Abstract
Nematodes play an important role in ecological processes and are one of the most abundant meiofaunal organisms associated with seaweeds. Yet, knowledge on seaweed bed ecosystems is limited. Nematodes associated with Sargassum polyceratium and Halimeda opuntia were compared in two transects, 80 m apart and parallel to the beach line in Cupe Beach, Brazil. The temporal variation during the dry and rainy seasons and the effect of sediment retention by the seaweed on nematode density and composition were investigated. The differences in nematode communities between the two seasons were mainly caused by the increase in density of the most abundant genera in the rainy season. A significant difference was observed between the nematode communities of the two transects for H. opuntia. The nematode communities of both seaweed species did not differ significantly in the same transect. The genus Euchromadora was dominant in both seaweed species. The amount of sediment retained by the seaweeds did not affect the overall nematode density. However, it was positively correlated with the density of Draconema and Euchromadora in both seaweeds, and both genera were exclusively found associated with seaweeds. This result opposes the idea that the more sediment retained by the seaweed, the higher the nematode overall density and the higher the number of nematodes originally coming from the sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweed beds and associated fauna form a highly productive ecosystem in shallow water coastal areas (De Troch et al. 2001). Seaweeds harbor a variety of organisms belonging to almost all trophic levels of the food web and also serve as a shelter, reproduction and/or grazing site for many organisms (Brewer et al. 1994; Kenyon et al. 1998; Ferreira et al. 2000; NagelkerkenI et al. 2000; Da Rocha et al. 2006). They provide oxygen and are involved in many mineralization and chemical cycling processes (Vidotti and Rollemberg 2004).
Seaweed beds in tropical areas are frequently associated with geological formations such as sandstone or biological reefs, which provide protection by dissipating the wave energy (Ferreira Júnior 2005). The local hydrodynamics can strongly affect the macrophytal and epiphytal biomass, abundance and density, which in turn affect the distribution and activity of organisms that are grazing on the seaweeds (Schanz et al. 2002). Seaweed beds provide protection from currents and desiccation and can influence the spatial distribution of the associated organisms (Muralikrishnamurty 1983). Moreover, seaweed beds also play a role in decreasing the current velocity and increasing the sedimentation rate of sediment and other particles present in the water column (Fonsêca and Calahan 1992). It has been suggested that the accumulation of detritus by the seaweed correlates with the ramification and structure of the macrophytes and increases microhabitat complexity, which would allow for a higher density of small-sized metazoans (Taylor 1967; Hicks 1980; Da Rocha et al. 2006). Seaweed beds are under the influence of tides and seasonality which also affect the associated organisms (Toyohara et al. 1999). However, for some small-sized organisms, examples are known where seasonality does not appear to be an important population driver, especially for those species which reproduce throughout the year (Coull and Vernberg 1975; Song et al. 2010).
Small-sized metazoans such as nematodes have a high capacity of colonizing seaweeds (Warwick 1977; Derycke et al. 2007) and play a fundamental role in the maintenance of the benthic ecosystem (Rieras and Hubas 2003). They are involved in processes such as biomineralization, bacterial population regulation, serve as food source for higher trophic levels and predate on the same and on lower trophic levels (Rysgaard et al. 2000; Schmid-Araya et al. 2002). With respect to seaweed, a very specific relationship with the associated fauna exists and can cause differentiation between communities from different seaweed species (Warwick 1977; Gibbons 1991; Gee and Warwick 1994a, b). Epistrate feeders are the most abundant nematode feeding type on seaweeds (Da Rocha et al. 2006) which may be related to the abundances of epiflora, and more specifically, of diatoms (Hagerman 1966; Tientjen and Lee 1973; Warwick 1977; Wetzel et al. 2002). Hence, nematodes may play an important role in controlling the densities of epiphytic organisms that compete for light and nutrients with the macroalgae (Van Donk 1998; Ghobrial et al. 2007). Information on temporal and spatial variation of nematode communities associated with seaweeds is extremely limited. Such a knowledge would provide insights on the dynamics of small-sized organisms associated with macrophytal ecosystems, allowing for a better understanding of physical factors that are important for structuring the communities.
In this study, the nematode communities associated with seaweed beds from the northeastern coast of Brazil were investigated. The seaweed species Halimeda opuntia (Linnaeus) Lamouroux (1816) and Sargassum polyceratium Montagne (1837) are abundantly present throughout the year. H. opuntia is a green calcareous seaweed that tends to make mats over hard substrate, while S. polyceratium is a brown seaweed which can stand up perpendicularly to the substrate (Fig. 1).
The specific goals of this study were fourfold. First, the diversity, community and feeding type structure of nematodes associated with H. opuntia and S. polyceratium were characterized and compared. Due to the different architectural structure of the two seaweed species, seaweed species-specific communities were expected. Moreover, a dominance of epistrate feeders was expected in the nematode communities of both seaweeds, because diatoms and cyanobacteria are abundant on the seaweed surface. Second, the temporal variability in nematode communities of H. opuntia and S. polyceratium was investigated by comparing the dry and rainy seasons and by comparing nematode communities over five months. Temporal fluctuations in abiotic parameters (e.g., the amount of rain, salinity) in Cupe beach may influence nematode abundances associated with H. opuntia and S. polyceratium and may cause shifts in the nematode community because of different tolerances of nematode species to abiotic changes. Third, spatial variation of nematode communities associated with H. opuntia in two transects parallel to the coast was investigated. These transects differed in their distance to the shore and in the degree of exposure to wave action. A higher variability in the nematode communities over time and lower nematode diversity and density were expected in the wave impacted zone because of the higher physical disturbance. Finally, the influence of sediment retention by the seaweeds H. opuntia and S. polyceratium on the nematode communities was assessed. The different architecture of H. opuntia and S. polyceratium may cause different sediment retention capacity, resulting in a higher density and richness of nematodes in the seaweed with the highest sediment retention capacity because of an increase in habitat complexity. Due to the tendency to form mats over hard substrate, it is expected that H. opuntia would accumulate more sediment.
