Abstract
The Yellow Sea in China has experienced annual large-scale green tides since 2007. Ulva prolifera, the dominant causative species, originating from Pyropia yezoensis aquaculture rafts, experiences periodic tidal emersion in the intertidal nursery. It was proposed that physiological adaptations of U. prolifera may enable it to survive the harsh intertidal environment and contribute to subsequent blooms. In this paper, we measured the responses of photosynthesis and nitrogen assimilation of U. prolifera during desiccation of 0–6 h and subsequent rehydration for 0–24 h. The results suggested that both PSII photosynthesis and nitrogen assimilation were significantly reduced at the onset of desiccation. These reductions were reflected by decreases in the maximum quantum yield (F v/F m), effective photochemical quantum yield (YII), maximal electron transport rate (ETRmax), light utilization efficiency (α), NO3-N and NH4-N uptake rates, tissue nitrogen concentration (TN), and nitrate reductase activity (NRA). Desiccation temporarily lowered PSII photosynthesis, but rates recovered after algae were submerged for 24 h. Assimilation of nitrogen was also reduced during exposure to air. Although both NO3-N and NH4-N uptake rate recovered within 12 h upon rehydration, TN and NRA were not completed within 24 h. U. prolifera therefore appears well adapted to survive prolonged periods of desiccation, as it experiences growing in Pyropia farms along the Chinese Jiangsu coast. These traits, along with its ability to grow fast, may explain its success and one of the mechanisms behind the formation of the extreme blooms in the Yellow Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intertidal macroalgae are subjected to fluctuations in physicochemical variables, such as high light intensities, high (or low) salinity, temperature extremes, nutrient deficiency, and desiccation (Kuwae et al. 2003; Liu and Pang 2010; Cabello-Pasini et al. 2011). This exposure can alter physiological features in the macroalgae (Holzinger and Karsten 2013; Niwa and Harada 2013). Parages et al. (2014) found that MAPK-like proteins in six representative intertidal macroalgae (Ulva rigida and Chaetomorpha aerea in Chlorophytes, Corallina elongata and Jania rubens in Rhodophytes, and Dictyota dichotoma and Dictyota spiralis in Phaeophytes) were highly phosphorylated in response to desiccation stress. Some intertidal macroalgae significantly decrease their photosynthetic rates following desiccation but quickly recover their photosynthetic abilities after rewetting (Kawamitsu et al. 2000; Karsten and Holzinger 2012). Additionally, a previous study indicated that the magnitude of the effects caused by desiccation on the early development of Mazzaella laminarioides, Scytosiphon lomentaria, and Lessonia nigrescens is one of the most important factors determining the position of the species in the intertidal zone (Contreras-Porcia et al. 2012). Kim et al. (2008, 2009) found that the physiological adaptations associated with nitrogen assimilation by species during repetitive exposure to severe desiccation and rehydration were related to their vertical distribution.
The Yellow Sea in China has experienced annual large-scale green tides since 2007. The main causative species has been identified as Ulva prolifera, which grows as macroalgal blooms, as seen in many estuaries and coastal seas around the world (Martins and Marques 2002; Lin et al. 2011; Liu et al. 2013). In China, between May and July 2008, prior to the Olympic sailing competition, large-scale blooms of Ulva prolifera caused the world’s largest green tide (Zhang et al. 2011; Xu et al. 2012). This green tide consisted of more than 1 million tons of drifting biomass and covered an area of 13,000–30,000 km2 (Sun et al. 2008). Fast growth, stress tolerance, an extraordinary propagation capability, and very high nutrient uptake are believed to be the physiological characteristics that allowed U. prolifera to act as the causative species of the world’s largest green-tide blooming event (Ye et al. 2011). The increased physiological ability to take up and store nutrients is considered to be an important factor that allows the species to dominate canopies of attached algae in coastal ecosystems (Runcie et al. 2003, 2004; Luo et al. 2012).
