Abstract
This study deals with meiofauna associated with a sublittoral population of the kelp Laminaria ochroleuca located on the northern coast of Spain. By sampling once a year over a 4-year period, we examined patterns of faunal distribution as a function of some environmental factors at the meso-scale level (depth, and exposure to waves and surge). We also examined the relationship between L. ochroleuca abundance (as dry weight biomass and number of plants per sampling quadrat) and abundance and diversity of meiofauna. Finally, we investigated patterns of within-plant distribution (algal frond vs. algal holdfast), using also the meiofauna of the adjacent bottom as a referent to estimate the level of "phytal dependence" of the meiofauna collected on L. ochroleuca. We found that the bulk of permanent meiofauna consisted of nematodes, copepods, mites, polychaetes, tanaids and ostracods, with copepods being predominant on the fronds of the alga and nematodes in the holdfasts. The temporary meiofauna consisted of juvenile amphipods, bivalves and gastropods, together with barnacle nauplii and cyprids. Abundance and major composition of meiofaunal taxa were unrelated to both depth and hydrodynamic exposure of the sampling quadrats. However, we detected significant qualitative and quantitative faunal differences as a function of microhabitat. All meiofaunal groups were more abundant in holdfast samples than in frond and bottom samples. The gross taxonomic composition of meiofauna in bottom samples was similar to that in holdfast samples, but substantially different from that of meiofauna associated with the fronds. The L. ochroleuca holdfasts, in which dense aggregations of meiofauna can occur, appear to function as ecotone between phytal and rocky-bottom microhabitats. All together, our results suggest that the distribution of meiofauna within the Laminaria bed is mostly affected by factors operating at the microhabitat level rather than the meso-scale level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diverse studies on sublittoral meiofauna associated with macroalgae suggest that the faunal abundance increases significantly with decreasing depth and increasing complexity in alga body shape. In the upper sublittoral zone, maximum abundance and diversity have been reported from shallow algal communities in sheltered areas, typically characterised by species with complex frond structure (Wieser 1952; Hicks 1980). In contrast, minimum values appear to occur in algal populations either established on very exposed shores or composed of species of simple morphology (Hicks 1985; Gibbons 1988a; Hull 1997).
It has been suggested that algae of small, simple fronds offer insufficient protection to most meiofaunal organisms against predation, desiccation and wave abrasion (Coull et al. 1983; Gibbons 1988b). Unlike complex fronds, simple algal forms are also inadequate substrata to accumulate both sediment and potential food for meiofaunal organisms (Whatley and Wall 1975; Hicks 1977a, 1980; Gibbons 1988a, 1988b; Edgar 1990; Hull 1997). Such a deficiency particularly prevents the establishment of many psammic organisms, which are an important component of the meiofauna associated with algae of complex morphology (Dahl 1948; Moore 1971, 1972a). The most accepted view is that the more complex the algal frond, the larger the available surface for colonization by meiofauna (Gunnill 1982a, 1982b, 1983; Gee and Warwick 1994a, 1994b), macroepifauna and epiphytic algae. The presence of the latter epibionts, in turn, will add further intricacy to the microhabitat structure, facilitating the development of meiofaunal communities (Moore 1971; Kangas 1978; Gunnil 1982b; Johnson and Scheibling 1987). Therefore, it is not surprising that several studies have reported that meiofaunal organisms tend to "aggregate" on algae of relatively complex morphology, on which they form richer and more diverse communities than those found on morphologically simpler species from the same geographical area (e.g. Hicks 1977a, 1980; Coull et al. 1983; Gee and Warwick 1994a, 1994b).
The initial view that phytal meiofauna abundance decreases with increasing depth within the sublittoral zone (e.g. Hicks 1985) probably favoured several decades of research focussed on the intertidal and the upper sublittoral zone. Additionally, the increasing complexity of the methods required with increasing depth has traditionally discouraged exhaustive investigations of meiofaunal assemblages associated with deep sublittoral algae. Here, we contribute to reduce this gap in knowledge by investigating the meiofauna associated with a sublittoral Laminaria bed, established between 5 and 20 m deep.
Several species of Laminaria are common in the intertidal and rocky subtidal bottoms of many temperate seas, forming either dense mono-specific aggregations or patchy communities along with other organisms (Seoane-Camba 1966; John 1969; Kain 1979). Laminarians are usually large algae. Most species possess a complex system of haptera (the holdfast) for attachment to the substratum. A variously long stalk emerges from the holdfast, bearing distally a relatively extended, flat frond. Therefore, from a morphological point of view, several levels of complexity occur within each plant, making laminarians potentially suitable substrata to host meiofauna. One of the earliest approaches to the meiofauna of laminarians goes back to Colman (1940), who examined and identified the meiofauna of six holdfasts, one frond and one stalk of L. digitata (Hudson) Lamoroux, as part of a broader study on phytal communities. In a similar way, several subsequent studies, mostly concerned with macrofauna, have included diverse pieces of information on laminarian meiofauna (Velmirov et al. 1977; Allen and Griffiths 1981; Tzetlin et al. 1997). One of the earliest studies specifically addressed to phytal meiofauna was published by Hicks (1980), who exclusively investigated the copepods associated with the fronds of L. digitata, disregarding the holdfasts. He found relatively low abundances and species numbers compared to other algae from the same area and attributed this finding to the comparatively low micro-spatial complexity provided by the Laminaria fronds. Conversely, a study series by Moore (1971, 1972a, 1972b, 1973, 1978) focussed on the total meiofauna from the holdfasts of L. hyperborea (Gunnerus) Foslie, disregarding the fronds. Nevertheless, Moore gave an unprecedented ecological dimension to his approach, investigating the effects of holdfast exposure to waves and on the meiofauna surge. He concluded that these factors affected the meiofauna whenever the quality and quantity of the sediment retained by the holdfast were also affected. After Moore' works, the meiofauna occurring on large macrophytes and on the adjacent bottom has rarely been quantitatively compared (Norton 1971; Sheppard 1976). Yet several recent studies suggest that these latter substrata may function as relevant sources of migrating organisms, enhancing maintenance of phytal meiofaunal communities (Edgar 1983b; Gibbons and Grifiths 1986; Somerfield and Jeal 1996; Atilla and Fleeger 2000). The scarcity of quantitative ecological data in this regard prevents a clear understanding of the dynamics of such meiofaunal communities.
