton and other particulates available to the salps seawater samples were collected at site 2 with a 12-m long sampling tube that integrated particulate matter over the top 12 m of the water column. Samples were preserved in 1% acidified Lugol’s solution or returned on ice to the laboratory for further processing. A CTD was deployed to record environmental parameters including temperature, salinity and dissolved oxygen. Although the phytoplankton and salps used in this study came only from Site 2, we have presented CTD data as averages of three sites, 1, 2 and 3 to highlight broader environmental conditions experienced by the salps as they swim around the bay.
Laboratory procedures
Phytoplankton samples were concentrated by sedimentation, in a two-step process reducing a 1-L sample to 15 mL. Samples were enumerated by phase contrast light microscopy (Leica DMLB2) using a Sedgewick-Rafter chamber. The entire chamber was scanned at low power (50×) to count large or rare species and then re-examined at 200×. Transects were followed until 400 squares had been viewed, or at least 200 cells of the dominant species had been counted. Higher magnification (400×) was used to confirm species identification. Cell biovolume for each taxon was calculated by assigning an appropriate geometric shape, as per Hillebrand et al. (1999) and Leblanc et al. (2012). Size parameters were measured from samples, or taken from references where available.
Twenty guts of each aggregate and solitary stage of S. fusiformis and T. democratica were removed from their tests using a pair of fine goose-necked forceps, then rinsed with deionised water to remove salt. Each gut was macerated in a centrifuge tube using a glass rod, then centrifuged at 2000 rpm for 10 min to concentrate gut contents. 50 μL of polylysine was placed onto a clean coverslip. Polylysine was used because it can effectively bind particulates and nanoplankton from intestine samples onto coverslips for SEM. This technique consistently results in better-preserved organisms that are less concealed by detritus than samples dried directly onto glass coverslips (Mazia et al. 1975; Marchant and Thomas 2011). After 5 min, excess polylysine was gently washed off and excess water was removed by touching filter paper wedges to the margin of the coverslip. Finally, the coverslip was placed, with polylysine side up, in a petri dish lined with filter paper. In a fume hood, 100 μL of one gut sample was placed on each coverslip. 50 μL of 4% Osmium tetroxide was added to each petri dish and covered, and the samples left covered for 30 min. SEM preparations were critical point dried on 12 mm stubs using a Tousimis Autosamdri-815 and sputter-coated with 5 nm Pt using a Cressington 208HR sputter coater. Once sputter-coated with gold, the sample was viewed under a field emission scanning electron microscope: a JEOL JSM-6701F with a Gatan Alto 2500 cryo-chamber.
Salps that were used for HPLC analysis were snap-frozen in liquid nitrogen, then maintained in a −30 °C freezer until analysis within 4 weeks of collection. Guts of each stage and species of salp were dissected from their bodies and the weight of five pooled guts per replicate (N = 3) was recorded. The guts were ground with a small amount of 100% acetone using a glass mortar and pestle, which was settled in ice. The ground guts and acetone were transferred to a 10-mL centrifuge tube and sonicated in an ice-water bath for 15 min in the dark. The samples were then kept in the dark at 4 °C for approximately 15 h. After this time 200 μL water was added to the samples, which were sonicated once more in an ice-water bath for 15 min. The extracts were transferred to a clean centrifuge tube and centrifuged to separate the gut material. The final extract was filtered through a 0.2-µm membrane filter (Whatman, Anatop) prior to analysis by HPLC using a Waters Alliance high-performance liquid chromatography system. This system comprised a 2695XE separation module with column heater and refrigerated autosampler and a 2996 photo-diode array detector. HPLC was carried out using a C8 column and binary gradient system with an elevated column temperature, following a modified version of the Van Heukelem and Thomas (2001) method. Pigments were identified by retention time and absorption spectra from a photo-diode array (PDA) detector and concentrations of pigments were determined from commercial and international standards (Sigma; DHI, Denmark).
For SIA entire salps were freeze-dried (JAVAC SB9) to constant mass at −40 °C for 24 h. Specimens were homogenised to fine powder and subsampled and a known weight of subsample encapsulated in a silver cup. When necessary, small individuals of the same species and stage were pooled to obtain sufficient material for analysis (0.7–1.7 mg dry sample weight). Salps were not acidified because acidification treatments are still under debate and their effect on both carbon and nitrogen isotopic compositions are not clear (Brodie et al. 2011). Further, lipids were not pre-extracted because salps are known to comprise only about 1% of their wet weight as lipid (Phleger et al. 2000). To analyse particulate organic matter (POM), a known volume of seawater was filtered onto a pre-combusted (450 °C for 12 h) quartz microfibre filter (Satorius; nominal pore size 0.3 μm). POM filters were demineralised over 1% HCl fumes for 24 h and then dried at 60 °C for at least 24 h. Subsamples were taken from each filter using 5 mL syringes as ‘punches’ and known weights were encapsulated in silver cups (Kennedy et al. 2005).