Extensive studies have shown that U. prolifera grows naturally in shallow waters, but coastal eutrophication and tide action caused large-scale blooming (Lin et al. 2011; Wang et al. 2011). Liu et al. (2009) proposed that the cause of the U. prolifera green-tide blooms was the rapid expansion of Pyropia yezoensis aquaculture along the Jiangsu coastline and that the particular oceanographic conditions found there favored rapid growth of the bloom. During the daily emersion cycle, U. prolifera attached to P. yezoensis aquaculture rafts usually undergo several hours of desiccation stress during periodic tidal emersion. Although Gao et al. (2011, 2014a) have found that U. prolifera performed a higher tolerance to desiccation stress of photosystem I than that of photosystem II, the survival mechanism associated with nutrient uptake has been little reported.
In addition to having high photosynthetic activity, U. prolifera also has a higher nutrient uptake capacity than other intertidal macroalgae, such as U. linza (Luo et al. 2012), Gracilaria lichvoides (Xu et al. 2012), and Gracilaria lemaneiformis (Gao et al. 2014b). Higher affinity for nitrogen uptake may allow U. prolifera to contribute to the macroalgal blooms in the Yellow Sea; however, few data are available on the response magnitude and recovery sequence between photosynthesis and nitrogen assimilation in U. prolifera under desiccation stress. To improve our understanding of how U. prolifera responds after different periods of desiccation and subsequent rehydration, PSII photosynthetic performance and nitrogen uptake, storage and reduction were determined simultaneously in the laboratory using algae collected from the shores of Qingdao.
Materials and methods
Sampling and culture conditions
Floating specimens of U. prolifera were collected in July 2012 from the intertidal zone at Zhanqiao Wharf, Qingdao, China (35°35′N, 119°30′E). In the laboratory, the intact samples were washed several times with sterile seawater, sterilized with 1 % sodium hypochlorite for 2 min, and then rinsed with autoclaved seawater. They were then precultured in sterile seawater enriched with f/2 medium (Berges et al. 2001) but without additional N at a constant temperature of 20 °C and light intensity of 100 μmol photons m−2 s−1 in a 12:12 h light/dark cycle in a GXZ-280 C intelligent illumination incubator (Ningbo Jiangnan Instrument, China) for 48 h before running experiments. The culture medium was completely renewed every 2 days. The pH and salinity of the seawater used for experiments were 8.0 and 30 PSU, respectively.
Experimental design
To determine the physiological responses of U. prolifera to desiccation and rehydration, fresh algae were gently blotted dry with filter paper to remove excess water and 0.3 g placed on each of 36 culture dishes. The algae were left to dry naturally at 25 °C, 100 μmol photons m−2 s−1, and 40 % humidity for 0 h (D0) 2 h (D2), 4 h (D4), and 6 h (D6), respectively. After desiccation, the relative water content (RWC) of a thallus was calculated according to the formula:
where W t is the weight of the thallus subjected to desiccation treatment, W d is the dry weight of the thallus after drying for approximately 24 h at 80 °C, and W 0 is the wet weight of the hydrated thallus before desiccation. The RWC was 100, 80, 50, and 20 % after dehydration for 0, 2, 4, and 6 h, respectively. At each time point (t = 0, 2, 4, or 6 h), nine samples of each desiccation treatment (D0, D2, D4, and D6) were collected to measure the algal physiological responses. Three of the algal samples were used to determine photosynthetic characteristics, three samples were ground and stored at −80 °C for tissue NO3 −, NO2 −, and NH4 + analyses, and three samples were thoroughly blotted dry, frozen, and stored in liquid N2 for later nitrate reductase activity (NRA) analysis.