In summary, despite the preliminary evidence that the study of laminarian algae may provide a substantial contribution to current knowledge on the taxonomy and ecology of phytal meiofauna, this meiofaunal habitat has received comparatively little research attention. More importantly, the available information is clearly biased towards the taxonomic approach, quantitative ecological data being scarce. As a consequence, some basic questions, such as the magnitude of differences in taxonomic composition and abundance distribution of meiofaunal groups between both algae belonging to the same population and parts of an alga, remain to be addressed.
From 1996 to 1999, a series of research projects was conducted to characterise the benthic assemblages surrounding the Island of Mouro in the Bay of Biscay (Cantabrian Sea, Spain), in an attempt to obtain scientific information to vindicate the status as a protected marine area for these bottoms. Initial results of these surveys suggested that the spatial distribution of the macrofauna associated with L. ochroleuca was affected by meso-scale environmental factors, such as bottom morphology, exposure to waves and surge, and depth of the sampling locations (García-Castrillo et al. 2000b). Interestingly, many of these macrobenthic organisms also showed a consistent preference to occupy a particular microhabitat within each Laminaria alga, regardless of the position of the alga within the bed (García-Castrillo et al. 2000b). Several studies involving macrofauna associated with other laminarian species have revealed similar patterns (Norton 1971; McKenzie and Moore 1981; Schültze et al. 1990). The prediction is that animals included in the meiobenthic size range (62 μm–1 mm) are likely to be affected differently than macrofauna by environmental factors that operate at a meso-scale level (Gibbons and Griffiths 1986), as a consequence of body size differences. Likewise, factors controlling within-plant distribution of meiofauna and those controlling the within-plant distribution of macroinvertebrates are likely to be different (Edgar 1983a; Gee and Warwick 1994a, 1994b).
Here we describe the meiofauna associated with a population of L. ochroleuca and investigate the faunal distribution as a function of both meso-scale factors (depth, exposure to waves and surge) and micro-scale factors (within-alga microhabitat, substratum type).
Materials and methods
Study site
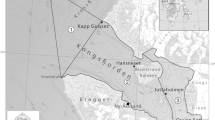
We investigated a Laminaria ochroleuca population established around Mouro Island (Fig. 1; 43°28′24″N; 3°45′22″W; Bay of Santander, north coast of Spain). The island is a 200-m-long rocky outcrop located about 1 km offshore, emerging from a submerged rocky shelf that extends up to a maximum depth of 25 m. Most of the rocky bottom is covered by the encrusting coralline alga Mesophyllum lichenoides (Ellis) Lemoine. The individuals of L. ochroleuca attach to either the bare rock or the M. lichenoides crusts. Various amounts of sediment accumulate between successive growth layers of M. lichenoides and between these and the rhizomes of L. ochroleuca, depending on substratum orientation. A detailed description of bottom topography and algal communities in the area is given elsewhere (García-Castrillo et al. 2000a, 2000b; Puente 2000).
The host alga
At Mouro Island, L. ochroleuca showed a relatively homogeneous distribution, though slightly more abundant in the exposed areas of the island. It was consistently found on the top of boulders or rocky surfaces, presenting the greatest abundance in the 10–15 m depth range. After the first 2 years of study, the population of L. ochroleuca experienced an unexplained, drastic decline, which initially affected only the vitality of fronds but ended in a substantial mortality of plants, its status changing from being the dominant macrophyte to presenting only scattered plants, with poorly developed fronds and small holdfasts.
The mean density in the years in which the population showed a healthy appearance was ca. 12 plants m−2, with a maximum density of 18 plants m−2. In subsequent years, following algal decline, mean density was reduced, reaching an average of ca. 5 plants m−2 in 1999.
L. ochroleuca shows the typical morphology of other species in the digitate section of the genus, that is, a wide, digitate lamina (the frond), a long, erect stipe and a complex system of haptera by which the alga attaches to the substrata (the holdfast). Unlike L. hyperborea, the rugose stipe of which favours settlement of numerous epiphytes, L. ochroleuca has a very smooth stipe, virtually lacking epiphytes. Nevertheless, the holdfast usually harbours a dense assemblage of epiphytes, typically sub-canopy algae, barnacles, sponges and other sessile macrofauna. The fronds may be colonised by a few species of bryozoans or hydrozoans and grazed by the gastropod Patina pellucida (L.), which occasionally feed on the lower portions of the alga as well (Braud 1974).
Collection and preservation procedures
The Laminaria bed was sampled by SCUBA once a year over a 4-year period (1996–1999). We sampled during July–August in 1996, May in 1997, July–August in 1998 and May–June in 1999. Between-year variability in sampling date was due to weather conditions, as underwater work was conducted in a high-risk diving zone. Sampling sites were selected at random on the island shelf, and their positions were identified by using a portable Magellan GPS (Fig. 1). At each sampling site, we recorded diving depth by using an ALADIN PRO diving computer, then corrected depth according to local tidal tables to average the effect of tidal oscillations. At each site, we sampled two different substrata. First, we marked 0.5×0.5 m quadrats and collected all individuals of L. ochroleuca in each quadrat, storing holdfasts and fronds separately in plastic bags. Then, we scraped a 0.25×0.25 m bottom area within each quadrat to collect whatever potential substratum for meiofauna occurred, i.e. the sediment veneer, the crusts of Mesophyllum, the soft algal canopy and the biofilm. All the elements of each bottom sample were stored together in a plastic bag.
During the first 2 years of study (1996 and 1997), we collected nine samples a year. In subsequent years, due to the decline in the L. ochroleuca population, only eight quadrats contained L. ochroleuca in 1998 and six in 1999 (Fig. 1).
Once in the laboratory, samples (including L. ochroleuca tissue, their associated fauna and the sediment retained among the rhizomes) were wet-weighed, then frozen until subsequent faunal extraction. Macrofaunal organisms were picked out by using forceps under a binocular microscope. Then, we sieved L. ochroleuca and bottom samples through 1-mm and 62-μm meshes, collecting the animals retained and preserving them in 4% buffered formalin with rose-bengal until taxonomic study. For samples containing important amounts of sediment or debris, we used an alternative method to isolate the meiofauna. Such samples were suspended in Ludox (colloidal silica polymer, density 1.15) and, after a 40-min settlement period, the supernatant fraction was filtered through a 62-μm mesh. The sediment was then re-suspended in Ludox, and the whole process was repeated three times. During each round, we examined sub-samples of sediment to determine whether or not the meiofauna had been extracted appropriately. We realised that the efficiency of this separation method was still low for ostracods, bivalves and gastropods. Therefore, in samples in which these taxa prevailed, the organisms were extracted manually under a binocular microscope. These latter groups were preserved in 70% alcohol until taxonomic study.
