Abstract
The present study sought to quantify the infauna and environmental characteristics of the deep SE Levantine Basin, Mediterranean Sea (33.4–31.7 N, 31.3–34.9E; 100–1900 m water depth). Box corer samples were collected at 50 sites in June–July 2013. The infauna (>250 μm) were enumerated and identified to the lowest possible taxonomic level (~37 % to the species level). Three faunal assemblages were identified, inhabiting three biotopes, the shelf margin (SM), the continental slope (CS) and the bathyal plain (BP). The within-biotope heterogeneity was evaluated by the average Chao-Sørensen similarity index and the beta diversity (exp(gamma diversity)—exp(alpha diversity)), revealing values of 0.64–0.7 and 1.73–2.77, respectively, upon applying the pooled bathyal plain samples. The corresponding non-pooled samples revealed higher heterogeneity, with values of 0.26 and 6.27, respectively, likely due to insufficient sampling. The infaunal density in the various biotopes (geometric mean 59–2220 ind m− 2) exhibited a logarithmic decline with water depth. The estimated number of taxa per biotope was calculated by rarefaction analysis in relation to the sampled area and the number of sampled individuals, resulting in 203 (sampled area)−206 (number of individuals) (SM), 170–163 (CS) and 144–130 (BP) taxa. The rarified alpha diversity in relation to the sampled area revealed decreasing values toward the deeper biotopes. However, similar rarified alpha diversities were found in all the biotopes in relation to the number of sampled individuals. Water depth, sediment grain size mode, CaCO3%, and clay% were jointly correlated with faunal composition; water depth provided the single strongest correlate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mediterranean deep sea is characterized by unique environmental conditions in comparison to the adjacent eastern Atlantic. Its water temperatures below ~300 m are homogenous and high (~13–14 °C), its salinity is high, and its primary production is relatively low. These parameters vary from the west to the east through the Mediterranean, reaching their extremes in the Levantine Basin (LB), where the highest temperature and salinity and the lowest primary production occur (Sardà et al. 2004; Danovaro et al. 2010). Oligotrophy was identified by Pusceddu et al. (2010) and Baldrighi et al. (2014) as the major determinant of the decreasing abundance and biomass of benthic fauna along the Mediterranean west–east axis, with the LB as an extremely low-nutrient, low-chlorophyll, and ultra-oligotrophic environment (Kress and Herut 2001; Siokou-Frangou et al. 2010). Understanding the Mediterranean ecology and its shaping factors requires, among other studies, the elucidation of the poorly investigated ecology of the southeastern LB, the easternmost point of these biotic and oceanographic gradients.
The deep-sea infauna of the southeast corner of the LB off the Israeli coast has been qualitatively reported previously, including its zoogeographic relationships with the western Mediterranean and the eastern Atlantic (Galil 2004 and literature therein). However, the quantitative characterization of this province in terms of its infaunal density, diversity, and composition in relation to environmental variation has only been partially performed (Kröncke et al. 2003; Baldrighi et al. 2014). Kröncke et al. (2003) sampled the entire LB from Crete to Israel and from Egypt to Cyprus, and Baldrighi et al. (2014) studied the deep sea south of Crete as part of a wider Mediterranean survey. Tselepides et al. (2000) studied the comparable environment of the adjacent deep Aegean Sea. The latter authors included detailed determinations of faunal assemblages from the shelf, continental slope, and bathyal plain, with data on faunal density and diversity and a variety of environmental parameters. Here, we aim to provide a comparable quantitative assessment of the infaunal benthos of the LB based on a particularly extensive broad-scale survey of the region.
Materials and methods
Surveyed area and sampling design
The area covered by the present study encompasses approximately 23,000 km2 of the southeastern LB. It was recently mapped (Hall et al. 2015), enabling the distinction of a variety of bottom terrain features (supplementary Fig. S1), and providing insight into the studied area geological evolution (Gvirtzman et al. 2015). The continental shelf margin (SM) and its continuation on the continental slope (CS) are located between 100 and 1000 m water depths and contains diverse terrain, including a steep canyon area in the north and the prominent Dor and Palmahim gravitational sediment slumps in the center of the coast, with a moderately inclined CS between them, marked with numerous smaller sediment slides (Katz et al. 2015). The bathyal plain (BP), located between 1000 and 1900 m water depths, is generally flatter than the CS but is crossed by southeast-northwest channels, frequent ridges and trenches.
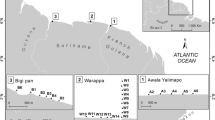
Seabed sediments were sampled at 50 sites (Fig. 1 and supplementary Fig. S1) from the RV Shikmona (Israel Oceanographic and Limnological Research). Offshore transects on the SM and the CS crossed the canyon area in the north and the Palmahim gravitational slump in the south. Other transects were located in the relatively featureless CS. BP samples were collected along approximate continuations of these transects. Details of the sampling operations are given in supplementary Table S1. A total of 150 box core sediment samples, three at each site, were collected with a 0.0625-m2 box corer (Ocean Instruments BX 700 AL) during June–July 2013. A 5.4-cm diameter sub-sample core was taken from each box corer sample for the measurement of the sediment chemical and sedimentological characteristics. The fauna from the remaining box corer content were sorted (0.0602 m2).