Materials and methods
Study area
Cupe beach was chosen to test the impact of spatial and temporal variation and seaweed species on nematode communities. The beach is located in the northeast of the Brazilian coastline (coordinates 8°45′48″–8°46′22″S and 34°98′85″–34°97′99″W) and belongs to Ipojuca city, Pernambuco State. The beach is characterized by arenite and stone reefs with natural swimming pools separating the beach from the open sea. Various seaweed species occur on the sandstone and its surrounding areas in the subtidal and intertidal zone. The water temperature ranges from 27.0 to 28.7 °C and the salinity varies between 28.88 and 37.16 according to the season. The sediment is composed mainly of quartz sand and is very rich in bioclast, such as gastropod shells and pieces of calcareous algae (Dominguez et al. 1992).
Sample collection and processing
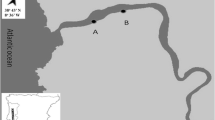
Based on their high abundance throughout the year, two species of seaweed were selected: Sargassum polyceratium and Halimeda opuntia. S. polyceratium and H. opuntia have architectural differences. The first one is a brown seaweed which can stand up perpendicularly to the substrate, whereas H. opuntia is a green calcareous seaweed that tends to make mats over hard substrate. The sampling occurred during the dry season (December 2005, January 2006) and the rainy season (May, June, July 2006) at low tide in the subtidal zone. Two transects of about 160 m length and parallel to the beach were demarcated with a distance between each other of about 80 m. Transect 1 (T1) was further from the shore compared to transect 2 (T2) (Fig. 2). For all five time points and for each transect, three equidistant sampling points were chosen, and from each point three samples from each seaweed species were collected (Fig. 2). The coordinates of each of the three sampling points are 8°45′78″S and 34°98′19″W, 8°45′86″S and 34°98′23″W, 8°45′94″S and 34°98′29″W for T1 and 8°45′73″S and 34°98′30″W, 8°45′81″S and 34°98′34″W, 8°45′87″ and 34°98′39″ for T2.
Location of transects in Cupe beach—Ipojuca—Pernambuco at the northeast of the Brazilian coast. T1 represents transect 1 which is more exposed to the waves, and T2 represents transect 2 which is closer to the beach and thus less exposed to the waves (modified from Da Rocha et al. 2006)
S. polyceratium only occurred in T2, while H. opuntia occurred in both transects. The seaweeds were collected by using a knife to detach the holdfast from the substrate, and the whole seaweed was put in a plastic bag and fixed with 4 % formalin. The seaweeds were washed under continuous water flow over a set of two sieves with mesh intervals for meiofauna of 500 and 44 micrometers and specimens retained on the latter were investigated. The volume of the seaweed was measured according to the methodology of Montouchet (1979) by measuring the difference between the initial and final water volume after the inclusion of seaweed in a graduated cylinder. To test the sediment retention capacity of S. polyceratium and H. opuntia, the sediment that was retained by the sieves for each seaweed sample was put in Petri dishes, dried in an oven and weighted (g). The nematodes were counted under a dissection microscope Olympus SZ51. When present, at least 100 nematodes were randomly and manually picked out and mounted on slides for identification. In case less than 100 specimens were present in the sample, all were mounted on slides. Preparation and mounting of the nematode specimens occurred according to De Grisse (1969). The nematodes were identified under the light microscope Olympus CX31 to genus level by using the pictorial identification keys (Platt and Warwick 1983; Warwick et al. 1998) and dichotomous keys in Abebe et al. (2007). Additionally, the nematode community was classified according to the feeding types proposed by Wieser (1953) based on the buccal cavity morphology: 1A Selective deposit feeders, 1B non-selective deposit feeders, 2A epistrate feeders and 2B predators or omnivores.
Data analyses
The richness, densities and relative abundance of the nematode community per seaweed sample were calculated. To compare the temporal (dry and rainy period, both seaweeds) and spatial variation (H. opuntia only) of the nematode community associated with S. polyceratium and H. opuntia, the abundance of the nematode community was converted to density (individuals/ml), transformed to square roots and standardized by the total number of nematodes in the sample (relative abundance) before the similarity analysis. All multivariate analyses (nMDS, PERMANOVA and SIMPER) were performed based on Bray-Curtis similarity matrix using the software PRIMER v. 6.1.6 (Clarke and Gorley 2006). The fixed factors used in PERMANOVA were: seaweed species, season and transect (H. opuntia only). The factor month was treated as random variable and nested within the factor season. PERMANOVA was used to compare 1) the nematode community between H. opuntia and S. polyceratium occurring in the same transect over time (season [months]) and 2) compare the nematode community in both transects over time (season [months]) for H. opuntia. When significant differences were found, a SIMPER analysis was performed to determine the taxa that contributed to those differences. The amount of sediment retained by the seaweeds was standardized to g/ml. The standardized amount of sediment retained by the seaweed, nematode densities and nematode richness were fourth-root-transformed to fulfill the assumptions for a parametric test. Two-way ANOVAs were performed to test whether there were: 1) differences in nematode density and richness over time between the seaweeds in T2, 2) differences in nematode density and richness over time between transects for H. opuntia, 3) differences in sediment retention by H. opuntia over time between transect and 4) differences in sediment retention between the seaweeds over time in T2. To test whether the amount of retained sediment correlated with the nematode density on the seaweeds, a Spearman’s correlation was done. The ANOVA and correlation analyses were performed using the statistical software STATISTICA v. 7 (StatSoft, Inc. 2004).