For the taxonomic study, we considered all meiobenthic organisms, except Protozoa, classifying them at fairly high taxonomic levels. Organisms were also assigned to the category of either temporary (organisms only transitorily in the meiofauna, such as developmental macrofaunal stages, etc.; sensu McIntyre 1969) or permanent (usually consisting of adult life-cycle stages) meiofauna. Because we sampled in different seasons each year and this may have had an effect on the abundance of temporary meiofauna, only the permanent meiofauna was considered for the ecological analyses.
Effects of depth on meiofauna
We investigated potential effects of depth on the abundance of meiofauna by examining the correlation (Pearson product moment correlation coefficient) between depth of each sampling quadrat and number of organisms in the quadrat.
We also examined potential differences in meiofaunal community structure as a function of depth by means of multivariate techniques. Faunal affinity based on permanent meiofaunal taxa was calculated between samples by Bray–Curtis distances on fourth-root transformed data. Matrices of pairwise faunal distances were then processed by applying non-metric multidimensional scaling (NMDS). Samples were assigned to a depth rank (6–10 m=1, 11–15 m=2 and 16–20 m=3), and this factor was used as a label for the different samples. Significance tests for predicted differences in the distribution of taxa according to these depth ranks were performed using the ANOSIM permutation test (Clarke 1993).
Preliminary analyses detected no between-year differences in abundance or community structure of meiofauna per sampling quadrat (Arroyo 2002); hence, samples from all years (n=32) were pooled for these analyses.
Effects of surge on meiofauna
The prevailing direction of waves approaching Mouro Island is N–NE. According to wave regime (height and frequency of the waves provided by the Asheville National Climatic Data Center, N.C., USA) and island topography (García-Castrillo et al. 2000a), most of the bottom surrounding the island is clearly exposed to surge. Nevertheless, the E–SW sector, located on the lee side, can be considered semi-exposed (Fig. 1).
We investigated differences in the abundance of both L. ochroleuca and its associated meiofauna as a function of the hydrodynamic exposure (exposed vs. semi-exposed) of sampling site. Patches of L. ochroleuca were more abundant at the exposed side of the island, which was sampled more intensively (N exposed=14 quadrats) than the semi-exposed side (N semi-exposed=4). Because the population of L. ochroleuca experienced epidemic mortality during the last 2 years of study, the analysis only involved data from the first 2 years (1996 and 1997). This analysis included samples from all the depth ranges mentioned above (5–20 m), under the assumption that the magnitude of the effect of depth on exposure may be considered negligible when compared with that between the lee and the exposed side of Mouro Island.
In these conditions, we examined differences in L. ochroleuca biomass (dry weight) per sampling quadrat as a function of exposure to surge by using the unpaired t-test after confirming that data met the assumptions of normality and homoscedasticity. We also examined differences in the distribution of taxonomic abundance of meiofauna as a function of exposure to surge by using ordination techniques. First, we conducted classification analyses of exposed and semi-exposed sampling quadrats using as descriptors fourth-root transformed abundance data of the most relevant meiofaunal groups. Pairwise faunistic similarities between quadrats were calculated by using the Bray–Curtis similarity index. Similarity matrices were submitted to an NMDS analysis, and the output was plotted in bi-dimensional space. A gross significance test for the level of faunal similarity detected between the two predicted areas of exposure was performed by using the ANOSIM permutation test. We also examined potential differences in mean abundance (number of individuals per sampling quadrat) of meiofauna as a function of hydrodynamic exposure of sampling site. Analyses were made for abundance values of total meiofauna, as well as for the major meiobenthic groups (N exposed=14, N semi-exposed=4). We used the t-test for parametric data sets and the Mann–Whitney U-test for data that did not fit the parametric model after transformation.
Effect of algal biomass on meiofauna
We investigated the relationship between L. ochroleuca biomass and meiofaunal abundance. Algal biomass was estimated as dry weight (65°C for 48 h in a drying oven) of L. ochroleuca per sampling quadrat, also considering separately holdfast and frond values per quadrat for some analyses. Meiofaunal abundance was estimated as the number of individuals per sampling quadrat. Additionally, we examined the relationship between L. ochroleuca biomass and meiofaunal diversity per sampling quadrat and algal tissue fraction (holdfast vs. frond) by using Pearson product moment correlation. Different estimates of faunal diversity were considered, such as the number of taxa, the Shannon–Wiener diversity index (H′, log base 2), and its evenness component (J), calculating their values for frond and holdfast samples separately. We also examined the correlation between the number of L. ochroleuca individuals per quadrat and abundance of meiofauna per quadrat, as well as between the former and meiofaunal diversity per sampling quadrat. A methodological mistake in sample labelling and storage caused a loss of data on holdfast and frond biomass per quadrat, as well as on number of algal individuals per quadrat for all quadrats collected in 1996 and half of those collected in 1997. Therefore, a total of just 18 quadrats (N=4 in 1997, N=8 in 1998, N=6 in 1999) was used for these analyses.
Within-plant distribution of meiofauna
We investigated differences in gross taxonomic composition and abundance of meiofauna as a function of within-alga microhabitat (frond vs. holdfast). Due to significant between-year differences in frond–meiofauna abundance and community structure (Arroyo 2002), analyses were run separately for each of the study years. The faunal affinity between frond and holdfast samples was evaluated by an ordination approach. First, we used the Bray–Curtis similarity index to calculate pairwise faunal similarities between holdfast and frond samples. The index was applied to fourth-root transformed data of numerical abundance per plant fraction and quadrat for each of the most relevant meiofaunal groups. The similarity matrix was then submitted to a NMDS analysis, and the output was plotted in bi-dimensional space. A one-way ANOSIM was used as a gross significance test for predicted differences between factions in each of the 4 years of study.