To further explore recovery capacity from desiccation stress, 27 algal samples of each desiccation treatment (D0, D2, D4, and D6) were subsequently subjected to one of four rehydration periods: no rehydration (R0) or rehydration for 6 h (R6), 12 h (R12), or 24 h (R24) by placing the fronds in 200 mL Erlenmeyer flask with 120 mL of 20 °C seawater culture medium supplemented with NH4NO3. The initial concentration of NO3 − and NH4 + in culture medium during rehydration is 180 and 95 µmol L−1, respectively. At each time point (t = 0, 6, 12, or 24 h), nine samples were collected to monitor physiological response as mentioned above and 5 mL of the culture medium collected for NH4 + and NO3 − analyses. All cultures were shaken manually every three hours to make all algal samples could get sufficient light.
Photosynthetic responses to dehydration and rehydration
The photosynthetic properties of the blades were measured during desiccation and rehydration. The PSII chlorophyll fluorescence was determined at room temperature using a Dual-PAM-100 fluorometer (Walz, Effeltrich, Germany) connected to a PC with WinControl software. Before measurement, samples were kept in the dark for 15 min and the minimum fluorescence (F 0) was determined under low light. A saturation light pulse was applied to obtain maximum fluorescence (F m) in the dark-adapted samples. The difference between F m and F 0 was referred to as the variable fluorescence (F v), and the maximum quantum yield (F v/F m) was calculated using the formula:
The F m and F 0 yield in the illuminated samples was denoted as \(F_{\rm m}^{'}\), and F t. YII was the effective photochemical quantum yield of PSII and was calculated using:
Rapid fluorescence light-response curves (RLC) were then constructed using the same samples that had been exposed to increasing irradiances for 15 s (PAR: from 11 to 830 μmol photons m−2 s−1). Then, the maximal electron transport rate (ETRmax) and light utilization efficiency (α) was obtained from the Dual-PAM software.
Determination of the nitrogen uptake rate (NUR), nitrate reductase activity (NRA), and tissue nitrogen concentration (TN) during dehydration and rehydration
Water samples used to determine nitrate (NO3-N) and ammonium (NH4-N) were analyzed photometrically using an AutoAnalyzer (BRAN and LUEBBE AA3, Germany). The nutrient uptake rates were calculated as:
where NUR is the nutrient uptake rate (μmol g−1 fresh weight day−1); C 0 and C t are the nutrient concentrations (μmol L−1) at the beginning and the end of the experiment, respectively; V is the volume of water (in L); FW is algal fresh weight (g−1); and t is the time interval (in h).
Nitrate reductase activity (NRA) was measured as follows: Frozen algal samples were ground to a powder in liquid nitrogen and estimated using an in vitro assay method described by Young et al. (2005), with a slight modification regarding the assay temperature, which was 20 °C to be consistent with the preculture temperature. To determine tissue nitrogen content, the frozen ground powder mixed with Milli-Q water was vortexed and extracted in a boiling water bath for 60 min. Then, they were cooled and filtered. The tissue nitrate (NO3-N) and ammonium (NH4-N) in filtrate were determined photometrically using an AutoAnalyzer (BRAN and LUEBBE AA3, Germany).
Statistical analysis
Statistical analyses were carried out with SPSS 17.0. Two-way ANOVA was used to analyze the combined effect of desiccation and rehydration on tested physiological traits. The significance level was set at 0.05 for all tests unless otherwise stated.