Carbon and nitrogen stable isotopes were analysed using an Iso-Prime100 mass-spectrometer coupled with an elemental analyser (Elementar vario PYRO cube, Germany). Samples were flash combusted to convert into N2 and CO2 and the purified gases were released into the mass spectrometer. Isotope ratios were reported as parts per thousand (‰) deviations from the conventional standards, Pee Dee Belemnite (PDB) for carbon and atmospheric N2 for nitrogen. Stable isotope concentrations are expressed in delta (δ) notation as parts per thousand according to the following equation:
where X = 13C or 15N, and R = 13C/12C or 15N/14N. Internal laboratory standards with known isotopic composition were run after every fifth sample. The stability of the instrumentation, including analytical precision, drift correction and linearity performance, was determined from the analysis of these standards.
Results
Water temperature on the two collection dates averaged 14.5 and 16.1 °C on 16 December 2013 and 23 January 2014, respectively, while mean salinity, dissolved oxygen and chlorophyll a concentrations were similar on both dates (Table 1). Thalia democratica was collected on both sampling dates, while S. fusiformis was only present in January. Aggregates of T. democratica were the most abundant stage collected (2397 individuals m−3), followed by solitary T. democratica and aggregate S. fusiformis. The solitary stage of S. fusiformis was collected in much lower numbers (Table 1). The guts of S. fusiformis were larger than those of T. democratica for both stages, although both species had similar gut morphology: a slender, elongated V-shape, as described by Fredriksson et al. (1988). The guts of the two species were different in colour when they were freshly collected: purple-blue in T. democratica and brown-green in S. fusiformis.
Comparison of gut contents with phytoplankton
Thirty different species of phytoplankton were observed in the guts of the salps using SEM (Fig. 2). These species included Chrysochromulina sp., Pyramimonas sp., Navicula spp., Pseudo-nitzschia sp., Phaeocystis sp., Prorocentrum spp., Leptocylindrus spp., Rhizosolenia sp., Cocconeis spp., Protoperidinium spp., Tripos sp., Emiliania huxleyi, Malacanosma minidiscus, Pyramimonas grossi, Cylindrotheca sp., Cylindrotheca closterium, Scrippsiella trochoidea, Corethron sp., Thalassionema sp., Fragilariopsis rhombica, Thalassiosira cf rotula, Proboscia alata, Nitzschia spp. and five unidentified taxa. Portions of the exoskeletons of copepods were observed in the guts of solitary stages of both T. democratica and S. fusiformis using SEM. Additionally, using a light microscope (Leica M165C) we observed whole copepods in the guts of solitary S. fusiformis.
Examples of gut contents. Salpa fusiformis solitary: a Copepod mandible, b Copepod (red box); Thalia democratica solitary: c Copepod swimming legs, d Prorocentrum minimum; Salpa fusiformis aggregates: e Box scale from Pyramimonas grossi, f Cocconeis sp.; Thalia democratica aggregates: g Protoperidinium sp., h Emiliana huxleyi
Representative chromatograms for each species and stage are presented in Fig. 3. Several peaks were identifiable, including fucoxanthin (a biomarker for diatoms), astaxanthin (indicative of crustaceans) and diatoxanthin (found in crytomonads and chlorophytes). A summary of the pigments observed in this study, and examples of groups where they dominate, is presented in Table 2.
Few peaks were identified in the solitary stages of T. democratica and the fucoxanthin and fucoxanthin-like peaks (Fig. 3a) suggested the presence of diatoms only (Table 2). For aggregates of T. democratica (Fig. 3b), small peaks were observed for chlorophyll a and fucoxanthin, though there were also several peaks that had absorption spectra similar to fucoxanthin. Elution times were indicative of small contributions from antheraxanthin, alloxanthin and β,β-carotene. The pigments identified suggest that the gut contents of aggregates of T. democratica included diatoms (fucoxanthin) and cryptophytes (alloxanthin).