We also investigated differences in mean meiofaunal abundance (number of individuals per quadrat) as a function of algal tissue fraction (holdfast vs. frond) in each of the 4 years. These analyses were run separately for total meiofauna and for each of the major taxa. Comparisons of mean abundances were made by using the Mann–Whitney U-test, due to non-compliance of the data with parametric assumptions. Due to the high number of comparisons involved in these analyses, the sequential Bonferroni procedure for adjusting significance levels was used in order to control the type I error rate (Quinn and Keough 2002).
Effects of algal epibiosis on meiofauna
We examined the hypothesis that the heavier the epiphytic load on L. ochroleuca the richer its associated meiofauna. Given that the L. ochroleuca population experienced a drastic decline in 1998 and 1999 and that the presence of epibionts on the algal fronds is extremely rare, we constrained this test to involve just the meiofauna found on the holdfasts collected during the first 2 years of study (1996–1997). First, we identified the main epiphytes associated with the holdfasts of all samples. Then, we obtained dry weight values for both total epibionts and each major epiphytic taxa in each sampling quadrat (N=18). We examined the relationship between weight of epibionts and numerical abundance of each major meiofaunal group by using the Pearson product moment correlation coefficient. We also examined the correlation between the abundance of each major meiobenthic taxon and the biomass of each of the most abundant epiphytic organisms. All data were log(n+1) transformed prior to analysis.
Substratum specificity of phytal meiofauna
To investigate substratum specificity of meiofauna associated with L. ochroleuca, we examined the faunal similarity among three different substrata on which meiofauna was collected: fronds and holdfasts of L. ochroleuca, and the underlying bottom. This latter substratum consists of a heterogeneous mix of elements, including a thin veneer of unclassified debris and sediment, hard fragments of M. lichenoides, the bacterial biofilm and the filamentous algal canopy that covers the rocks.
We conducted a joint classification analysis of holdfasts (N=32), fronds (N=28) and bottom samples (N=21) using as descriptors the presence–absence of the major meiofaunal groups in each sample during the 4 years of study. Pairwise faunal similarities between substratum samples were obtained by using the Sørensen similarity index. The resulting similarity matrix was then processed by the un-weighed arithmetic average algorithm of clustering to produce a hierarchical dendogram. We also performed ordination by submitting the similarity matrix to NMDS analysis and plotting the output in bi-dimensional space. The ANOSIM permutation test was used as a gross significance test for predicted levels of faunal similarity between substrata. Utilisation of presence–absence data allowed comparisons considering samples from the 4 years of study, since quantitative differences between years and differences due to variation in sample size were obviated by this procedure.
Results
Taxonomic composition
We collected a total of ca. 172,000 individuals belonging to 12 phyla, including permanent and temporary meiofauna (Table 1). Permanent meiofauna accounted for 79% of total organisms, while temporary meiofauna represented 21%. Mean density of total meiofauna per quadrat was 4,895.45±4,774.3 individuals, which means about 10,000 organisms m−2 of Laminaria ochroleuca bed.
Of the major meiofaunal taxa, nematodes were the most abundant group (52%), followed by copepods (29%) and polychaetes (15%). Other relevant groups were mites (2%), ostracods (1%) and tanaids (1%). For the temporary forms, barnacle nauplii dominated in abundance (65%), followed by polychaete postrocophoran stages (11%), and juveniles of bivalves (8%) and gastropods (5%).
Effects of depth on meiofauna
There was no significant association between depth and abundance values of meiofauna (Pearson correlation, P>0.05). The ANOSIM test did not detect significant differences in the level of faunal similarity between samples from the different ranks of depth (R=−0.001, P>0.05; Fig. 2).
Effects of surge on meiofauna
We found no significant difference in the mean biomass of L. ochroleuca per sampling quadrat between hydrodynamically exposed and semi-exposed sampling sites (t=−1.075, df=16, P>0.05). The ANOSIM test did not show significant differences (R=0.169, P>0.05) in the level of faunal similarity between samples from the exposed and the semi-exposed areas. The ordination analyses based on permanent meiofauna (Fig. 3) show that samples were grouped irrespective of sampling year and exposure factor. Similarly, no significant differences were found in mean abundance of each major meiofaunal taxon between exposed and semi-exposed quadrats (P>0.05).
Effect of algal biomass on meiofauna
The abundance of meiofauna showed significant positive correlation with frond biomass (r 2=0.69, P<0.001). However, no significant association was found between abundance of meiofauna and holdfast biomass (r 2=0.09; P>0.05).
Data on mean number of taxa, diversity, evenness of meiofauna per quadrat and per plant fraction (frond vs. holdfast) are summarised in Table 1. The number of taxa found on fronds and holdfasts, respectively, correlated with the biomass of L. ochroleuca in each of the plant fractions (r 2 fronds=0.51, P<0.01; r 2 holdfasts=0.39, P<0.01). In contrast, for meiofauna, the Shannon–Wiener diversity index did not correlate with biomass values of any algal fraction (r 2 fronds=0.18, P>0.05; r 2 holdfasts=0.12, P>0.05).
The number of L. ochroleuca individuals per quadrat did not correlate with abundance of meiofauna (r 2=0.074, P>0.05). Neither, did it correlate with number of meiobenthic taxa (r 2 fronds=0.064, P>0.05; r 2 holdfasts=0.0006, P>0.05), or Shannon–Wiener diversity (r 2 fronds=0.034, P>0.05; r 2 holdfasts=0.0125, P>0.05).
Within-plant distribution of meiofauna
The algal holdfasts consistently hosted higher abundance of meiofauna than the fronds. All permanent taxa were significantly more abundant on holdfasts than on fronds in the 4 years of study (Tables 1, 2; Figs. 4, 5), with some groups showing only occasional presence in frond samples. Barnacles, cumaceans, entoprocts, insect larvae, oligochaetes, picnogonid larvae, polyplacophorans, polychaete larvae and tardigrades were exclusive to the holdfasts.
The taxonomic analysis of the permanent meiofauna on fronds indicated that copepods were the most abundant organisms (45%), followed by nematodes (25%), polychaetes (18%), ostracods (8%), mites (3%) and tanaids (1%). On the holdfasts, nematodes predominated (53%), followed by copepods (28%), polychaetes (15%), mites (2%), ostracods (1%) and tanaids (1%).
The results of the one-way ANOSIM for each of the 4 years clearly corroborate the above-mentioned faunal differences, indicating differences in community structure between frond and holdfast samples, in the 4 years of study (Fig. 5).