Results
Variation in PSII photosynthetic variables
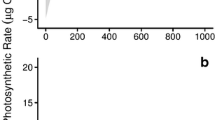
The PSII photosynthetic variables varied significantly during the course of desiccation and rehydration (Two-way ANOVA, df = 9, p < 0.001, Table 1; Fig. 1). The maximum quantum yield (F v/F m) decreased by an order of magnitude after desiccation lasting longer than 4 h (Fig. 1a). Maximum yield in algae desiccated for 2 h recovered after 6 h of hydration and algae desiccated for 4 or 6 h returned to initial values after rehydration for 24 h. Variations in the effective quantum yield of PSII (YII), following desiccation and then rehydration, were also seen (two-way ANOVA, df = 9, F = 55.768, p < 0.001, Fig. 1b). It declined significantly with desiccation and decreased to zero after desiccation for 6 h with only 20 % RWC. Moreover, YII levels in the samples desiccated for 6 h did not recover until they had been rehydrated for 24 h. The other two PSII photosynthetic variables, the maximal electron transport rate ETR max (two-way ANOVA, df = 9, F = 14.757, p < 0.001, Fig. 1c) and the light utilization efficiency (α) (two-way ANOVA, df = 9, F = 23.789, p < 0.001, Fig. 1d), showed similar variation trends to YII during the course of desiccation and rehydration.
Variations in the PSII photosynthetic variables F v/F m (a), YII (b), ETR max (c), and α (d) in U. prolifera thalli after different periods of desiccation and rehydration. D0, D2, D4, and D6 represent desiccation of 0, 2, 4, and 6 h, respectively. R0, R6, R12, and R24 represent rehydration for 0, 6, 12, and 24 h, respectively. Vertical bars represent the SD of three incubations
Variation in nitrogen uptake rate
U. prolifera could take up NO3 − and NH4 + simultaneously, but preferred NH4-N to NO3-N. In samples that had not been desiccated (D0), the average uptake rate of NH4-N during periods of 6 and 12 h was 0.15 and 0.85 times higher, respectively, than that of NO3-N (Fig. 2). There were significant differences in the nitrogen uptake rates during the course of desiccation and rehydration (two-way ANOVA, Table 1; Fig. 2). The NO3-N uptake rates in samples desiccated for 2 h showed similar trends in variation to D0 treatment in that they decreased markedly during the course of the experiment. NO3-N uptake in samples desiccated for 4 and 6 h increased during the first 12 h of rehydration, but then decreased (Fig. 2a). The NH4-N uptake rates in all desiccated samples increased to a maximum after rehydration for 12 h and then declined (Fig. 2b). In D0, NH4-N uptake rate declined continuously. Additionally, both NH4-N and NO3-N uptake rates during the first 6 h of dehydration were less than those in D0, but they were higher or nearly so during the next 18 h of dehydration.
Nitrogen uptake rate during rehydration process of desiccated thalli. a NO3-N uptake rate; b NH4-N uptake rate. D0, D2, D4, and D6 represent desiccation of 0, 2, 4, and 6 h, respectively. R0, R6, R12, and R24 represent rehydration for 0, 6, 12, and 24 h, respectively. Vertical bars represent the SD of three incubations
Variation in the tissue nitrogen concentration
The tissue NO2 − concentrations were consistently below 0.004 mmol g−1 fresh wt (close to 0), so they are not reported here. During the course of desiccation and rehydration, both tissue NO3 − (two-way ANOVA, df = 9, F = 5.435, p < 0.001, Table 1; Fig. 3a) and NH4 + (two-way ANOVA, df = 9, F = 28.962, p < 0.001, Table 1; Fig. 3b) changed dramatically. In the process of rehydration, tissue NO3 − generally increased markedly from the onset of rehydration (Fig. 3a). In contrast, tissue NH4-N first increased after rehydration for 6 h and then decreased (Fig. 3b). Additionally, although both tissues NO3-N and NH4-N in the desiccated samples increased during rehydration, they did not reach the levels seen in D0 after 24 h.
Variation in nitrate reductase activity (NRA)
Nitrate reductase activity (NRA) changed significantly during the course of desiccation and rehydration (two-way ANOVA, df = 9, F = 3.367, p = 0.005, Table 1, Fig. 4). With the increase in duration of desiccation, NRA deceased significantly. On the other hand, during rehydration, there were significant differences among treatment of R6 and R12. But after rehydration for 24 h, it was shown that there were not any significant differences among samples of D0, D2, D4, and D6.