Results
Nematode communities and feeding type structure of H. opuntia and S. polyceratium
In total, 96 samples were analyzed: 35 for S. polyceratium (T2) and 61 for H. opuntia (T1 and T2). Identification of the nematode communities in these samples yielded 59 genera that were associated with both seaweeds (Table 1), 36 genera that were found only on S. polyceratium (T2: mean 6.74 ± 0.48) and 55 genera that were only associated with H. opuntia (T1: total = 49, mean 9.19 ± 0.61; T2: total = 41, mean 9.25 ± 0.75). The most abundant genera were Euchromadora, Paracanthonchus and Halalaimus for H. opuntia (35, 10 and 8 %, respectively), and Euchromadora, Paracanthonchus and Hypodontolaimus for S. polyceratium (34, 14 and 9 %, respectively). Acanthonchus and Chromadora reached two to threefold higher abundances in June compared to the other months, but only for H. opuntia (Fig. 3).
Densities of the most abundant genera associated with S. polyceratium and H. opuntia in Cupe Beach (Brazil) in 2005–2006. a Overall average densities per seaweed and transect; b–d average densities of the most abundant genera per seaweed along the months of December, January, May, June and July. The corresponding abbreviations are: Acan (Acanthonchus), Chro (Chromadora), Drac (Draconema), Euch (Euchromadora), Hala (Halalaimus), Hypo (Hypodontolaimus), Para (Paracanthonchus) and Poly (Polygastrophora)
H. opuntia attained a significantly higher nematode richness (two-way, seaweed, ANOVA, F = 13.04, P = 0.003) compared to S. polyceratium. No significant compositional difference (PERMANOVA, seaweed, Pseudo-F = 2.95, P = 0.057) was observed between the nematode communities of both seaweeds (Table 2a).
The most frequent feeding type with more than 50 % of the relative abundance in both seaweeds were epistrate feeders (2A) (53 and 56 %), followed by predators (2B) (20 and 28 %), selective deposit feeders (1A) (20 and 14 %) and non-selective deposit feeders (1B) (7 and 3 %) in H. opuntia and S. polyceratium, respectively.
Temporal variation of nematode communities associated with H. opuntia and S. polyceratium
Comparing the nematode density pattern between H. opuntia and S. polyceratium over time in T2, no significant differences were observed within each season (two-way ANOVA, season × seaweed, F = 0.25, P = 0.639), but significant differences (Table 2a and Fig. 4a) were observed between the seaweeds within each of the five months (two-way ANOVA, month × seaweed, F = 3.23, P = 0.029). The pairwise comparison revealed that the nematode density in the month of June for H. opuntia (Table 4) was significantly higher compared with the months December, January and also higher than the data of June for S. polyceratium (Tukey HSD, H. opuntia January × S. polyceratium December; January; June = P < 0.001; P < 0.001; P = 0.032, respectively). No significant temporal variation in richness patterns (Fig. 4b) was observed (two-way ANOVA, season, F = 2.58, P = 0.175 − month, F = 4.79, P = 0.115 – season × seaweed, F = 1.32, P = 0.27 − month × seaweed, F = 0.39, P = 0.754).
In terms of community structure, no clear distinction was observed in the nMDS plot (Fig. 5). No significant interaction between seaweed and seasons (PERMANOVA, seaweed × season, Pseudo-F = 1.60, P = 0.133) or seaweed and months (PERMANOVA, seaweed × months Pseudo-F = 1.54, P = 0.066) was observed, indicating that community structure between seaweed species over time was similar. However, nematode community structure was significantly different between the dry and rainy seasons (PERMANOVA, season, Pseudo-F = 3.45, P = 0.002). The genera that contributed the most for the differences between seasons were Euchromadora, Chromadora and Acanthonchus (SIMPER, 12.78, 7.75, and 7.69 %). Moreover, nematode communities were also significantly different between the months (PERMANOVA, month, Pseudo-F = 2.62, P = 0.001). Pairwise comparisons revealed that the nematode community significantly fluctuated over the months (Table 3a).
Spatial variation of the nematode community of H. opuntia
A total of 49 and 41 genera were found associated with H. opuntia in T1 and T2, respectively. The genera that presented the highest densities were Euchromadora, Chromadora and Acanthonchus in T1 (1.01; 0.91; 0.88 individuals/ml, respectively) and Euchromadora, Paracanthonchus and Halalaimus in T2 (2.07; 0.80; 0.44 individuals/ml, respectively). The genera that reached the highest relative abundance in each transect (Fig. 6) were Chromadora, Euchromadora and Draconema in T1 (17, 16, and 16 %, respectively), and Euchromadora, Paracanthonchus and Halalaimus in T2 (35, 10, and 8 %, respectively).