Effect of algal epibiosis on meiofauna
Mean dry-weight values of epiphytes per sampling quadrat (Fig. 6A) represent a moderate percentage of the total phytal weight per quadrat. The most abundant epibionts were four species of algae (Fig. 6B), Cryptopleura ramosa (Hudson) Kylin ex Newton, Plocamium cartilagineum (Linnaeus) Dixon, Pterosiphonia complanata (Clemente) Falkenb. and Rhodymenia pseudopalmata (Lamoroux) P. Silva.
Biomass (dry weight) of Laminaria ochroleuca and its epiphytes in 1996 and 1997 samples. Values are mean dry weight per sampling quadrat. Error bars: standard error of the mean. B Contribution of the most abundant epiphytes (Cryptopleura ramosa, Pterosiphonia complanata, Plocamium cartilagineum, Rhodymenia pseudopalmata) to overall epiphyte biomass in 1996 and 1997 samples
Neither total meiofauna abundance nor that of any of the major groups was correlated with biomass of total epiphytes. In contrast, correlation analyses considering each epiphyte separately revealed a weak negative correlation (not shown) between abundance of copepods and ostracods and biomass of C. ramosa (Pearson: r 2=0.40, P<0.05 and r 2=0.30, P<0.05, respectively). The abundance of the remaining taxa and that of total meiofauna did not correlate with the biomass of any epiphyte.
Substratum specificity of phytal meiofauna
Classification and ordination analyses (Fig. 7) consistently detected higher faunal affinities between holdfast and bottom samples than between bottom and frond samples. In consistency with these results, the ANOSIM test detected significant faunal differences between frond and holdfast samples (R=0.252, P<0.01) and between frond and bottom samples (R=0.073, P<0.05), but not between holdfast and bottom samples (R=−0.002, P>0.05). The analysis of relative abundances shows that nematodes (36%) are the predominant permanent taxon in bottom samples, followed by polychaetes (33%) and copepods (24%). Mites (4%), ostracods (2%) and tanaids (1%) were clearly less abundant in bottom samples than in L. ochroleuca samples (Fig. 4), particularly regarding the holdfasts. Among the temporary meiofauna, barnacle nauplii (30%) dominated in bottom samples, followed by juvenile gastropods (26%), polychaete larvae (19%), juvenile bivalves (18%), gammaridean (6%) and caprellid amphipods (1%).
Classification analysis (A) and NMDS ordination (B) of fronds, holdfasts and bottom samples from the 4 years of study, using presence–absence of major meiofaunal taxa (in the dendrogram: F, H and S; in the ordination: squares, triangles and circles indicate frond, holdfast and bottom samples, respectively)
Discussion
Taxonomic distribution of meiofaunal abundance
The total meiofauna associated with Laminaria ochroleuca at Mouro Island shows somewhat lower abundance values per surface unit than those reported from studies on meiofauna associated with other macroalgae (Colman 1940; Hagerman 1966; Gibbons and Griffiths 1986; Johnson and Scheibling 1987). Copepods dominated the fronds and nematodes the holdfasts, which is a pattern similar to that found in other macrophytes (Hagerman 1966; Moore 1972a; Sarma and Ganapati 1972; Pallares and Hall 1974; Hicks 1977a, 1977b; Novak 1982; Jarvis and Seed 1996; De Troch et al. 2001). The meiofauna of L. ochroleuca rhizome samples consists of a heterogeneous mix of phytal and psammic organisms, the latter ones favoured by the occurrence of sand grains and debris attached to the algal rhizomes. A mix of phytal and psammic meiofauna has also been described in the basal-most portion of other macroalgae (Hagerman 1966; Moore 1972a, 1972b; Sarma and Ganapati 1972; Hicks 1977b; Novak 1982; De Troch et al. 2001). The meiofauna of fronds appears to be relatively unrelated to that of rhizome and bottom samples. This pattern is also consistent with the results of several studies on algae and seagrasses, in which many of the copepods dominating the fronds were found to possess special adaptations to live on these flat, undulating substrata and cope with the abundant mucilagenous secretions produced by frond cells (Hicks and Grahame 1979; Hicks 1980, 1985; Bell et al. 1987). It is noteworthy that meiobenthic polychaetes were unexpectedly abundant on the fronds (Fig. 4), as this group has usually been described in association with Laminaria rhizomes and bottom samples (Colman 1940; Moore 1973). Polychaetes were also very abundant in our holdfast samples.
Tanaids and halacarid mites were secondary groups in terms of numerical abundance, usually occurring in holdfast samples. Tanaids have been described in association with the sediment trapped by L. digitata tissues (Colman 1940), while mites occur in association with very specific microhabitats within plants (Colman 1940; Pugh and King 1985; Somerfield and Jeal 1996).
A distinctive feature of the meiofauna found on L. ochroleuca is the low density of ostracods, a group usually abundant on other macrophytes (Hagerman 1966; Kangas 1978; Hull 1997), including holdfast samples of other Laminaria species (Whatley and Wall 1975). A study by Colman (1940) on L. digitata also showed an uncommonly low abundance of ostracods. According to some studies, these animals are rare on hydrodynamically exposed shores, where they aggregate on finely branching or filamentous algae (Hagerman 1966; Whatley and Wall 1975; Kangas 1978; Gibbons 1988b; Hull 1997). The absence of these algal morphologies in the studied area and the severe surge around Mouro Island may explain the scarcity of ostracods in our samples.
As part of the temporary meiofauna, the amphipods were one of the most abundant groups. Similarly high abundance has been reported in other phytal studies (Fenwick 1976; Moore 1978), in which benthic amphipods were found in close association with the algal tissue, with sessile invertebrates that colonise the holdfasts, and within tubes built with the sediment attached to the algal rhizomes (Fenwick 1976). Nauplii were also abundant members of the temporary meiofauna. Most collected nauplii belonged to Verruca stroemia O.F. Müller, as did the cyprids. Copepod and ostracod nauplii were also collected, but in much lower abundance than nauplii of Cirripedia.