Discussion
Previous studies have demonstrated that large-scale Pyropia aquaculture during annual March–May along the Jiangsu coast provided a nursery bed for U. prolifera (Sun et al. 2008; Liu et al. 2009; Pang et al. 2010). Algae attached on farming facilities experience a daily emersion cycle and this exposure usually lasts no more than six hours. Then, winds transport the macroalgae along with nutrient-rich coastal waters northwards where inorganic nitrogen concentration could exceed more than 60 μmol L−1 and the ratio of NO3 −/NH4 + was more than 2 in summer (Wang 2013). It was suggested that physiological adaptations of U. prolifera may enable it to survive the harsh intertidal environment and contribute to subsequent blooms as floaters. Although the algal sample used in our study was collected from Qingdao seashore, it has been confirmed as the exact species of U. prolifera, based on genetic analysis before experiment. In the study, experimental design mimicked the process of desiccation and rehydration experienced by U. prolifera in intertidal. Treatments of desiccation (D2, D4, and D6) and rehydration (R24, R12, and R6) represented that the algae inhabiting different tidal heights were differently exposed to air at low tide and submerged in seawater at high tide (Gao et al. 2014a).
Desiccation is one of the most important factors influencing the photosynthetic states of intertidal algae and has therefore received considerable attention (Henley et al. 1992; Xie et al. 2013). Most intertidal species significantly decreased photosynthesis following desiccation. However, some intertidal macroalgae showed a constant or even increased level of photosynthesis from the onset of emersion and declined thereafter as desiccation became more severe (Sven and Eshel 1983). Our PSII photosynthetic performance results for U. prolifera were consistent with the declining trend during desiccation (Fig. 1).
In contrast, less information has been reported on the effects of desiccation on nutrient uptake by intertidal macroalgae. Some studies have indicated that desiccation stress can determine upper distribution limits and may enhance short-term nutrient uptake in some eulittoral algal species (Thomas et al. 1987b; Hurd and Dring 1991). However, the effects were species specific and may be related to the species’ position in the eulittoral zone. Kim et al. (2009) compared the growth and nitrate uptake of New England Porphyra species from different tidal elevations in relation to desiccation. Their study found that eulittoral Porphyra umbilicalis, which is subjected to longer exposure times, had higher time-use efficiency than sublittoral Porphyra amplissima in terms of nitrate uptake. In our study, desiccation treatment reduced both NH4-N and NO3-N uptake in U. prolifera during the first 6 h of rehydration compared to the control (Fig. 2). This demonstrated that desiccation stress had lasting negative effects on nitrogen assimilation by the alga. During the later stages of rehydration (12–24 h), the uptake rate in the desiccated samples recovered to levels that were similar to the control.
Inorganic nitrogen and carbon deficiency occurred during emersion when seaweed was isolated from its source of nitrogen (Cabello-Pasini et al. 2011). It has been found that some Porphyra species could prevent a decrease in their tissue carbon and nitrogen for a certain period of time (Kim et al. 2009). U. prolifera was different from Porphyra in that the tissues NH4-N and NO3-N decreased dramatically compared to the control during desiccation (Fig. 3). This may be due to the low photosynthesis during emersion, and no energy was available to uptake and retain inorganic nitrogen. It has been reported that the degree of N and P release in eulittoral seaweeds is dependent on their vertical habitats (Thomas et al. 1987a; Hurd and Dring 1991). The upper littoral macroalga Gracilaria pacifica lost nitrogen after 50 % water loss, while sublittoral plants lost nitrogen after only 10 % water loss (Thomas et al. 1987a).