No significant differences in nematode density (two-way ANOVA, season × transect, F = 0.37, P = 0.571 − month × transect, F = 1.21, P = 0.314) or richness (season × transect, F = 1.36, P = 0.285 − month × transect, F = 0.65, P = 0.584) between both transects over time were observed, indicating that the observed pattern in density and richness was very similar over time in both transects (Table 2b). No significant difference in nematode density (two-way ANOVA, transect, F = 1.72, P = 0.248) or richness (two-way ANOVA, transect, F = 0.09, P = 0.769) between the T1 and T2 was found. For the factor time, only a significant difference in nematode density between the months was observed (two-way ANOVA, month, F = 13.81, P = 0.029, Table 3b). The nMDS plot did not show a clear separation between transects (Fig. 7), and the interaction between the transects and season or transects and months did not show a significant difference over time (PERMANOVA, transect × season, Pseudo-F = 1.56, P = 0.263; transect × month, Pseudo-F = 0.75, P = 0.820). However, the main effects were significantly different (Table 2b), revealing a difference in community structure between the two transects. (PERMANOVA, transect, Pseudo-F = 5.57, P = 0.045) and months (PERMANOVA, month, Pseudo-F = 1.97, P = 0.004). The taxa that contributed the most for the differences between transects were Euchromadora, Chromadora and Draconema (SIMPER: 8.13, 8.06, and 7.57 %, respectively), with the last two being more abundant in T1, while Euchromadora was more abundant in T2. The pairwise analysis revealed that the differences in months for both transects were between May and June (PERMANOVA, pairwise, P = 0.001). The genera that contributed the most for the differences were Paracanthonchus, Euchromadora and Acanthonchus (SIMPER: 9.03 %; 8.09 %; 8.01 %, respectively), with the two first more abundant on May.
Comparison on sediment retention between seaweeds and for H. opuntia between transects
In total, 90 samples for H. opuntia (9 replicates per transect over 5 months) and 35 samples for S. polyceratium were analyzed. There were no differences in sediment retention over time between H. opuntia and S. polyceratium in T2 (two-way ANOVA, seaweed × season, F = 0.09, P = 0.761 − seaweed × month, F = 0.67, P = 0.572). Yet, the difference in architecture of the two seaweeds yielded differences in overall sediment retention capacities in T2 (Table 2a) where H. opuntia retained significantly more sediment than S. polyceratium (two-way ANOVA, seaweed, F = 10.53, P = 0.010). No significant differences between season (two-way ANOVA, season, F = 0.05, P = 0.828) or between months (two-way ANOVA, month, F = 0.41, P = 0.391) were observed. For H. opuntia, no spatial pattern (Table 2b) was observed in sediment retention between transects over time (two-way ANOVA, transect × season, F = 0.73, P = 0.416 – transect × month, F = 0.46, P = 0.707) or between the transects (two-way ANOVA, F = 0.40, P = 0.539). Performing the Spearman’s correlation, no correlation was found between the nematode density and the amount of sediment retained for H. opuntia or S. polyceratium. However, a positive correlation was observed between the amount of retained sediment and nematode richness for both seaweeds (H. opuntia: R = 0.32, P = 0.011—S. polyceratium: R = 0.40, P = 0.014). Three of the most abundant genera showed a positive correlation between the amount of retained sediment and genus density in both seaweeds: Draconema (H. opuntia, R = 0.26, P = 0.03—S. polyceratium, R = 0.34, P = 0.04), Euchromadora (H. opuntia, R = 0.41, P < 0.001—S. polyceratium, R = 0.37, P = 0.02) and Paracanthonchus only in H. opuntia (R = 0.28, P = 0.026). No correlation was found for Acanthonchus, Chromadora, Eurystomina or Hypodontolaimus.
Discussion and conclusions
Co-occurring seaweed species harbor similar nematode communities and similar trophic composition
Overall nematode densities, community structures and community compositions were similar on both seaweeds, which is in agreement with the observations described in a study involving four different macrophyte species (Da Rocha et al. 2006). Despite the similarity in density and community structure (P value = 0.057 fairly in the limit), the genera richness was significantly different between H. opuntia and S. polyceratium. In terms of average relative abundance, some nematodes appeared to prefer one seaweed species over the other as illustrated by Hypodontolaimus for S. polyceratium. In contrast, on H. opuntia a higher average relative abundance of the family Draconematidae was observed, also the occurrence of Epsilonematidae, which were not associated with S. polyceratium. Both families are typically found associated with corals and other hard substrate (Raes and Vanreusel 2006; Raes et al. 2008; Armenteros et al. 2012); their occurrence on H. opuntia is most likely related to the calcareous nature of H. opuntia. This kind of preference was already mentioned by other authors (Hopper and Meyers 1967a, b; Warwick 1977). In epiphytic amphipods, no correlation has been found between seaweed morphology or complexity (ratio between surface area and biomass) and their abundance or species richness (Russo 1990). In contrast, ostracod species from California did show a strong correlation with complexity levels of the seaweed they were associated with (Frame et al. 2007). Therefore, it seems that different organisms have a different relationship with the macroalgal substrate. Regarding the feeding types, in this study, the epistrate feeders (2A) were the most dominant in both seaweeds, as has been previously observed for seaweeds (Ólafsson et al. 1995; Da Rocha et al. 2006; Jaya et al. 2012). However, this is in contrast with the nematode community associated with the seagrass Zostera in which 1B was the most dominant feeding type (Alves et al. 2015) and with Caulerpa taxifolia which was dominated by the genus Halichoanolaimus, a predator/omnivore or 2B (Soetaert and Heip 1995; Pape et al. 2013).