Factors affecting distribution of meiofauna
The spatial distribution of the studied meiofauna seems to be affected by microhabitat factors, such as type of substratum (holdfast, frond, bottom), rather than by meso-scale factors, such as depth and exposure to surge. Neither depth differences (within the 5–25 m sampled range) nor differences in surge (exposed vs. semi-exposed quadrats) had any apparent effect on the abundance and gross taxonomic composition of the meiofauna. Nevertheless, we realise that such a conclusion is only moderately supported by our observational data, as the number of samples taken from the semi-exposed side (N=4) was relatively low compared with that from the exposed side (N=14). On the other hand, the idea that the distribution of sublittoral meiofauna is more dependent on microhabitat conditions than on general environmental features, whenever changes in the latter features have no substantial repercussion at the microhabitat level, is consistent with the results of several other studies (Gibbons and Griffiths 1986; Gibbons 1988a; Somerfield and Jeal 1996; De Troch et al. 2001; Prathep et al. 2003). Abundance and diversity of macroinvertebrates have generally been found to be positively correlated with plant biomass (Heck and Wetstone 1977; Gunnill 1982b). In our study, both diversity of total meiofauna on fronds and abundance of several taxa in frond samples were also positively correlated with the dry-weight biomass of this algal fraction. A slightly different pattern characterised the meiofauna of holdfasts, in which meiofaunal diversity positively correlated with holdfast biomass, but the abundances of meiofaunal organisms did not. Indeed, the numerical abundances of meiofauna are unlikely to be linearly correlated with rhizome weight (Moore 1972a; Preston and Moore 1988; Edgar 1990). Whereas the surface provided by the fronds is directly dependent on their biomass, this is not the case for the holdfasts. Rather, the intricate structure of the holdfast provides a multiplicity of microhabitats for meiofauna and favours occurrence of dense faunal aggregations. By contrast, the comparative structural simplicity of the L. ochroleuca fronds, along with the mucous exudation characterising the frond surface, make them a generally unsuitable habitat.
The affinity in meiofaunal community structure between holdfast and bottom samples, along with the dissimilarity between those and that from the frond, suggests that only the meiofauna associated with the L. ochroleuca frond can be considered as strictly phytal. The variation in abundance between fractions (Fig. 4) indicates that most groups found in holdfast samples also occur with similar abundances in bottom samples. Only cirripedes were absent from bottom samples, despite the fact that barnacle nauplii and cyprids were found in this fraction.
Holdfast meiofauna is not strictly phytal, but a mixture of inhabitants from phytal, epibenthic and interstitial rocky-bottom habitats, associated mainly with the sediment retained by the holdfast structure and the variety of niches and refuge provided by them. This is bound to result in much higher abundances of the meiofauna associated with this fraction compared to the fronds of the plant, particularly considering that meiofauna is a typically benthic taxon. Further, it may also explain the apparent lack of "response" of the meiofauna associated with the holdfasts to L. ochroleuca–die-back during the last 2 years of the study. Contrary to the fronds, the holdfasts maintained a relatively "healthy" structure until the last year of the study, with similar sediment-retention capabilities, and remained available as attachment surface for macroepifauna and other epiphytic algae. Hence, the associated meiofauna may not necessarily not be affected by a global loss in biomass, which was mainly due to frond deterioration (Arroyo 2002). This hypothesis is consistent with other data from the literature, in which several epiphytic animals have been found to respond more to the physical structure of the algae they inhabit than to biological aspects such as primary production, growth, or reproduction. In general, an indirect relationship between the animals and their host plant has been pointed out, often mediated by the presence of other epiphytic algae (Edgar 1983a; Johnson and Scheibling 1987; Hall and Bell 1988; Viejo 1999).
In our study, the presence of epiphytes on L. ochroleuca had a negligible effect on the abundance of the associated meiofauna, except for the epiphytic alga C. ramosa. The increasing abundance of this species was weakly associated with a decrease in abundance of copepods and ostracods. Sulphated galactans with high cytotoxic concentrations have been isolated from this alga (Carlucci et al. 1997), which may be of detriment to some meiofaunal taxa. Several authors have suggested the occurrence of slimes or the exudation of particular metabolites as factors determining the suitability of a substratum to host epifauna (Hornsey and Hide 1976; Lippert et al. 2001). Because the biomass of the epiphytes on L. ochroleuca was just moderate to low, the lack of correlation between epiphytic biomass and abundance of meiofauna was not surprising (Hicks 1977b; Edgar 1983a, 1983c; Hall and Bell 1988).
Other studies analysing the importance of habitat complexity in macrophytic communities have found that the total number of individuals was not affected directly by this factor, but taxonomical changes at species levels occurred (e.g. Young and Young 1977; Sánchez-Jerez et al. 1999), and this may have been the case in our study. Similarly, specific differences in depth distribution or in tolerance to exposure (regardless of complexity differences at the microhabitat level), undetectable by an analysis at the high taxonomic level, may have occurred at Mouro Island, and should not be discounted. Indeed, because our study deals with meiofauna at high taxonomic levels, the interpretation of most of the results is limited and should be considered a preliminary examination of the meiofauna associated with L. ochroleuca, which is to be complemented with further studies focussed on the most relevant groups (Arroyo et al., in preparation). Nevertheless, despite limitations, some general trends emerge clearly. The distribution of the meiofauna associated with the population of L. ochroleuca appears to be affected mainly by within-plant factors. The holdfasts of L. ochroleuca host a substantially higher abundance of meiofauna than both fronds and the adjacent bottom. The holdfasts appear to be an ecotone for meiofauna, because they transiently contain organisms from the fronds and the adjacent bottoms, in addition to their "own" particular fauna.