Sampling locations and biotopes identified. The red dotted line designates the division added by the clustering of the NT data. The blue lines delimit the areas of the pooled samples and PBP1-5 are the designations of the pooled samples. SM shelf margin, US upper continental slope, LS lower continental slope, CS continental slope, BP bathyal plain
Environmental parameters
The 0–1 cm sediment horizons of the three sub-samples at each site were mixed, dried and used for all required sediment-related measurements. A Mastersizer (Malvern, UK) was used to characterize the grain size distribution following the protocol of Crouvi et al. (2008). The samples were characterized by their grain size mode and their clay (<8 μm), silt (8–63 μm), and sand (>63 μm) volume proportions. The upper clay limit (<8 μm) was set following Konert and Vandenberghe (1997). The sediment total organic carbon (TOC) weight percentage was measured from the 0–1 cm sediment horizon by dichromate digestion and potentiometric titration according to Gaudette et al. (1974) with a detection threshold of 0.02 %. The sediment CaCO3 weight percentage was evaluated by reaction with concentrated HCl (Jones and Kaiteris 1983). The water temperatures at three depth ranges, 100–200, 200–1000 and 1000–1900 m were estimated from a large dataset collected from 1980 present off the Mediterranean coast of Israel (http://isramar.ocean.org.il/isramar2009/).
Faunal sampling and processing
Previous experience at water depths of 40–1900 m off the Israeli coast suggested the effective absence of live infauna below a sediment depth of 7 cm (Hadas Lubinevsky, perscomm); hence, only the 0–10 cm sediment horizon was sampled in the present study. The samples were processed on a 250-µm mesh size sieve, and the resultant residue was preserved in 99 % ethanol on board. In the laboratory, the samples were stained in an ethanol/rose bengal solution of ~1 mg/ml and left for at least 24 h prior to further processing. The stained specimens were sorted under a stereoscope and identified to the lowest possible taxonomic rank with the assistance of a group of taxonomists (see Acknowledgements). All identified infaunal taxa were included in the subsequent analyses regardless of their status as macrofauna or meiofauna or the Linnaean rank of their identification.
Statistical methods
Very low specimen counts per sample initially led us to pool the three replicates from each site, corresponding to a sampled site area of 0.18 m2. However, in the case of the BP site data, the specimen counts remained rather low. Consequently, the samples from the 35 deep sites were pooled into groups of seven sites in depth order to yield five “pooled samples”, each having a sampled area of 1.26 m2 (Fig. 1 and supplementary Table S1). Following these manipulations, the average specimen counts per site were 403 and 138 individuals, respectively, for the SM and the CS and 99 individuals per pooled sample from the BP.
Differences among univariate sets of faunal and abiotic parameters were tested by ANOVA followed by post hoc pairwise t tests (Excel, Microsoft) or by the non-parametric Kruskal–Wallis rank sum test with Bonferroni’s P value adjustment, followed by the post hoc pairwise Wilcoxon rank sum test with Bonferroni’s P value adjustment, using the R software environment. Spearman’s rank correlation was used to test the correlation between two univariate variables (R Core Team 2016).
Faunal assemblages were determined by cluster analysis of the quantitative sample data (specimen counts normalized to the area sampled). Both non-transformed (NT) and square root (SR)-transformed sample profiles were subjected to the PRIMER-v7 (Clarke et al. 2014; Clarke and Gorley 2015) and PERMANOVA+ (Anderson et al. 2008) software packages. The Bray–Curtis similarity measure was applied to both the NT- and SR-transformed data matrices. Sample clusters were visualized using a dendrogram that was created by applying the group average clustering method and relative differences among samples were visualized by the nMDS ordination. The resultant clusters were tested by pairwise PERMANOVA (applying a maximum of 10,000 permutations and using the Monte Carlo correction) to test for significant differences among the identified assemblages. The bottom area encompassing a significantly different faunal assemblage was termed a biotope.
The Chao–Sørensen similarity index between samples at a scale of 0 (no similarity) to 1 (complete identity) (Chao et al. 2005) was calculated by the EstimateS 9.1.0 software package (Colwell 2013), evaluating the homogeneity of the faunal assemblages of each biotope by averaging the Chao–Sørensen similarity values obtained for each pair of compared samples. The entropy Shannon “number equivalents” (exp(gamma diversity) – exp(alpha diversity)) were calculated to evaluate the beta diversity values in different biotopes following the approach of Jost (2007; equations 17a–c), also a measure of heterogeneity within each biotope.