Seasonal variation reveals higher nematode abundances during the rainy season, but the community composition was very similar
Overall nematode density was significantly higher during the rainy season and varied differently among months on both seaweeds. In June, H. opuntia presented a significantly higher nematode density compared with S. polyceratium in the same month. Although the same general trend was observed for both seaweeds (increase of nematode density toward June), the magnitude of this increase appeared to be seaweed species specific. Temporal variation in density of nematodes associated with seaweeds peaking in certain periods of the year has already been observed (Kito 1982). However, comparisons between nematode communities from different seaweeds species over time are extremely rare. In the current work, no variation in richness was observed between seasons and months for both seaweeds and for H. opuntia in both transects, showing a fairly stable composition throughout the year. In contrast, a significant difference in nematode community structure has been found between the rainy and the dry seasons. Although the composition was very similar between the dry and rainy seasons, some abundant genera reached significantly higher relative abundances during the rainy season (e.g., Euchromadora). Temporal variation of the epifauna living on macrophytes can be related to seasonal change of the thallus (Travizi et al. 2004) or to preferences for different structures of the seaweed (Venekey et al. 2008). Microarthropod species associated with the macrophyte Ascophyllum nodosum have also shown temporal variation (Jarvis and Seed 1996), with some species showing an increased density at a particular time point while the density decreased for other species. Meiofauna associated with the seagrass Posidonia oceanica showed higher temporal variability in density present on the leaf region than on the stem region, where the densities were higher with little variation throughout the year (Novak 1982). These differences were correlated with the seasonal development of the seagrass. Seaweeds, as Sargassum muticum, also show seasonal developmental variation which in turn may affect the associated fauna (Taylor 1997; Baer and Stengel 2010). However, it is important to emphasize that the mentioned studies were performed in temperate higher latitudes (>42°N or >35°S) where there is a marked seasonal variation affecting the organism’s life cycle. In contrast, the current work was performed in a tropical low latitude (8°S) region with fairly stable temperatures averaging at 26.5 °C during the rainy season and at 27.9 °C during the dry season (Machado 2015).
The nematode community structure differed between transects, but no differences in the density or richness were observed
Although there were no significant differences in nematode density or richness, for H. opuntia between transects, there was a significant difference in nematode community structure. Spatial variation on epiphytic meiobenthic communities has been attributed to food source availability and environmental complexity (Novak 1982; Bell et al. 1984). The level of shelter from wave action appears to be a factor influencing nematode communities associated with the seaweed Sargassum in Brazil (Venekey et al. 2008) and with Gelidium pristoides in South Africa (Gibbons 1988). However, such effect of wave exposure was not observed by Arroyo et al. (2004) studying the meiofauna and nematode community associated with the seaweed genus Laminaria in Spain. In the present investigation, Euchromadora was the genus that contributed most to spatial differences; it preferred areas closer to the beach and thus more sheltered (T2), where it could reach twice the density of the area further away from the beach line (T1). This may indicate that the changes in community structure were mostly resulting from a higher degree of exposure rather than from temporal fluctuation. The community associated with macrophytes reached a higher average density in more sheltered areas, although the data were not always statistically significant.
Sediment retention capacity differed between seaweeds, affecting the density of some specific genera but not the density of the whole community
There was no significant difference in sediment accumulation between the two transects over time. The sediment retention capacity related more to the seaweed species rather than to degree of exposure and appears to be also related to the level of architectural complexity of the seaweed. Despite a significant difference in sediment retention capacity of the two seaweeds studied, the overall nematode density on the seaweeds was not affected. However, the retained sediment showed a positive correlation with the nematode richness for both seaweeds (H. opuntia: R = 0.32; P = 0.011—S. polyceratium: R = 0.40; P = 0.014). For some genera, a positive correlation was observed between nematode density and seaweed species, for example in Draconema and Euchromadora. This may suggest that the effect of the amount of retained sediment is species specific, affecting the community structure and richness, but not the overall nematode density. Interestingly, Draconema and Euchromadora did not occur in the bottom sediment (de Oliveira et al. 2014), maybe due to morphological and locomotion adaptations of the former (Raes et al., 2008), while Hypodontolaimus occurred in the bottom sediment and on the seaweed but did not show any correlation with the retained sediment. This suggests that the retained sediment by the seaweed was a more important factor affecting the nematode genera that were restricted to seaweeds rather than the genera occurring in seaweed and sediment. This result contrasts with two general paradigms described in a number of articles (Wieser 1951, 1952; Ott 1967; Hopper and Meyers 1967a, b; Moore 1971; Warwick 1977; Da Rocha et al. 2006): (1) the more sediment on the seaweed, the higher the density of nematodes and (2) the more sediment, the more nematodes originating from the sediment are also found on the seaweed. However, none of the above mentioned studies quantified the amount of retained sediment and tested its correlation with the nematode community density or structure on seaweeds. Nematodes choose actively the substrate on which they settle (Ullberg and Ólafsson 2003; Arroyo et al. 2006) and are rather not just passively transported along with the sediment through currents and retained by the seaweed. Experiments on colonization of macrophytes by nematodes have demonstrated that through time, the community is dominated by species that are typically found associated with macrophytes (Arroyo et al. 2006; Derycke et al. 2007). This result opposes the idea that the more sediment retained by the seaweed, the higher the nematode overall density and the higher the number of nematodes originally coming from the sediment.