In the chromatograms from solitary stages of S. fusiformis peaks were eluted at 3.647, 5.495, 5.939, 8.551, 17.603 and 22.920 min (Fig. 3c); these peaks had absorption spectra that bear no resemblance to any well-known pigment. There was a very small peak for chlorophyll a, and peaks for pyro-phaeophorbide and pyro-phaeophytin, which are degradation products of chlorophyll a. Notably, there was no peak identified as fucoxanthin, though there were several peaks that had absorption spectra like that of fucoxanthin, but which had eluted at different times. There was a peak that appeared similar to chlorophyll c, though with a shift in the absorption spectrum; it did elute at a similar time to chlorophylls c2 and c1. The dominant peak in the chromatogram corresponded to alloxanthin (an indicator of cryptophytes), and peaks corresponding to chlorophyll b (green algae) and β,β-carotene (not specific to any one algal group) were also present. Based on this information the pigmented prey of S. fusiformis solitary stages included diatoms, cryptophytes and green algae.
Chromatograms for the aggregate stages of S. fusiformis revealed peaks eluted at 5.503, 14.784 and 18.0291 min (Fig. 3d); these had absorption spectra that bear no resemblance to any well-known pigment. There were small peaks for chlorophyll a, pyro-phaeophorbide and pyro-phaeophytin. Peaks that eluted at standard times and had absorption spectra that matched known standards were astaxanthin, alloxanthin, diatoxanthin, zeaxanthin and β,β-carotene. Two other peaks eluted at the correct time of a known pigment, but the absorption spectra had significant shifts; these were diadinoxanthin and antheraxanthin. The pigments identified suggest that the gut content of the aggregate stages of S. fusiformis contained diatoms (fucoxanthin) and cryptophytes (alloxanthin).
The composition of the phytoplankton community present in Storm Bay at the same time as the salps were collected is presented in Fig. 4. Diversity was lower in January, with many dinoflagellates, including Protoperidinium sp., Prorocentrum spp., Peridinium cf. quinquecorne and silicoflagellates (Ebria tripartite and Dictyocha fibula) not collected. The diatoms Proboscia alata and Skeletonema pseudocostatum and the dinoflagellates Noctiluca scintillans, Tripos spp. and Scrippsiella trochoidea were the dominant species based on cell biovolume. Only nine species of phytoplankton observed in our samples via light microscopy were found in the gut contents of salps using the SEM method. These were Fragilariopsis rhombica, Leptocylindrus spp., Navicula spp., Proboscia alata, Prorocentrum spp., Protoperidinium spp., Scripsiella trochoidea, Thalassionema sp. and Thalassiosira cf rotula. Other species that were present in the gut contents, such as the coccolithophorid Emiliana huxleyi, were too small to be enumerated in the phytoplankton samples using our light microscopy method.
Trophic positions of salps in Storm Bay
The carbon and nitrogen isotopic profiles of POM and each species and stage of salp in Storm Bay are presented in Fig. 5. Mean δ13C for POM was −23.4, while the mean δ13C for the salps fell between −23.0 and −24.3. δ15N values of the salps exhibited little enrichment (+0.1 to +1.3 ‰) from the mean POM δ15N value of 7.6. Aggregates of S. fusiformis were the most enriched (8.9 ± 1.3), while aggregates of T. democratica were the least (7.7 ± 0.4).
Discussion
We employed three methods to study the diets of Thalia democratica and Salpa fusiformis in Storm Bay. The combination of SEM and HPLC highlighted that diatoms were a key group ingested by the salps. The δ13C profile of the salps falling within the range of ±1 ‰ of the POM indicated that salps were obtaining their carbon primarily from POM sources in Storm Bay, while shifts in δ15N of <2 ‰ above POM suggested little trophic enrichment. The size range of food particles found in solitary stages of T. democratica and S. fusiformis was similar, while a smaller size range of food particles was found in aggregates. The pigments identified suggest that the gut content of the salps contained fucoxanthin, alloxanthin and astaxanthin, confirming that the salps had predominately ingested diatoms, cryptophytes and green algae.