The EstimateS 9.1.0 software was used also to evaluate the relationships between alpha diversity and the sampled area or the number of sampled individuals using rarefaction analysis. The estimated series of alpha diversity values enables diversity comparisons between biotopes based on a common number of sampled individuals or number of samples. The sample size or the number of samples which is related to an asymptotic value of alpha diversity signifies a sufficient sampling effort to represent the examined biotope. Rarefaction was also used to determine the maximal estimated number of taxa in each biotope (Colwell et al. 2012).
A matrix of the abiotic parameters sampled at each site was constructed: water depth, TOC%, CaCO3%, grain size mode, clay%, and clay + silt%. These variables were rescaled prior to assessing their potential correlations with faunal composition (SR-transformed data) using the BIOENV program (Clarke and Ainsworth 1993; PRIMER v7). The analyses were based on the Bray–Curtis similarity of faunal composition and on Euclidean distance measures for the abiotic variables.
Results
Faunal variation
A total of 232 taxa were identified; 92 were identified to the species level, 86 to the genus level, 37 to the family level, and 17 to higher taxonomic levels. Three assemblages were identified by the analysis of the SR-transformed faunal composition (Fig. 2a): the shelf margin (SM), the continental slope (CS), and the bathyal plain (BP). Analysis of the NT data divided the CS into two units, the upper (US) and the lower slope (LS) (Fig. 2b). The nMDS presentation of the similarity among samples is presented in Fig. 3. Supplementary Table S2 provides primary data on the infauna of each assemblage, and average biotope-specific biotic characteristics are presented in Table 1.
Cluster analysis of a SR-transformed, and b non-transformed sample profiles based on Bray-Curtis similarity index and group average clustering. The black dots label roots of statistically significant clusters (in a, pairwise PERMANOVA, t statistic(7–9) = 1.86–2.53, P(MC) = 0.0019 − 0.0011; b pairwise PERMANOVA, t statistic(7–13) = 1.83–2.74, P(MC) = 0.0005–0.003). SM shelf margin, US upper continental slope, LS lower continental slope, CS continental slope, BP bathyal plain. Depth ranges of the assembled samples are depicted in the vertical framed rectangles
nMDS analysis of a non-transformed, and b SR-transformed sample profiles. The ellipses label roots of statistically significant clusters (see Fig. 2). SM shelf margin, US upper continental slope, LS lower continental slope, CS continental slope, PBP bathyal plain, pooled samples
The relationship between faunal density and water depth at each sampled site is presented in Fig. 4, described by a log-linear function. Significant variation in the infaunal density was detected among all of the biotopes (ANOVA, post hoc multiple t tests, log-transformed data, Table 1).
The maximal numbers of taxa per biotope and their 95 % confidence limits were estimated using two rarefaction analyses in relation to the number of samples and the number of sampled individuals. The results are presented in Fig. 5a, b. As not all the sampled individuals were identified to the species level, the obtained maximal numbers of taxa represent a minimum number of species. The alpha diversity values calculated by both rarefaction methods within the range of the actual samples or number of sampled individuals are presented in Fig. 6.
Rarefied, intrapolated and extrapolated number of taxa in the three identified biotopes related to the number of samples (a) and to the numbers of sampled individuals (b). The black dot in each graph designates the last actually sampled site or the actual number of individuals sampled. Black line the estimated number of taxa, dashed lines −95 % confidence limits. SM shelf margin, CS continental slope, BP bathyal plain, PBP BP pooled samples
Two approaches were followed to evaluate the heterogeneity within each biotope, the average Chao–Sørensen similarity index and the beta diversity, presented in Table 1.
Environmental variation
Table 2 presents the average values for the available environmental parameters in each of the identified biotopes and general biotope characteristics, size, depth and multiannual temperature regime which were at the LB minimum for the CS and the BP. The BP values of the CaCO3% and the sediment mode were significantly different from their values in the other two biotopes. Other parameters revealed minor differences or no differences at all.
Two parameters, water depth and grain size mode, were significantly correlated with faunal composition (Table 3). No such correlation was observed for the other four parameters, tested individually, but certain tests of these latter parameters in concert with other ones, revealed significant correlations. Water depth revealed the strongest rank correlation of a single parameter with sample faunal composition (Rho of 0.78). Water depth, CaCO3%, and clay% revealed the strongest rank correlation of multiple parameters (Rho of 0.81).
Discussion
General
This study is the first to comprehensively sample the benthic infauna of the LB in the southeastern Mediterranean and to examine the correlations between three defined biotopes and environmental parameters. The Levantine Basin is the warmest, saltiest, and least productive area in the Mediterranean. It should be noted that the studied area did not encompass the deepest part of the LB (>2000 m) and is characterized by a relatively shallow CS in comparison to many other Mediterranean CSs. The density in the studied area is low and logarithmically decreases with depth. The Shannon diversity index rarified according to the largest mutually sampled area unit (4 samples) also decreased with increasing depth, assumedly due to the decreasing density. However, almost no change in the Shannon index was observed among the biotopes rarified to the unit of sampled individuals (494 ind). The biotopes identified are clearly water depth-related, but their distributions are likely governed by a suite of depth-related factors rather than exclusively water depth per se. These parameters include the characteristics of the fine sediment grain fraction and the sediment CaCO3 percentage but are hypothesized also to include local bottom terrain variables and the quantity and quality of food sources.