References
Allen JC, Griffiths CL (1981) The fauna and flora of a kelp bed canopy. S Afr J Zool 16:80–84
Arroyo NL (2002) Meiofauna asociada al alga Laminaria ochroleuca de la Pylaie en la isla de Mouro (Santander, Cantabria). PhD thesis, Universidad Complutense de Madrid, Madrid
Atilla N, Fleeger JW (2000) Meiofaunal colonization of artificial substrates in an estuarine embayment. Mar Ecol 21:69–83
Bell SS, Walters K, Hall MO (1987) Habitat utilization by harpacticoid copepods: a morphometric approach. Mar Ecol Prog Ser 35:59–64
Braud JP (1974) Etude de quelques parametres ecologiques, biologiques et biochimiques chez une pheophycee des cotes Bretonnes. Laminaria ochroleuca. Revue Trav Inst (Sci Tech) Pêch Marit 38:115–204
Carlucci MJ, Scolaro LA, Errea MI, Matulewicz MC, Damonte EB (1997) Antiviral activity of natural sulphated galactans on herpes virus multiplication in cell culture. Planta Med 63:429–432
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Colman J (1940) On the faunas inhabiting intertidal seaweeds. J Mar Biol Assoc UK 24:129–183
Coull BC, Creed EL, Eskin RA, Montagna PA, Palmer MA, Wells JBJ (1983) Phytal meiofauna from the rocky intertidal at Murretts Inlet, South Carolina. Trans Am Microsc Soc 102:380–389
Dahl E (1948) On the smaller Arthropoda of marine algae, especially in the polyhaline waters off the Swedish west coast. Dissertation, Lund. Undersökningar över Öresund 35:1–193
De Troch M, Gurdbeke S, Friers F, Vincx M (2001) Zonation and structuring factors of meiofauna communities in a tropical seagrass bed (Gazi Bay, Kenya). J Sea Res 45:45–61
Edgar GJ (1983a) The ecology of south-east Tasmanian phytal animal communities. I. Spatial organization on a local scale. J Exp Mar Biol Ecol 70:129–157
Edgar GJ (1983b) The ecology of south-east Tasmanian phytal animal communities. II. Seasonal change in plant and animal populations. J Exp Mar Biol Ecol 70:159–179
Edgar GJ (1983c) The ecology of south-east Tasmanian phytal animal communities. III. Patterns of species diversity. J Exp Mar Biol Ecol 70:181–203
Edgar GJ (1990) The influence of plant structure on the species richness, biomass and secondary production of macrofaunal assemblages associated with Western Australian seagrass beds. J Exp Mar Biol Ecol 137:215–240
Fenwick GD (1976) The effect of wave exposure on the amphipod fauna of the alga Caulerpa brownii. J Exp Mar Biol Ecol 25:1–18
García-Castrillo G, Rodríguez C, Puente A, Preciado I, Serrano A, Juanes J (2000a) Cartografiado bentónico sublitoral de la Isla de Mouro (Cantabria). Ozeanografika 3:69–83
García-Castrillo G, Serrano A, Preciado I, Rodríguez C, Puente A, Juanes J (2000b) Estructuración biocenótica de la comunidad de laminariales de la Isla de Mouro (Mar Cantábrico, Santander). Ozeanografika 3:85–99
Gee JM, Warwick RM (1994a) Metazoan community structure in relation to the fractal dimensions of marine macroalgae. Mar Ecol Prog Ser 103:141–150
Gee JM, Warwick RM (1994b) Body-size distribution in a marine metazoan community and the fractal dimensions of macroalgae. J Exp Mar Biol Ecol 178:247–259
Gibbons MJ (1988a) The impact of wave exposure on the meiofauna of Gelidium pristoides (Turner) Kuetzing (Gelidiales: Rhodophyta). Estuar Coast Shelf Sci 27:581–593
Gibbons MJ (1988b) The impact of sediment accumulations, relative habitat complexity and elevation on rocky shore meiofauna. J Exp Mar Biol Ecol 122:225–241
Gibbons MJ, Griffiths CL (1986) A comparison of macrofaunal and meiofaunal distribution and standing stock across a rocky shore, with an estimate of their productivities. Mar Biol 93:181–188
Gunnill FC (1982a) Macroalgae as habitat patch islands for Scutellidium lamellipes (Copepoda: Harpacticoida) and Amphitoe tea (Amphipoda: Gammaridae). Mar Biol 69:103–116
Gunnill FC (1982b) Effects of plant size and distribution on the numbers of invertebrate species and individuals inhabiting the brown alga Pelvetia fastigiata. Mar Biol 69:263–280
Gunnill FC (1983) Seasonal variations in the invertebrate faunas of Pelvetia fastigiata (Fucaceae): effects of plant size and distribution. Mar Biol 73:115–130
Hagerman L (1966) The macro- and microfauna associated with Fucus serratus L., with some ecological remarks. Ophelia 3:1–43
Hall MO, Bell SS (1988) Response of small motile epifauna to complexity of epiphytic algae on seagrass blades. J Mar Res 46:613–630
Heck KL, Wetstone GS (1977) Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J Biogeogr 4:135–142
Hicks GRF (1977a) Species composition and zoogeography of marine phytal harpacticoid copepods from Cook Strait, and their contribution to total phytal meiofauna. NZ J Mar Freshw Res 11:441–469
Hicks GRF (1977b) Species associations and seasonal population densities of marine phytal harpacticoid copepods from Cook Strait. NZ J Mar Freshw Res 11:621–643
Hicks GRF (1980) Structure of phytal harpacticoid copepod assemblages and the influence of habitat complexity and turbidity. J Exp Mar Biol Ecol 44:157–192
Hicks GRF (1985) Meiofauna associated with rocky shore algae. In: Moore PG, Seed R (eds) The ecology of rocky coasts. Hodder and Stoughton, London
Hicks GRF, Grahame J (1979) Mucus production and its role in the feeding behaviour of Diarthrodes nobilis (Copepoda: Harpacticoida). J Mar Biol Assoc UK 59:321–330
Hornsey IS, Hide D (1976) The production of antimicrobial compounds by British marine algae. II. Seasonal variation in production of antibiotics. Br Phycol J 11:63–67
Hull SL (1997) Seasonal changes in diversity and abundance of ostracods on four species of intertidal algae with differing structural complexity. Mar Ecol Prog Ser 161:71–82
Jarvis SC, Seed R (1996) The meiofauna of Ascophyllum nodosum (L.) Le Jolis: characterization of the assemblages asociated with two common epiphytes. J Exp Mar Biol Ecol 199:249–267
John DM (1969) An ecological study on Laminaria ochroleuca. J Mar Biol Assoc UK 49:175–187
Johnson SC, Scheibling RE (1987) Structure and dynamics of epifaunal assemblages on interidal macroalgae Ascophyllum nodosum and Fucus vesiculosus in Nova Scotia, Canada. Mar Ecol Prog Ser 37:209–227