References
Abebe E, Traunspurger W, Andrássy I (2007) Freshwater nematodes: ecology and taxonomy, 1st edn. Cabi Publishing, Oxfordshire
Alves AS, Caetano A, Costa JL, Costa MJ, Marques JC (2015) Estuarine intertidal meiofauna and nematode communities as indicator of ecosystem’s recovery following mitigation measures. Ecol Indic 54:184–196. doi:10.1016/j.ecolind.2015.02.013
Armenteros M, Ruiz-Abierno A, Sosa Y, Pérez-García JA (2012) Habitat heterogeneity effects on macro- and meiofauna (especially nematodes) in Punta Francés coral reef (SW Cuban Archipelago). Rev Invest Mar 32(1):50–61
Arroyo NL, Maldonado M, Pérez-Portela R, Benito J (2004) Distribution patterns of meiofauna associated with a sublittoral Laminaria bed in the Cantabrian Sea (north-eastern Atlantic). Mar Biol 144:231–242. doi:10.1007/s00227-003-1191-8
Arroyo NL, Aarnio K, Bonsdorff E (2006) Drifting algae as a means of re-colonizing defaunated sediments in the Baltic Sea. A short-term microcosm study. Hydrobiologia 554:83–95. doi:10.1007/s10750-005-1008-5
Baer J, Stengel DB (2010) Variability in growth, development and reproduction of the non-native seaweed Sargassum muticum (Phaeophyceae) on the Irish west coast. Estuar Coast Shelf S 90:185–194. doi:10.1016/j.ecss.2010.08.011
Bell SS, Walters K, Kern JC (1984) Meiofauna from seagrass habitats: a review and prospectus for future research. Estuaries 7(4):331–338. doi:10.2307/1351617
Brewer DT, Blaber SJM, Salini JP, Farmer MJ (1994) Feeding ecology of predatory fishes from Groote Eylandt in the Gulf of Carpentaria, Australia, with special reference to predation on Penaid prawns. Estuar Coast Shelf S40:577–600. doi:10.1006/ecss.1995.0039
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Coull BC, Vernberg WB (1975) Reproductive periodicity of meiobenthic copepods: seasonal or continuous? Mar Biol 32(3):289–293
Da Rocha CMC, Venekey V, Bezerra TNC, Souza JRB (2006) Phytal marine nematode assemblages and their relation with the macrophytes structural complexity in a Brazilian tropical rocky beach. Hydrobiologia 553:219–230. doi:10.1007/s10750-005-0923-9
De Grisse AT (1969) Redescription ou modification de quelques techniques utilisés dans l’ étude des nématodes phytoparasitaires. Meded. Rijksfakulteit Landbouwwetenschappen Gent 34:251–369
De Oliveira DAS, Dos Santos GAP, Derycke S, Moens T, Decraemer W (2014) Biodiversity and connectivity of marine nematodes associated with algae from two tropical beaches. J Nematol 46(2):152
De Troch M, Gurdebeke S, Fiers F, Vincx M (2001) Zonation and structuring factors of meiofauna communities in a tropical seagrass bed (Gazi Bay, Kenya). J Sea Res 45:45–61. doi:10.1016/S1385-1101(00)00055-1
Derycke S, Vynckt RV, Vanaverbeke J, Vincx M, Moens T (2007) Colonization patterns of Nematoda on decomposing algae in the estuarine environment: community assembly and genetic structure of the dominant species Pellioditis marina. Limnol Oceanogr 52(3):992–1001. doi:10.4319/lo.2007.52.3.0992
Dominguez JML, Bittencourt ACSP, Martin L (1992) Controls on quaternary coastal evolution of the east-northeastern coast Brazil: roles off sea-level history, trade winds and climate. Sediment Geo l80:213–232
Ferreira Júnior AV (2005) Mapeamento da Zona Costeira Protegida por Arenitos de Praia (Beachrocks) em Anísia Floresta—RN. Master thesis, Federal University of Rio Grande do norte
Ferreira CEL, Gonçalves JEA, Coutinho R (2000) Communities structure of fishes and habitat complexity on a tropical rocky shore. Environ Biol Fish 61:353–369
Fonsêca MS, Calahan JA (1992) A preliminary evaluation of wave attenuation by four species of seagrass. Estuar Coast Shelf S35:565–576. doi:10.1016/S0272-7714(05)80039-3
Frame K, Hunt G, Roy K (2007) Intertidal meiofaunal biodiversity with respect to different algal habitats: a test using phytal ostracodes from Southern California. Hydrobiologia 586(1):331–342. doi:10.1007/s10750-007-0707-5
Gee JM, Warwick RM (1994a) Body-size distribution in a marine metazoan community and fractal dimensions of macroalgae. J Exp Mar Biol Ecol 178:247–259. doi:10.1016/0022-0981(94)90039-6
Gee JM, Warwick RM (1994b) Metazoan community structure in relation to the fractal dimensions of marine macroalgae. Mar Ecol-Prog Ser 103:141–150. doi:10.1016/0022-0981(94)90039-6
Ghobrial MG, Okbah MA, Gharib SM, Soliman AM (2007) Influence of barley straw and submerged macrophytes on fishpond wastewater quality. Egypt J Aquat Res 33(3):68–87
Gibbons MJ (1988) The impact of wave exposure on the meiofauna of Gelidium pristoides (Turner) Kuetzing (Gelidiales: Rhodophyta). Estuar Coast Shelf Sci 21:581–593. doi:10.1016/0272-7714(88)90070-4