Faunal assemblages
Three significantly different faunal assemblages were identified here using the SR-transformed abundance profile of the various samples. Repeating the analysis with the NT data enabled the division of the CS assemblage into two depth-related ones, likely due to the different densities of several taxa between the two parts of the CS and slight differences in the faunal composition. The CS assemblage was demonstrated to be more heterogeneous than the SM and the BP by its relatively high beta diversity (Table 1) and by the larger differences among its samples as demonstrated by the nMDS presentation distances, both indicating the potential existence of more than one assemblage (Fig. 2; NT data). The differences among the faunal compositions in the various biotopes are also demonstrated by the high number of biotope-specific taxa, 53% of the total number. A study by Tselepides et al. (2000) along the north coast of Crete was conducted considering a bottom terrain and depth range that were similar to those of the present studied area. Their study revealed similar biotopes to those of the present one, located at similar depths, 100–200 m (comparable to the present shelf margin), 540–940 m (continental slope) and 1570 m (bathyal plain).
The present assessment of faunal assemblages was based on data of variable taxonomic resolution and that crossed the traditional boundaries of meio- and macrobenthos. The representation of faunistic groups containing individuals that cross the sieving threshold distorts the abundance profile of certain taxa but on the other hand is assumed to enhance the power of distinction among biotopes, providing more comprehensive data. The identification of fauna to the species level was not always achieved, and it was assumed that identification to higher taxonomic levels reduces the distinctions among faunal assemblages but would not introduce any false distinctions. Bett and Narayanaswamy (2014) previously assessed the influence of taxonomic level on the ecological assessment of deep-sea macrobenthos, noting that genus-level alpha and beta diversity measures were highly correlated to and are good predictors of their species-level equivalents. They further argued that given the complexity of the West Shetland slope environment, it may be reasonable to expect these conclusions to hold for other deep-sea environments. We suggest that the ecological coherence of our results generally support this approach. Nevertheless, the need to improve taxonomic accuracy in ecological studies using both conventional and genetic approaches is evident.
Faunal density
The infaunal density logarithmically declined with water depth (Fig. 4). Table 4 presents density data from the present study along with those from three previous studies in the eastern Mediterranean (Tselepides et al. 2000; Kröncke et al. 2003; Baldrighi et al. 2014). Comparisons among these studies are hampered by the different years of sampling, low sample numbers, and different sieve mesh sizes, permitting only general conclusions: (a) a well-established exponential decline in faunal density with water depth (Fig. 4 and Tselepides et al. (2000)); (b) relatively low densities (100–1000 s ind m− 2 on the continental slope and 10–100 s ind m− 2 on the bathyal plain); (c) consistently higher values observed by Kröncke et al. (2003) in comparison to those found in the present study and those of Baldrighi et al. (2014) may reflect temporal changes; (d) no obvious west–east faunal density reduction in the LB was observed, applicable at the scale of the entire Mediterranean (Sardà et al. 2004; Baldrighi et al. 2014).
It has been indicated on a global scale that specific biotic groups display different density proportions along an increasing depth gradient accompanied by a smaller size and lower biomass of individuals, namely, the larger megafauna and macrofauna are replaced by smaller meiofaunal species. This leads to a faunal change into denser communities containing taxa that are smaller-sized but more adaptable to shortages in available food and its differential availability to different biotic groups (Rex et al. 2006; Wei et al. 2010). The global-scale findings are also supported by the foraminiferal density demonstrated in the framework of the present sampling effort, showing only minor density changes in the various biotopes (Orit Hyams-Kaphzan and Ahuva Almogi-Labin, unpubl data).
Primary production in the eastern Mediterranean is rather low, e.g., 59 g C m−2 year−1 recorded in the Cretan Sea (Siokou-Frangou et al. 2010), with export flux through the water column likely declining exponentially with water depth (Marsay et al. 2015). Hence, Danovaro et al. (1999) reported for the Cretan Sea flux values of 1–2 g C m− 2 year− 1 at 1540 m water depth. This latter study evaluated a 10 % contribution of vertically transported labile carbon to the deep water in the western Mediterranean compared to 2–3 % in the Cretan trough. The low rate of vertical sedimentation described in the LB supports the low vertical supply of particulate organic matter from the euphotic zone. Van Santvoort et al. (1996) demonstrated for the deep eastern Mediterranean, >2000 m depth, 3 cm k year− 1 , converted to 0.0021 g cm− 2 year− 1 using a conversion factor of 0.7 g cm− 3 (Basso et al. 2004). The preliminary results of Schirone et al. (2014) in the present study area showed a vertical sedimentation rate of 0.08 ± 0.01 g cm− 2 year− 1 in the Israeli CS and even a much lower, non-significant level for its BP. Hence, the above indications of low vertical flux to the LB deep waters may explain at least partially the sharp density decline with depth of the > 250 μm infauna. The comparable characteristics of the smaller meiofauna in the studied area are still missing.