The ecological consequences of punctuated, extreme climate events depend not only upon a species’ physiological capacity to tolerate such stresses but also upon their capacity to recover from such stressful events (Wieters et al. 2013). Wieters et al. (2013) showed that short-term exposure of Gelidium chilense to UVB radiation during daytime low tides can critically compromise the ability of the fronds to recover once the stressor is removed. Moreover, the recovery ability differed between tidal heights and among populations from different sites along the central coast. Extensive studies (Proctor and Smirnoff 2000; Kim et al. 2008, 2009; Gao et al. 2011; Gao and Wang 2012) have shown that although desiccation significantly reduced photosynthesis during diurnal low tides, some intertidal macroalgae fully recovered their photosynthetic abilities when rehydrated. In this study, U. prolifera also presented the recovery capacity of photosynthesis during the cycle of desiccation and rehydration (Fig. 1).
The recovery in nitrate uptake after periods of desiccation is possibly another factor that regulates onshore algal distribution (Kim et al. 2009). Previous studies have shown that the sublittoral species Porphyra amplissima did not recover its nitrate uptake function after severe desiccation stress, whereas nitrate uptake by Porphyra umbilicalis did not differ between desiccation and nondesiccation treatments (Kim et al. 2009). In this study, desiccation stress had lasting effects on nitrogen assimilation by U. prolifera. The uptake rate (Fig. 2), tissue nitrogen concentration (Fig. 3), and NRA during rehydration (Fig. 4) were all lower than those of the control, which was continuously immersed in seawater during the experiment. Nitrate reductase is considered as the rate-limiting enzyme in nitrogen assimilation pathway. The ratio nitrate/ammonium is of prime relevance since NH4 + usually inhibits nitrate uptake and nitrate reductase in Ulva (Corzo and Niell 1992; Wang et al. 2015). In the present, although the initial concentration of NO3 − was one time higher than NH4 + in culture medium, U. prolifera preferred NH4 + than NO3 −.
The opportunistic species U. prolifera is well known for its high rates of nutrient uptake, photosynthesis, and growth, which has given it competitive advantages over other species (Fan et al. 2014). Moreover, it has evolved a good photosynthetic response mechanism to address different light, temperature, and UV stresses (Dong et al. 2012; Xu et al. 2013). In our study, the alga also showed plastic photosynthetic abilities during the desiccation to rehydration period. In contrast, its nitrogen assimilation recovered more slowly than photosynthesis and could not be fully restored within 24 h. However, it does have some nitrogen uptake capacity because the desiccated alga samples continued to grow after rehydration, and biomass increased over the following days (data not shown). It could be that its nitrogen uptake after desiccation takes more than 24 h to fully recover. To confirm whether the alga possesses nitrogen metabolism plasticity, an experiment investigating the length of time needed for nitrogen assimilation by U. prolifera to fully recover from severe desiccation will be undertaken in the future. The physiological responses of photosynthesis and nitrogen assimilation may be an important adaptive mechanism for U. prolifera to survive the harsh intertidal environment and for its successful colonization of coastal ecosystems.