Although both S. fusiformis and T. democratica filter phytoplankton using their mucus nets (Silver 1981) examination of their gut contents revealed that they ingested slightly different size profiles of particles, despite being collected at the same time and being exposed to the same food environment. Salps are extremely efficient, nonselective filter feeders, retaining particles over a wide size range between 1 μm and 1 mm (Harbison and McAlister 1979; Kremer and Madin 1992; Licandro et al. 2006). All particles observed in both the aggregate and solitary stages of T. democratica and S. fusiformis were within the size ranges published for other species, though the methods employed in the present study were biased towards species that leave hard parts, such as diatoms and thecate dinoflagellates. In solitary T. democratica, individual scales of Pyramimonas sp. with size of 200 nm were found. If Pyramimonas sp. were consumed as aggregated materials and the scales were released from cells once consumed, the ingested particles ranged in size from 10–15 to 1400 μm (the maximum measurement of the whole body size of a copepod, calculated from those body parts found). For the aggregate stage of T. democratica, food particles ranged from 2 to 250 μm. For solitary S. fusiformis, the minimum particle size found was 8 μm and the maximum was 1400 μm (copepod remains), while the size range of particles found in aggregates was 5–100 μm.
There was limited evidence of differential ingestion by T. democratica and S. fusisformis. Isotopic ratios highlighted that salps in Storm Bay were isotopically not enriched over POM, indicating that they were feeding largely indiscriminately on available food sources. SEM highlighted only minor differences between species ingested: there were five species of Haptophytes (flagellates), two Prasinophytes (flagellates), eight diatoms, four dinoflagellates, three unidentified species and some copepod body parts observed in images obtained from T. democratica guts. In contrast, only two species of Haptophytes (flagellates), six diatoms and two unidentified species were observed in images captured from the guts of S. fusiformis. Coccolithopores were common in aggregates of both species and were abundant in the guts of the solitary form of S. fusiformis, while partially digested copepods were only found in solitary phases of both T. democratica and S. fusiformis. Copepods have been recorded previously in salp diets: specimens of aggregate S. thompsoni had the copepod Rhincalanus gigas in their branchial cavities (Perissinotto and Pakhomov 1997). Salpa thompsoni has also been shown to ingest copepod nauplii and the copepod genera Oithona and Oncaea (Hopkins and Torres 1989).
To our knowledge, there are only two other studies of SEM analyses of salp gut contents (Madin and Purcell 1992; Harbou et al. 2011). These studies have reported several species of diatoms and dinoflagellates in the fresh guts of Cyclosalpa bakeri, including Denticulopsis seminae, Nitzshia sp., Corethron hystrix, Thalassiosira spp., Chaetoceros sp., Coccolithus pelagicus, Rhizosolenia alata, dinoflagellates and Emiliania huxleyi (Madin and Purcell 1992).
This paucity of studies could be due to methodological problems similar to those encountered in the present study. Freshly collected specimens are essential for SEM studies. Because they are fragile gelatinous species, mucus from the feeding nets of salps can get incorporated into gut contents if preparations are not done carefully during the gut washing and centrifuging (Madin and Purcell 1992). Improper preservation techniques have also been a problem for SEM examination of gut contents on some other invertebrates. For example, no identifiable organic materials were observed in the shrimp Rimicaris exoculata, possibly a consequence of the delay in preservation of the material until several hours after collection (Van Dover et al. 1988). Thaliaceans are known for their unpredictable occurrence, which might also partly explain why studies of SEM analyses of gut contents of (freshly collected) thaliaceans are scarce.
Pigments obtained from salps differed slightly according to species and stage. Fucoxanthin, fucoxanthin-like pigments and chlorophyll a were identified in all species and stages of salps. Alloxanthin, astaxanthin and diatoxanthin were present in both stages of S. fusiformis. Meanwhile, β,β-carotene was only present in the guts of aggregate stages of both species. Carotenoids such as peridinin and fucoxanthin are considered characteristic of dinoflagellates and diatoms, respectively, while fucoxanthin derivatives are present in prymnesiophytes, chrysophytes and some dinoflagellates (Kozlowski et al. 1995). No peridinin or its derivatives (peridinol) were detected in the guts of salps in this study, suggesting that dinoflagellates were not an important prey item, though dinoflagellates were observed by SEM. Most salps in this study clearly contained a predominance of fucoxanthin over other pigments in the guts. This predominance could be a function of the degree of digestion, as flagellates are digested more completely than hard-shelled diatoms. Only the chromatograms of S. fusiformis (both stages) recorded the presence of the carotenoids astaxanthin and zeaxanthin. Zeaxanthin is one of the carotenoid pigments found in Pyramimonas grossi (Ackman 1989), which concurs with our SEM analysis, where scales of Pyramimonas grossi were observed in the guts of S. fusiformis.