Lateral transport from terrestrial or shelf sources across the CS may partially explain the density gradient towards the BP. A phenomenon termed “downwelling” of downhill wintertime currents was reported by Rosentraub and Brenner (2007) and Rosentraub et al. (2010) flowing from the Israeli shelf to the slope down to the 500-m depth contour. In addition, a turbidity current across the CS was indicated to be created by dense water development along continental margins of the LB (Oszoy et al. 1989; Chronis et al. 2000). Transport from the Nile delta by the general counter-clockwise Mediterranean current (Schattner et al. 2015) is another assumed mechanism of lateral transport. Chemosynthetic bacterial mats were recently found on the CS, estimated to cover 3 % of the CS bottom (Rubin-Blum et al. 2014), and may also be considered a potential food source.
The distributions of TOC concentrations on the sediment surface here are quite uniform, and although their small changes are correlated with depth (Table 5), they are unlikely to explain the huge differences in biotope densities. This difference is likely caused by labile carbon and its availability to different biotic groups, which should be evaluated in the future. The TOC percentages found in this study are in agreement with previously presented levels, which are twice those found on the western side of the LB (Romankevich 1984).
Faunal diversity
Assumed faunal assemblage uniformity within each of the biotopes underlies the comparison of a variety of ecological aspects among them. The area required to fully represent a biotope’s fauna depends on the faunal density. However, within-biotope variation at different spatial scales may also exist, increasing the required representative area. The pooling of the BP samples demonstrates the effect of density. The increased representative area reduced the heterogeneity of the pooled samples, as demonstrated by the increased Chao–Sørensen and decreased beta diversity indices (Table 1). Higher within-biotope heterogeneity in the CS in comparison to other biotopes is indicated by its higher beta diversity and by the larger area for CS in the nMDS representation (Fig. 3), leading also to the separation of the CS into two biotopes when using the NT data. Within-biotope heterogeneity is a well-documented phenomenon even in communities with much higher population densities than the present one (Grassle and Maciolek 1992). The existence of natural patchiness resulting from the presence of a variety of micro-habitats in an apparently uniform muddy bottom is well established in the deep-sea literature (Grassle and Morse-Porteous 1987; Grassle and Maciolek 1992; Snelgrove et al. 1992; Gage 2004 and literature therein).
The relationships between alpha diversity and the number of sampled individuals indicates high alpha diversity similarity across biotopes, as the three biotope-specific curves overlap and reach a mutual asymptote at least for the SM and the CS and partially for the BP (Fig. 6B). However, a different sampling area is required in each biotope to approach this asymptotic level (Fig. 6a), likely due to the lower densities in deeper waters. However, this analysis needs to be interpreted with caution, as the ranges of both the sampling area and the number of sampled individuals are incomplete in the rarified alpha diversity curves. Hence, it is not known if all curves of the sample-dependent alpha diversity would reach a common asymptote similar to the one of the number of individuals-dependent alpha diversity of the CS and SM curves. It is also doubtful that the rarified alpha diversity level in the number of individuals-dependent BP curve would maintain its overlap with the curves of the SM and the CS at higher numbers of sampled individuals. This doubt is supported by the different estimated maximal numbers of taxa in the various biotopes (Fig. 5), which may lead to different asymptotic alpha diversity values.
Tselepides et al. (2000) presented taxa accumulation curves for their various sampled depth ranges. Their 23 samples did not reach an asymptote, and the expected number of taxa reached ~160 taxa in the SM, ~100–130 in the CS and ~60 in the BP, revealing a similar trend to that observed in the present study, showing a decreasing number of species with increasing depth. The alpha diversity in the study by Tselepides et al. (2000) declined with depth, similar to the present observed results; these results are compatible with the general trend in Mediterranean alpha diversity with depth (Danovaro et al. 2010). However, the study by Tselepides et al. (2000) lacks the evaluation of estimated alpha diversity, with its above-indicated similarity when rarified to the number of sampled individuals. The limited sampling effort of both Kröncke et al. (2003) and Baldrighi et al. (2014) at the relevant sites (Table 4), in addition to the other above-mentioned differences in identification accuracy, size threshold and sampling date, permits only a general statement claiming a roughly similar number of sampled taxa.