Gibbons MJ (1991) Rocky shore meiofauna: a brief overview. Trans R Soc S Afr 47:595–603
Hagerman L (1966) The macro and microfauna associated with Fucus serratus L., with some ecological remarks. Ophelia 3:1–43. doi:10.1080/00785326.1966.10409631
Hicks GRF (1980) Structure of phytal harpacticoid copepod assemblages and the influence of habitat complexity and turbidity. J Exp Mar Biol Ecol 44:157–192. doi:10.1016/0022-0981(80)90151-3
Hopper BE, Meyers SP (1967a) Populations studies on benthic nematodes within a subtropical seagrass community. Mar Biol 11(2):85–96. doi:10.1007/BF00386510
Hopper BE, Meyers SP (1967b) Foliicolous marine nematodes on turtle grass, Thalassia testudinum König, in Biscayne Bay, Florida. Bull Mar Sci Gulf Caribb 17:471–517
Jarvis SC, Seed R (1996) The meiofauna of Ascophyllum nodosum (L.) Le Jolis: characterization of the assemblages associated with two common epiphytes. J Exp Mar Biol Ecol 199:249–267. doi:10.1016/0022-0981(95)00184-0
Jaya P, Vijaya Bhanu Ch, Naveen Babu M, Annapurna C (2012) Phytal nematodes associated with Caulerpa fastigiata and Caulerpa taxifolia of Visakhapatnam coast. Int J Biol Pharm Allied Sci 1(3):331–336
Kenyon RA, Haywood MDE, Heals DS, Loneragan NR, Pendrey RC, Vance DJ (1998) Abundance of fish and crustacean post larvae on portable artificial seagrass units: daily sampling provides quantitative estimates of the settlement of new recruits. J Exp Mar Biol Ecol 232:197–216. doi:10.1016/S0022-0981(98)00107-5
Kito K (1982) Phytal marine nematode assemblage on Sargassum confusum Agardh, with Reference to the structure and seasonal fluctuations. J Fac Sci Hokkaido Univ Ser VI Zool 23(1):143–161
Machado RCA (2015) Estrutura da comunidade fitoplanctônica e hidrologia do ecossistema recifal de porto de galinhas (Pernambuco-Brasil). Ph.D. thesis, Universidade Federal de Pernambuco, Recife, Brazil
Montouchet PC (1979) Sur la communauté des animaux vagiles associés a Sargassum cymosum C. Agardh a Ubatuba, État de São Paulo, Brésil. Stud Neotrop Fauna Environ 14:33–64. doi:10.1080/01650527909360546
Moore PG (1971) The nematode fauna associated with holdfasts of kelp Laminaria hyperborea in North-East Britain. J Mar Biol 51:589–604. doi:10.1017/S0025315400014983
Muralikrishnamurty PV (1983) Intertidal phytal fauna of Gangavaram, east coast of India. Indian J Mar Sci 12(2):85–89
NagelkerkenI Van Der, Velde G, Gorissen MW, Meijer GJ, Van’t Hof T, Den Hartog C (2000) Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar Coast Shelf Sci 51:31–44. doi:10.1006/ecss.2000.0617
Novak R (1982) Spatial and seasonal distribution of the meiofauna in the seagrass Posidonia oceanica. Neth J Sea Res 16:380–388. doi:10.1016/0077-7579(82)90044-8
Ólafsson E, Johnstone RW, Ndaro SGM (1995) Effects of intensive seaweed farming on the meiobenthos in a tropical lagoon. J Exp Mar Biol Ecol 191:101–117. doi:10.1016/0022-0981(95)00055-V
Ott J (1967) Vertikalverteilung von Nematoden in Beständen nordadriatischer Sargassaceen. Helgoland Wiss Meer 15:412–428. doi:10.1007/BF01618638
Pape E, Oevelen D, Moodley L, Soetaert K, Vanreusel A (2013) Nematode feeding strategies and the fate of dissolved organic matter carbon indifferent deep-sea sedimentary environments. Deep-Sea Res Pt I 80:94–110. doi:10.1016/j.dsr.2013.05.018
Platt HM, Warwick RM (1983) Free-living marine nematodes. Part. I. British Enoplids. Cambridge University Press, Cambridge
Raes M, Vanreusel A (2006) Microhabitat type determines the composition of nematode communities associated with sediment-clogged cold-water coral framework in the Porcupine Seabight (NE Atlantic). Deep-Sea Res Pt I 53:1880–1894. doi:10.1016/j.dsr.2006.08.012
Raes M, Decraemer W, Vanreusel A (2008) Walking with worms: coral-associated epifaunal nematodes. J Biogeogr 35:2207–2222. doi:10.1111/j.1365-2699.2008.01945.x
Rieras P, Hubas C (2003) Trophic ecology of nematodes from various microhabitats of the Roscoff Aber Bay (France): importance of stranded macroalgae evidenced through δ13C and δ15N. Mar Ecol-Prog Ser 260:151–159. doi:10.3354/meps260151
Russo AR (1990) The role of seaweed complexity in structuring Hawaiian epiphytal amphipod communities. Hydrobiologia 194(1):1–12. doi:10.1007/BF00012107
Rysgaard S, Christensen PB, Sorensen MV, Funch P, Berg P (2000) Marine meiofauna, carbon and nitrogen mineralization in sandy and soft sediments of Disko Bay, West Greenland. Aquat Microb Ecol 21:59–71
Schanz A, Polte P, Asmus H (2002) Cascading effects of hydrodynamics on an epiphyte–grazer system in intertidal seagrass beds of the Wadden Sea. Mar Biol 141:287–297. doi:10.1007/s00227-002-0823-8