Kain JM (1979) A view of the genus Laminaria. Oceanogr Mar Biol Annu Rev 17:101–161
Kangas P (1978) On the quality of meiofauna among the epiphytes of Fucus vesiculosus in the Asko area, northern Baltic Sea. Contrib Asko Lab, Univ Stockholm 24:1–32
Lippert H, Iken K, Rachor E, Wiencke C (2001) Macrofauna associated with macroalgae in the Kongsfjord (Spitsbergen). Polar Biol 24:512–522
McKenzie JD, Moore PG (1981) The microdistribution of animals associated with the bulbous holdfasts of Saccorhiza polyschides (Phaeophyta). Ophelia 20:201–213
McIntyre AD (1969) Ecology of marine meiobenthos. Biol Rev 44:245–290
Moore PG (1971) The nematode fauna associated with holdfasts of kelp (Laminaria hyperborea) in N.E. Britain. J Exp Mar Biol Assoc UK 51:589–604
Moore PG (1972a) Particulate matter in the sublittoral zone of an exposed coast and its ecological significance with special reference to the fauna inhabiting kelp holdfasts. J Exp Mar Biol Ecol 10:59–80
Moore PG (1972b) The kelp fauna of northeast Britain. I. Introduction and the physical environment. J Exp Mar Biol Ecol 13:97–125
Moore PG (1973) The kelp fauna of northeast Britain. II Multivariate classification: turbidity as an ecological factor. J Exp Mar Biol Ecol 13:127–163
Moore PG (1978) Turbidity and kelp holdfasts. Amphipoda. 1. Wales and S.W. England. J Exp Mar Biol Ecol 32:53–96
Norton TA (1971) An ecological study of the fauna inhabiting the sublittoral marine alga Saccorhiza polyschides (Lightf.) Batt. Hydrobiologia 37:215–231
Novak R (1982) Spatial and seasonal distribution of the meiofauna in the seagrass Posidonia oceanica. Neth J Sea Res 16:380–388
Pallares RE, Hall MA (1974) Análisis bioestadístico-ecológico de la fauna de copépodos asociados a los bosques de Macrocystis pyrifera (Conclusion). Physis (B Aires) 33:409–432
Prathep A, Marrs RH, Norton TA (2003) Spatial and temporal variations in sediment accumulation in an algal turf and their impact on associated fauna. Mar Biol 142:381–390
Preston A, Moore PG (1988) The flora and fauna associated with Cladophora albida (Huds.) Küntz. from rockpools on Great Cumbrae Island, Scotland. Ophelia 29:169–186
Puente A (2000) Distribución y estructura de las comunidades de macroalgas de la Isla de Mouro (Cantabria, Golfo de Vizcaya). Consideraciones sobre su aplicación en la vigilancia ambiental de espacios litorales. PhD thesis, Universidad de Cantabria, Santander
Pugh PJA, King PE (1985) Vertical distribution and substrate association of the British Halacaridae. J Nat Hist 19:961–968
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Sánchez-Jerez P, Barberá Cebrián C, Ramos Esplá A (1999) Comparison of the epifauna spatial distribution in Posidonia oceanica, Cymodocea nodosa and unvegetated bottoms: importance of meadow edges. Acta Oecol 20:391–405
Sarma ALN, Ganapati PN (1972) Faunal associations of algae in the intertidal region of Visakhapatnam. Proc Indian Natl Sci Acad Part B Biol Sci 38:380–396
Schültze K, Janke K, Krüb A, Weidemann W (1990) The macrofauna and macroflora associated with Laminaria digitata and L. hyperborea at the island of Helgoland (German Bight, North Sea). Helgol Meeresunter 44:39–51
Seoane-Camba J (1966) Las laminariáceas de España y su distribución. Publ Tec Junta Estud Pesca 5:425–436
Sheppard CRC (1976) The holdfast ecosystem of Laminaria hyperborea (Gunn.) Fosl. and environmental monitoring: an ecological study. PhD thesis, Durham University, Durham, England
Somerfield PJ, Jeal F (1996) The distribution of Halacaridae (Acari: Prostigmata) among macroalgae on sheltered rocky shores. J Mar Biol Assoc UK 76:251–254
Tzetlin AB, Mokievsky VO, Melnikov AN, Saphonov MV, Simdyanov TG, Ivanov IE (1997) Fauna associated with detached kelp in different types of subtidal habitats of the White Sea. Hydrobiologia 355:91–100
Velmirov B, Field JG, Griffiths CL, Zoutendyk P (1977) The ecology of kelp bed communities in the Benguela upwelling system. Helgol Wiss Meeresunter 30:495–518
Viejo RM (1999) Mobile epifauna inhabiting the invasive Sargassum muticum and two local seaweeds in northern Spain. Aquat Bot 64:131–149
Whatley RC, Wall DR (1975) The relationship between Ostracoda and algae in littoral and sublittoral marine environments. In: Swain FM (ed) Biology and palaeobiology of Ostracoda. Bull Am Palaeontol Soc 65:173–203
Wieser W (1952) Investigations on the microfauna inhabiting seaweeds on rocky coasts. IV. Studies on the vertical distribution of the fauna inhabiting seaweeds below the Plymouth Laboratory. J Mar Biol Assoc UK 31:145–174
Young DK, Young MW (1977) Community structure of the macrobenthos associated with seagrass of the Indian River Estuary, Florida. In: Coull BC (ed) Ecology of marine benthos. University of South Carolina Press, Columbia
Acknowledgements
We are indebted to Dr. G. García-Castrillo for extending the scope of his grant to the meiobenthic domain. We thank the staff of ACEM for their help during sampling, A. Puente for her advice when dealing with seaweed, and I. Preciado for always being there when whatever information was needed. This study was carried out within the framework of two research projects developed by ACEM and funded by the Marcelino Botín Foundation. The study was also partially funded by two grants from the Spanish government (MEC-PB98-0485 and BOS 2000-0568, respectively) and a PhD fellowship from the Universidad Complutense of Madrid to N.L. Arroyo. M. Gibbons and K. Aarnio gave valuable comments on an earlier version of the manuscript. E. Ólafsson and two anonymous referees also provided helpful comments to improve the style and contents of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Hagerman, Helsingør
Rights and permissions
About this article
Cite this article
Arroyo, N.L., Maldonado, M., Pérez-Portela, R. et al. Distribution patterns of meiofauna associated with a sublittoral Laminaria bed in the Cantabrian Sea (north-eastern Atlantic). Marine Biology 144, 231–242 (2004). https://doi.org/10.1007/s00227-003-1191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1191-8