Ecology of the region
Two approaches were applied to examine the potential correlations between abiotic parameters and changes in the composition of the fauna: sample-wise (Table 3) and biotope-wise (Table 2). The sample-wise approach revealed water depth as the major correlated parameter, which is also evident from observing the depth-related distribution of the biotopes (Figs. 1, 2). However, it is likely not water depth per se (hydrostatic pressure) that is responsible for this correlation but a number of underlying parameters that co-vary with depth. Sediment is widely recognized to be a strong driver of infaunal ecology, both generally (Gray 1974) and in the deep sea (Etter and Grassle 1992), although it may be a complex relationship (Snelgrove and Butman 1994). In the present study, in addition to water depth, the grain size mode showed significant correlation with the sample biotic profiles. Percentage clay and CaCO3% in concert increased the rank correlation of both water depth and grain size mode with the faunal composition (Table 3). Table 5 provides the Spearman’s rank correlation between all the abiotic parameters and depth. Although all five examined abiotic parameters demonstrated a significant rank correlation with depth, only the grain size mode, the CaCO3% and the TOC% revealed strong correlations with depth. The importance of the effect of the TOC percentage on biotope densities is discussed above. The higher percentage of CaCO3 in the BP results from the higher biogenic content of planktonic foraminifera and pteropod shells (Elyashiv et al. 2014). The siliciclastic and less biogenic, closer-to-shore sediment originated from the sediment province formed by the Nile sediment transportation (Maldonado and Stanley 1976).
The three biotopes are related to three different bottom terrains; this strong affiliation is supported by several preliminary sedimentological indications that led us to put forward a working hypothesis for future studies suggesting that bottom sediment stability is a determining factor of faunal composition, with a much less stable CS in comparison to the SM and the BP. Sediment stability is generally a result of its fluidity, mixing processes, bottom inclination and overlying currents. Preliminary results reported higher sediment water content in the slope region of the studied area in comparison to both the shelf margin and the bathyal plain (Barak Herut, unpubl data).
Barsanti et al. (2011) studied and reviewed the sediment mixing processes in the Mediterranean deep sea (>2000 m) and in the LB, they found mixing processes mainly in the upper 2 cm caused by bioturbation. Bottom mixing was preliminarily examined in the SM, CS and BP of the present studied area by Schirone et al. (2014) using the 210Pb and 137Cs radionuclide and metal contaminant depth profiles. The present studied bottom area is much shallower than the bottom examined in Barsanti et al. (2011) and the results demonstrated a 2- and 4-cm mixed layer in the SM and the BP, respectively, and a much thicker mixed horizon in the CS. The intensively mixed, and hence less stable, CS bottom is assumed to result from the stronger inclination of the CS in comparison to its neighboring provinces coupled with the downhill currents and gravitational slides described and discussed above. Interestingly, one of the LB sites examined by Barsanti et al. (2011) in the much deeper mid-slope near Rhodes revealed a 6-cm mixed layer indicated to result from physical factors rather than bioturbation, similar to our interpretation of the results of Schirone et al. (2014) from the Israeli CS.
Future research directions
The present study comprehensively characterized the biotic parameters of the >250 μm benthic fauna of the southeastern corner of the LB and their assumedly shaping abiotic factors. This is the first detailed study of the benthic ecology of this region, the eastern edge of the Mediterranean west–east axis. Future studies in this area should technically include more accurate faunistics and more intensive sampling in view of the low infaunal density. Scientifically, the characterization of other benthic faunal communities, mainly those of smaller meiofauna and bacteria are needed, as well as a better characterization of the sediment features that potentially affect the faunal composition and density.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: Guide to software and statistical methods. PRIMER-E Ltd, Plymouth, UK, p 218
Baldrighi E, Lavaleye M, Aliani S, Conversi A, Manini E (2014) Large spatial scale variability in bathyal macrobenthos abundance, biomass, alpha and beta diversity along the Mediterranean continental margin. PLoS One 9:e107261
Barsanti M, Delbono I, Schirone A, Langone L, Miserocchi S, Salvi S, Delfanti R (2011) Sediment reworking rates in deep sediments of the Mediterranean Sea. Sci Total Environ 409:2959–2970