Schmid-Araya JM, Hildrew AG, Robertson A, Schmid PE, Winterbottom J (2002) The importance of meiofauna in food web: evidence from an acid stream. Ecology 83(5):1271–1285. doi:10.1890/0012-9658(2002)083[1271:TIOMIF]2.0.CO;2
Soetaert K, Heip C (1995) Nematode assemblages of deep-sea and shelf break sites in the North Atlantic and Mediterranean Sea. Mar Ecol-Prog Ser 125:171–183. doi:10.3354/meps125171
Song SJ, Ryu J, Khim JS, Kim W, Yun SG (2010) Seasonal variability of community structure and breeding activity in marine phytal harpacticoid copepods on Ulva pertusa from Pohang, east coast of Korea. J Sea Res 63:1–10. doi:10.1016/j.seares.2009.08.004
StatSoft, Inc. (2004) STATISTICA (data analysis software system), version 7. http://www.statsoft.com
Taylor WMR (1967) Species of Caulerpa (Chlorophyceae) collected on the International Indian Ocean Expedition. Blumea 15:45–53
Taylor RB (1997) Seasonal variation in assemblages of mobile epifauna inhabiting three subtidal brown seaweeds in northeastern New Zealand. Hydrobiologia 361:25–35. doi:10.1023/A:1003182523274
Tientjen JH, Lee JJ (1973) Life history and feeding types of the marine nematode Chromadora macrolaimoides Steiner. Oecologia 12:303–314
Toyohara T, Nakaoka M, Aioi K (1999) Population dynamics and reproductive traits of phytal gastropods in seagrass bed in Otsuchi Bay, north-eastern Japan. Mar Ecol 19(2–3):162–178. doi:10.1046/j.1439-0485.1999.2034082.x
Travizi A, Zavodnik N, Zavodnik N (2004) Phenology of Caulerpa taxifolia and temporal dynamics of its epibiontic meiofauna in the port of Malinska (Croatia, northern Adriatic Sea). Sci Mar 68:145–154
Ullberg J, Ólafsson E (2003) Free-living marine nematodes actively choose habitat when descending from the water column. Mar Ecol Prog Ser 260:141–149. doi:10.3354/meps260141
Van Donk E (1998) Switches between clear and turbid water states in a biomanipulated lake (1986–1996): the role of herbivory on macrophytes. In: Jeppesen E, Søndergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Springer, New York, pp 290–297
Venekey V, Fonsêca-Genevois VG, Da Rocha CMC, Santos PJP (2008) Distribuição espaço-temporal da meiofauna em Sargassum polyceratium Montagne (Fucales, Sargassaceae) de um costão rochoso do nordeste do Brasil. Atlântica 30(1):53–67. doi:10.5088/atlântica.v30i1.823
Vidotti EC, Rollemberg MCE (2004) Algas: da economia nos ambientes aquáticos à biorremediação e à química analítica. Quím Nova 27(1):139–145. doi:10.1590/S0100-40422004000100024
Warwick RM (1977) The structure and seasonal fluctuation of phytal marine nematode association on the Isles of Scilly. In: Keegan BF, Ceidigh PO, Boaden PJS (eds) Biology of benthic organisms. Pergamon Press, Oxford, pp 577–585
Warwick RM, Platt HM, Somerfield, PJ (1998) Free-living Marine Nematodes Part III Monhysterids. Synopses of the British fauna (New Series), 53. Field Studies Council: Shrewsbury. ISBN 1-85153-260-9. VII
Wetzel MA, Weber A, Giere O (2002) Re-colonization of anoxic/sulfidic sediments by marine nematodes alter experimental removal of macroalgal cover. Mar Biol 141:679–689. doi:10.1007/s00227-002-0863-0
Wieser W (1951) Untersuchungen über die algaenbewohnende Mikrofauna mariner Hartböden. I. Zur Oekologie und Systematik der Nematodenfauna von Plymouth. Ost Zool Z 3:425–480
Wieser W (1952) Investigations on the microfauna inhabiting seaweeds on rocky coasts. IV. Studies on the vertical distribution of the fauna inhabiting seaweeds below the Plymouth Laboratory. J Mar Biol Assoc UK 31:145–174. doi:10.1017/S002531540000374X
Wieser W (1953) Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Arkiv für Zoologie 4:439–484
Acknowledgments
We would like to thank Prof. Dr. Carl Vangestel for the statistical advice, Prof. Dr. Verônica da Fonsêca-Genevois (in memorian) for the incentive and for sharing her experience, The Federal Rural University of Pernambuco (UFRPE) for the logistic support and the Flemish Interuniversity Council—University Development Cooperation (VLIR-UOS) for the grant and financing of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Weinberger.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
De Oliveira, D.A.S., Derycke, S., Da Rocha, C.M.C. et al. Spatiotemporal variation and sediment retention effects on nematode communities associated with Halimeda opuntia (Linnaeus) Lamouroux (1816) and Sargassum polyceratium Montagne (1837) seaweeds in a tropical phytal ecosystem. Mar Biol 163, 102 (2016). https://doi.org/10.1007/s00227-016-2882-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2882-2