There are several limitations to the HPLC approach to diet analysis. Based on our experience, the separation of a salp’s gut from the body must be done extremely carefully, as soft gelatinous parts of the body could contaminate the chromatogram, resulting in many unidentifiable peaks. Since HPLC analysis is expensive, it would be more cost effective if all peaks in a chromatogram were identifiable. The HPLC chromatograms of the salp guts were quite cluttered, with several peaks that were not identifiable. The chromatograms obtained in this study were noisy, making it difficult to establish a baseline. This could be due to the gelatinous matter of the body walls contaminating the gut content samples. This was likely due to gelatinous body material from the specimens, i.e. the polysaccharides forming the tunicin of the tests, being inadvertently added during the extraction process. The presence of peaks that eluted in ‘known’ positions but which had no known absorption spectra (e.g. chlorophyll a-like and fucoxanthin-like) indicated the possibility of many of the parent pigments degrading into other forms as a result of the digestion process.
The three methods we employed in this study have their own strengths and weakness, so we suggest using a combination of methods to build a comprehensive picture of salp diets. In particular, including similar analyses of faecal pellets would enable the consideration of ingestion versus digestion. As our SEM images indicated, many of the food particles found in the guts were well preserved, most like to due to salps lacking jaws or any structure that macerates their prey.
Gelatinous species have a fundamentally different life cycle to crustacean zooplankton. They can reproduce rapidly and devour a very wide range of particle sizes. Thaliaceans are a natural component of temperate, marine ecosystems but their appearance in the water column appears to be less predictable than that of other zooplankton, especially crustacea. Blooms can be ephemeral and, in southeastern Tasmanian waters, are appearing more frequently in response to increased southwards flow of the East Australian Current (Ahmad Ishak 2014). As waters warm and the composition of the pelagic community changes zooplankton communities might shift from being crustacean-dominated to gelatinous-dominated, and this has important implications for energy transfer up the food chain.
References
Ackman RG (1989) Marine biogenic lipids, fats and oils, vol II. CRC Press, Boca Raton, Florida
Ahmad Ishak NH (2014) The bloom dynamics and trophic ecology of salps and doliolids in Storm Bay, Tasmania. PhD thesis, University of Tasmania
Boero F, Belmonte G, Bracale R, Simonetta F, Piraino S, Zampardi S (2013) A salp bloom (Tunicata, Thaliacea) along the Apulian coast and in the Otranto Channel between March–May 2013. F1000Research 2:181. doi:10.12688/f1000research.2-181.v1
Brodie CR, Leng MJ, Casford JS, Kendrick CP, Lloyd JM, Zong Y, Bird MI (2011) Evidence for bias in C and N concentrations and δ13C composition of terrestrial and aquatic organic materials due to pre-analysis acid preparation methods. Chem Geol 282:67–83
Fredriksson G, Ofverholm T, Ericson LE (1988) Iodine binding and peroxidase-activity in the endostyle of Salpa fusiformis, Thalia democratica, Dolioletta gegenbauri and Doliolum nationalis (Tunicata, Thaliacea). Cell Tissue Res 253:403–411. doi:10.1007/BF00222297
Harbison G, McAlister V (1979) The filter-feeding rates and particle retention efficiencies of three species of Cyclosalpa (Tunicata, Thaliacea). Limnol Oceanogr 24:875–892. doi:10.4319/lo.1979.24.5.0875
Harbou LVD, Pakhomov EA, Hunt BP, Hagen W, Bathmann UV (2011) Salps in the Lazarev Sea, Southern Ocean: I. Feeding dynamics. Mar Biol 158:2009–2026. doi:10.1007/s00227-011-1709-4
Henschke N, Everett JD, Richardson AJ, Suthers IM (2016) Rethinking the role of salps in the ocean. TREE 31:720–733. doi:10.1016/j.tree.2016.06.007
Hillebrand H, Durselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser 84:9–18
Hopkins TL, Torres JJ (1989) Midwater foodweb in the vicinity of a marginal ice zone in the western Weddell Sea. Deep Sea Res 36:543–560. doi:10.1016/0198-0149(89)90005-8
Kawahara M, Uye S, Ohtsu K, Iizumi H (2006) Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar Eco Prog Ser 307:161–173. doi:10.3354/meps307161