Basso D, Thomson J, Corselli C (2004) Indications of low macrobenthic activity in the deep sediments of the eastern Mediterranean Sea. Sci Marina 68:53–62
Bett BJ, Narayanaswamy BE (2014) Genera as proxies for species alpha and beta diversity: tested across a deep-water Atlantic-Arctic boundary. Mar Ecol 35:436–444
Chao A, Chazdon RL, Colwell RK, Shen T-J (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Chronis G, Lykousis V, Georgopoulos D, Zervakis V, Stavrakakis S, Poulos S (2000) Suspended particulate matter and nepheloid layers over the southern margin of the Cretan Sea (NE Mediterranean): seasonal distribution and dynamics. Prog oceanogr 46:163–185
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Gorley RN (2015) PRIMER v7: User Manual/Tutorial. PRIMER-E Ltd, Plymouth, UK, p 300
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: An approach to statistical analysis and interpretation, 3 PRIMER-E Ltd, Auckland 262
Colwell, RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User’s Guide and application published at: http://purl.oclc.org/estimates
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual based and sample based rarefaction, extrapolation, and comparison of assemblages. J Plant Ecol 5:321
Crouvi O, Amit R, Enzel Y, Porat N, Sandler A (2008) Sand dunes as a major proximal dust source for late Pleistocene loess in the Negev desert, Israel. Quarter Res 70:275–282
Danovaro R, Company JB, Corinaldesi C, D’Onghia G, Galil B, Gambi C, Gooday AJ, Lampadariou N, Luna G, Morigi C, Olu K, Polymenakou Ramirez-Llodra E, Sabbatini A, Sardà F, Sibuet M, Tselepides A (2010) Deep-Sea biodiversity in the Mediterranean Sea: The known, u, and the unknowable. PLoS One 5:e11832
Danovaro R, Dinet A, Duineveld G, Tselepides A (1999) Benthic response to particulate fluxes in different trophic environments: a comparison between the Gulf of Lions-Catalan Sea (western-Mediterranean) and the Cretan Sea (eastern Mediterranean). Prog Oceanog 44:287–312
Elyashiv H, Crouvi O, Almogi-Labin A, Harlavan Y, Hyams-Kaphzan O (2014) Characteristics of deep sea sediments from the Levantine basin (Israel economic zone)—Preliminary results, 19th International Sedimentological Congress, Geneva, Switzerland, pp 198
Etter RJ, Grassle JF (1992) Patterns of species diversity in the deep sea as a function of sediment particle size diversity. Nature 369:576–578
Gage JD (2004) Diversity in deep-sea benthic macrofauna: the importance of local ecology, the larger scale, history and the Antarctic. Deep-Sea Res Part II-Top Stud Oceanogr 51:1689–1708
Galil BS (2004) The limit of the sea: the bathyal fauna of the Levantine Sea. Sci Mar 68 (Suppl. 3):63–72
Gaudette HE, Flight, WR, Toner L, Folger DW (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sedim Petrol 44:249–253
Grassle JF, Maciolek NJ (1992) Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am Nat 139:313–341
Grassle JF, Morse-Porteous LS (1987) Macrofaunal colonization of disturbed deep-sea environments and the structure of deep-sea benthic communities. Deep-Sea Res 34:1911–1950
Gray JS (1974) Animal-sediment relationships. Oceanogr Mar Biol Ann Rev 12:223–261
Gvirtzman Z, Reshef M, Buch-Leviatan O, Groves-Gidney G, Karcz Z, Makovsky Y, Ben-Avraham Z (2015) Bathymetry of the Levant basin: interaction of salt-tectonics and surficial mass movements. Mar Geol 360:25–39
Hall JK, Lippman S, Gardosh M, Tibor G, Sade AR, Sade H, Golan A, Amit G, Gur-Arie L, Nissim I (2015) A New Bathymetric Map for the Israeli EEZ: Preliminary Results. Israeli Ministry of Energy and Water, Natural Resources Administration, report NEFT_239_2015, pp 11
Jones GA, Kaiteris P (1983) A vacuum-gasometric technique for rapid and precise analysis of calcium carbonate in sediments and soils. J Sedimen Petrol 53:655–660
Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88:2427–2439
Katz O, Reuven E, Aharonov E (2015) Submarine landslides and fault scarps along the eastern Mediterranean Israeli continental slope. Mar Geol 369:100–115
Konert M, Vandenberghe J (1997) Comparison of laser grain size analysis with pipette and sieve analysis: a solution for the underestimation of the clay fraction. Sedimentology 44:523–535
Kress N, Herut B (2001) Spatial and seasonal evolution of dissolved oxygen and nutrients in the southern Levantine Basin (eastern Mediterranean Sea): chemical characterization of the water masses and inferences on the N†¯: P ratios. Deep Sea Res Part I Oceanogr Res Pap 48:2347–2372
Kröncke I, Türkay M, Fiege D (2003) Macrofauna Communities in the Eastern Mediterranean Deep Sea. P.S.Z.N. Mar Ecol 24:193–216
Maldonado A, Stanley DJ (1976) The Nile Cone: Submarine fan development by cyclic sedimentation. Mar Geol 20:27–40
Marsay CM, Sanders RJ, Henson SA, Pabortsava K, Achterberg EP, Lampitt RS (2015) Attenuation of sinking particulate organic carbon flux through the mesopelagic ocean. PNAS 112:1089–1094
Ozsoy E, Hecht A, Ünlüata Ü (1989) Circulation and hydrography of the Levantine Basin: Results of POEM coordinated experiments 1985–1986. Prog Oceanogr 22:125–170