References
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol 37:1138–1145
Cabello-Pasini A, Macías-Carranza V, Abdala R, Korbee N, Figueroa FL (2011) Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). J Appl Phycol 23:363–369
Contreras-Porcia L, Callejas S, Thomas D, Sordet C, Pohnert G, Contreras A, Lafuente A, Flores-Molina MR, Correa JA (2012) Seaweeds early development: detrimental effects of desiccation and attenuation by algal extracts. Planta 235:337–348
Corzo A, Niell FX (1992) Inorganic nitrogen metabolism in Ulva rigida illuminated with blue light. Mar Biol 112:223–228
Dong MT, Zhang XW, Zou J, Ye NH, Xu D, Mou SL, Liang CW, Wang WQ (2012) Identification and expression analysis of the gene lhcSR associated with adaptation to light and low temperature stress in the green tide forming alga Ulva prolifera. Mar Biol Res 8:746–755
Fan X, Xu D, Wang YT, Zhang XW, Cao SN, Mou SL, Ye NH (2014) The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. J Appl Phycol 26:527–544
Gao S, Wang GC (2012) The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J Exp Bot doi:10.1093/jxb/ers082
Gao S, Shen SD, Wang GC, Niu JF, Lin AP, Pan GH (2011) PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol 52:885–893
Gao S, Zheng ZB, Gu WH, Xie XJ, Huan L, Pan GH, Wang GC (2014a) Photosystem I shows a higher tolerance to sorbitol-induced osmotic stress than photosystem II in the intertidal macro-algae Ulva prolifera (Chlorophyta). Physiol Plantarum 152:380–388
Gao ZQ, Xu D, Meng CX, Zhang XW, Wang YT, Li DM, Zou J, Zhuang ZM, Ye NH (2014b) The green tide-forming macroalga Ulva linza outcompetes the red macroalga Gracilaria lemaneiformis via allelopathy and fast nutrients uptake. Aquat Ecol 48:53–62
Henley WJ, Lindley ST, Levavasseur G, Osmond CB, Ramus J (1992) Photosynthetic response of Ulva rotundata to light and temperature during emersion on an intertidal sand flat. Oecologia 89:516–523
Holzinger A, Karsten U (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms. Front Plant Sci 4:327
Hurd CL, Dring MJ (1991) Desiccation and phosphate uptake by intertidal fucoid algae in relation to zonation. Brit Phycol J 26:327–333
Karsten U, Holzinger A (2012) Light, temperature, and desiccation effects on photosynthetic activity, and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microbial Ecol 63:51–63
Kawamitsu Y, Driscoll T, Boyer JS (2000) Photosynthesis during desiccation in an intertidal alga and a land plant. Plant Cell Physiol 41:344–353
Kim JK, Kraemer GP, Yarish C (2008) Physiological activity of Porphyra in relation to eulittoral zonation. J Exp Mar Biol Ecol 365:75–85
Kim JK, Kraemer GP, Yarish C (2009) Research note: comparison of growth and nitrate uptake by New England Porphyra species from different tidal elevations in relation to desiccation. Phycol Res 57:152–157
Kuwae T, Kibe E, Nakamura Y (2003) Effect of emersion and immersion on the porewater nutrient dynamics of an intertidal sandflat in Tokyo Bay. Estuar Coast Shelf Sci 57:929–940
Lin HZ, Jiang P, Zhang JX, Wang JF, Qin S, Sun S (2011) Genetic and marine cyclonic eddy analyses on the largest macroalgal bloom in the world. Environ Sci Technol 45:5996–6002
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Biol Ecol 382:82–87
Liu D, Keesing JK, Xing QG, Shi P (2009) World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull 58:888–895
Liu DY, Keesing JK, He PM, Wang ZL, Shi YJ, Wang YJ (2013) The world’s largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuar Coast Shelf Sci 129:2–10
Luo MB, Liu F, Xu ZL (2012) Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat Bot 100:18–24
Martins I, Marques JC (2002) A model for the growth of opportunistic macroalgae (Enteromorpha sp.) in tidal estuaries. Estuar Coast Shelf Sci 55:247–257
Niwa K, Harada K (2013) Physiological responses to nitrogen deficiency and resupply in different blade portions of Pyropia yezoensis f. narawaensis (Bangiales, Rhodophyta). J Exp Mar Biol Ecol 439:113–118