Kennedy P, Kennedy H, Papadimitriou S (2005) The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotope composition in algae and marine sediment. Rapid Commun Mass Spectrom 19:1063–1068
Kozlowski W, Vernet M, Lamerdin SK (1995) Palmer LTER: predominance of cryptomonads and diatoms in Antarctic coastal waters. Antarct J 30:267–268
Kremer P, Madin LP (1992) Particle retention efficiency of salps. J Plankton Res 4:1009–1015. doi:10.1093/plankt/14.7.1009
Leblanc K et al (2012) A global diatom database—abundance, biovolume and biomass in the world ocean. Earth Syst Sci Data 4:149–165
Licandro P, Ibanez F, Etienne M (2006) Long-term fluctuations (1974–1999) of the salps Thalia democratica and Salpa fusiformis in the northwestern Mediterranean Sea: relationships with hydroclimatic variability. Limnol Oceanogr 51:1832–1848. doi:10.4319/lo.2006.51.4.1832
Madin LP, Purcell JE (1992) Feeding, metabolism, and growth of Cyclosalpa bakeri in the subarctic Pacific. Limnol Oceanogr 37:1236–1251. doi:10.4319/lo.1992.37.6.1236
Marchant HJ, Thomas DP (2011) Polylysine as an adhesive for the attachment of nanoplankton to substrates for electron microscopy. J Microsc 131:127–129. doi:10.1111/j.1365-2818.1983.tb04239.x
Mazia D, Schatten G, Sale W (1975) Adhesion of cells to surfaces coated with polylysine. J Cell Biol 66:198–200. doi:10.1083/jcb.66.1.198
McClintock J, Ducklow H, Fraser W (2008) Ecological responses to climate change on the Antarctic Peninsula. Am Sci 96:302–310
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Paffenhöfer GA (2013) A hypothesis on the fate of blooms of doliolids (Tunicata, Thaliacea). J Plankton Res 35:919–924. doi:10.1093/plankt/fbt048
Perissinotto R, Pakhomov EA (1997) Feeding association of the copepod Rhincalanus gigas with the tunicate salp Salpa thompsoni in the southern ocean. Mar Biol 127:479–483. doi:10.1007/s002270050036
Phleger CF, Nelson MM, Mooney B, Nichols PD (2000) Lipids of Antarctic salps and their commensal hyperiid amphipods. Polar Biol 23:329–337
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Ridgway KR (2007) Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett 34:1–5
Silver MWBKW (1981) Differential feeding and fecal pellet composition of salps and pteropods, and the possible origin of the deep-water flora and olive-green “cells”. Mar Biol 62:263–273. doi:10.1007/BF00397693
Van Dover CL, Fry B, Grassle JF, Humphris S, Rona PA (1988) Feeding biology of the shrimp Rimicaris exoculata at hydrothermal vents on the Mid-Atlantic Ridge. Mar Biol 98:209–216. doi:10.1007/BF00391196
Van Heukelem L, Thomas C (2001) Computer assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J Chromatogr A 910:31–49. doi:10.1016/S0378-4347(00)00603-4
Acknowledgements
Funding was provided by the Winifred Violet Scott Foundation (to KMS), the Fisheries Research and Development Corporation (Project Numbers 2009/067 and 2014/031; to KMS and RE) and Institute for Marine and Antarctic Studies (Fisheries and Aquaculture Program). The Storm Bay project team—Jason Beard, Lisette Robertson, Hugh Jones and Andrew Pender—are thanked for help in the field. Justin Hulls produced the map and we thank two anonymous reviewers for helping to improve the manuscript. This work has been carried out while the lead author held Malaysian Ministry of Higher Education and Universiti Malaysia Terengganu scholarships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Winifred Violet Scott Foundation and the Fisheries Research and Development Corporation (Grant Numbers 2009/067 and 2014/031). The lead author held Malaysia Ministry of Higher Education and Universiti Malaysia Terengganu scholarships (Grant SLAB/SLAI).
Conflict of interest
Author Ahmad Ishak declares that she has no conflict of interest. Author Clementson declares that she has no conflict of interest. Author Eriksen declares that she has no conflict of interest. Author van den Enden declares that he has no conflict of interest. Author Williams declares that he has no conflict of interest. Author Swadling declares that she has no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Tasmania.
Additional information
Responsible Editor: X. Irigoien.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Ahmad Ishak, N.H., Clementson, L.A., Eriksen, R.S. et al. Gut contents and isotopic profiles of Salpa fusiformis and Thalia democratica . Mar Biol 164, 144 (2017). https://doi.org/10.1007/s00227-017-3174-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3174-1