Pusceddu A, Bianchelli S, Canals M, Vidal AS, Durrieu De Madron X, Heussner S, Lykousis V, deStigter H, Danovaro R (2010) Organic matter in sediments of canyons and open continental slopes along European continental margins. Deep-Sea Res I(57):441–457
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Rex MA, Etter RJ, Morris JS, Crouse J, McClain CR, Johnson NA, Stuart CT, Deming JW, Thies R, Avery R (2006) Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar Ecol Prog Ser 317:1–8
Romankevich EA (1984) Geochemistry of organic matter in the ocean. Springer-Verlag, Berlin, pp 336
Rosentraub Z, Brenner S (2007) Circulation over the southeastern continental shelf and slope of the Mediterranean Sea: Direct current measurements, winds, and numerical model simulations. J Geophys Res 112:C11011 
Rosentraub Z, Anis E, Goldman R (2010) Wintertime cross-shelf circulation and shelf/slope interaction off the central Israeli coast. 39th CIESM congress, Venice, 10–14 May, 2010
Rubin-Blum M, Antler G, Tsadok R, Shemesh E, Austin JA Jr, Coleman DF, Goodman-Tchernov BN, Ben-Avraham Z, Tchernov D (2014) First evidence for the presence of iron oxidizing Zetaproteobacteria at the Levantine continental margins. PLoS One 9:e91456
Sardà F, Calafat A, Mar Flexas M, Tselepides A, Canals M, Espino M, Tursi A (2004) An introduction to Mediterranean deep-sea biology. Sci Mar 68 (Suppl. 3):7–38
Schattner U, Gurevich M, Kanari M, Lazar M (2015) Levant jet system—effect of post LGM seafloor currents on Nile sediment transport in the eastern Mediterranean. Sedim Geol 329:28–39
Schirone A, Herut B, Delbono I, Barsanti M, Delfanti R (2014) Sedimentation and mixing rates in the Levantine Sea. Poster presented at the PERSEUS Scientific Conference, December 1–4, Marrakesh, Morocco
Siokou-Frangou I, Christaki U, Mazzocchi MG, Montresor M, Ribera d’Alcalà M, Vaquè D, Zingone A (2010) Plankton in the open Mediterranean Sea: a review. Biogeosciences 7:1543–1586
Snelgrove PVR, Butman CA (1994) Animal-sediment relationships revisited: cause versus effect. Oceanog Mar Biol Ann Rev 32:111–177
Snelgrove PVR, Grassle JF, Petrecca RF (1992) The role of food patches in maintaining high deep-sea diversity: Field experiments with hydrodynamically unbiased colonization trays. Limnol Oceanogr 37:1543–1550
Tselepides A, Papadopoulou N, Podaras D, Plaiti W, Koutsoubas D (2000) Macrobenthic community structure over the continental margin of Crete (South Aegean Sea, NE Mediterranean). Prog Oceanogr 46:401–428
Van Santvoort PJM, De Lange GJ, Thomson J, Cussen H, Wilson TRS, Krom MD, Strøhle K (1996) Active post-depositional oxidation of the most recent sapropel (Sl) in sediments of the eastern Mediterranean Sea. Geochim Cosmochim Acta 60:4007–4024
Wei CL, Rowe GT, Escobar-Briones E, Boetius A, Soltwedel T, Caley MJ, Soliman Y, Huettmann F, Qu F, Yu Z, Pitcher CR, Haedrich RL, Wicksten MK, Rex MA, Baguley JG, Sharma J, Danovaro R, MacDonald IR, Nunnally CC, Deming JW, Montagna P, Lévesque M, Weslawski JM, Wlodarska-Kowalczuk M, Ingole BS, Bett BJ, Billett DSM, Yool A, Bluhm BA, Iken K, Narayanaswamy BE (2010) Global patterns and predictions of seafloor biomass using random forests. PLoS One 5:e15323
Acknowledgements
The study was initiated and supported by the Israeli Ministry of Infrastructure, Energy and Water Resources. Special gratitude goes to Mr. Ilan Nissim, the head of the environmental department of the Ministry. The study was also partially supported by the PERSEUS project (EC Contract #287600) to B.H and M.T. The commitment and assistance of the crew of R/V Shikmona and of the research assistants who participated in the sampling cruises and performed the laboratory tasks are highly appreciated, especially Ms. Eva Misrahi. Ms. Hadar Elyashiv is thanked for the grain size analysis. The taxonomists who performed or supported the identification are highly appreciated. They are Dr. Sabrina Lo Brutto, University of Palermo, Italy (Amphipoda); Dr Graham Bird, Marine Biologist, Kāpiti, New Zealand (Tanaidacea); Dr. Jordi Corbera, Institut Cartografic de Catalunya, Spain (Cumacea); Dr. David Drumm, EcoAnalyst, USA (Copepoda); Dr. Bella Galil, Israel Oceanographic and Limnological Research (Decapoda); Dr. Cesare Bogi, Italy (Mollusca); Dr. Chip Barrett, EcoAnalyst, USA (Polychaeta). The anonymous reviewer and the associate editor are deeply thanked for their very helpful revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All applicable international, national and institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: J. Grassle.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lubinevsky, H., Hyams-Kaphzan, O., Almogi-Labin, A. et al. Deep-sea soft bottom infaunal communities of the Levantine Basin (SE Mediterranean) and their shaping factors. Mar Biol 164, 36 (2017). https://doi.org/10.1007/s00227-016-3061-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-3061-1