Pang SJ, Liu F, Shan TF, Xu N, Zhang ZH, Gao SQ, Chopin T, Sun S (2010) Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar Environ Res 69:207–215
Parages ML, Capasso JM, Niell FX, Jiménez C (2014) Responses of cyclic phosphorylation of MAPK-like proteins in intertidal macroalgae after environmental stress. J Plant Physiol 171:276–284
Proctor MCF, Smirnoff N (2000) Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. J Exp Bot 51:1695–1704
Runcie JW, Ritchie RJ, Larkum AWD (2003) Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat Bot 76:155–174
Runcie JW, Ritchie RJ, Larkum AWD (2004) Uptake kinetics and assimilation of phosphorus by Catenella nipae and Ulva lactuca can be used to indicate ambient phosphate availability. J Appl Phycol 16:181–194
Sun S, Wang F, Li C, Qin S, Zhou M, Ding L, Pang S, Duan D, Wang G, Yin B, Yu R, Jiang P, Liu Z, Zhang G, Fei X, Zhou M (2008) Emerging challenges: massive green algae blooms in the Yellow Sea. Nat Precedings 2266:1–5
Sven B, Eshel A (1983) Photosynthesis of Ulva sp. I. Effects of desiccation when exposed to air. J Exp Mar Biol Ecol 70:91–97
Thomas TE, Harrison PJ, Turpin DH (1987a) Adaptations of Gracilaria pacifica (Rhodophyta) to nitrogen procurement at different intertidal locations. Mar Biol 93:569–580
Thomas TE, Turpin DH, Harrison PJ (1987b) Desiccation enhanced nitrogen uptake rates in intertidal seaweeds. Mar Biol 94:293–298
Wang WT (2013) Study on the distribution and enrichment of nutrients in the sea-surface microlayer of the East China Sea. Dissertation, Ocean University of China
Wang XH, Qiao F, Lu J, Gong F (2011) The turbidity maxima of the northern Jiangsu shoal-water in the Yellow Sea, China. Estuar Coast Shelf Sci 93:202–211
Wang DS, Xu D, Wang YT, Fan X, Nai HY, Wang WQ, Zhang XW, Mou SL, Guan Z (2015) Adaptation involved in nitrogen metabolism in sea ice alga Chlamydomonas sp. ICE-L to Antarctic extreme environments. J Appl Phycol 27:787–796
Wieters EA, Medrano A, Quiroga G (2013) Spatial variation in photosynthetic recovery of intertidal turf algae from acute UVB and temperature stress associated with low tides along the central coast of Chile. J Exp Mar Biol Ecol 449:340–348
Xie X, Gao S, Gu W, Pan G, Wang G (2013) Desiccation induces accumulations of antheraxanthin and zeaxanthin in intertidal macro-alga Ulva pertusa (Chlorophyta). PLoS ONE 8:e72929
Xu D, Gao ZQ, Zhang XW, Fan X, Wang YT, Li DM, Wang WQ, Zhuang ZM, Ye NH (2012) Allelopathic interactions between the opportunistic species Ulva prolifera and the native macroalga Gracilaria lichvoides. PLoS ONE 7:e33648
Xu JF, Zhang XW, Ye NH, Zheng Z, Mou SL, Dong MT, Xu D, Miao JL (2013) Activities of principal photosynthetic enzymes in green macroalga Ulva linza: functional implication of C4 pathway in CO2 assimilation. Sci China Life Sci 56:571–580
Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, Zou J, Zhuang ZM, Wang QY (2011) ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol Res 26:477–485
Young E, Lavery P, Van Elven B, Dring M, Berges JA (2005) Nitrate reductase activity in macroalgae and its vertical distribution in macroalgal epiphytes of seagrasses. Mar Ecol Prog Ser 288:103–114
Zhang XW, Xu D, Mao YZ, Li YX, Xue SY, Zou J, Lian W, Liang CW, Zhuang ZM, Wang QY, Ye NH (2011) Settlement of vegetative fragments of Ulva prolifera confirmed as an important seed source for succession of a large-scale green tide bloom. Limnol Oceanogr 56:233–242
Acknowledgments
This work was supported by National Natural Science Foundation of China (41306179), Hi-Tech Research and Development Program (863) of China (2012AA052103).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: K. Bischof.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Xu, D., Zhang, X., Wang, Y. et al. Responses of photosynthesis and nitrogen assimilation in the green-tide macroalga Ulva prolifera to desiccation. Mar Biol 163, 9 (2016). https://doi.org/10.1007/s00227-015-2806-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-015-2806-6